SUMMARY
We discovered a class of naturally occurring human proteins with unusually high net positive charge that can potently deliver proteins in functional form into mammalian cells both in vitro, and also in murine retina, pancreas, and white adipose tissues in vivo. These findings represent new, diverse macromolecule delivery agents for in vivo applications, and also raise the possibility that some of these human proteins may penetrate cells as part of their native biological functions.
INTRODUCTION
Macromolecules serve as essential research tools and important human therapeutics but are limited by their general inability to cross cell membranes. Previously we discovered that engineered green fluorescent protein (GFP) variants with very high net positive charge can potently penetrate and deliver associated macromolecules into mammalian cells (Cronican et al., 2010; McNaughton et al., 2009). Since these “supercharged” GFPs were engineered to maximize net charge (Lawrence et al., 2007), we hypothesized that naturally occurring human proteins with high net positive charge may possess undiscovered cell-penetrating properties. Identifying such a class of proteins would enable many proteins with varying charge, structure, and molecular weight to be used as new agents for the delivery of macromolecular cargo into mammalian cells, and would also raise the possibility that these proteins may possess native biological functions associated with their ability to penetrate cells.
RESULTS
Identification of Naturally Supercharged Human Proteins
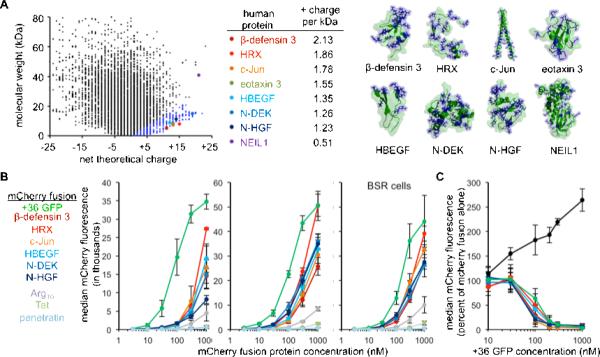
To identify naturally supercharged human proteins (NSHPs) we ranked all proteins within the human proteome by their net theoretical charge to molecular weight ratio. Based on our previous cell-penetration results with +15, +25 and +36 GFPs (McNaughton et al., 2009), we hypothesized that proteins with a ratio of charge units per kDa greater than 0.75 could be potent cell-penetrating proteins. Of the 13,417 human proteins with evidence of existence at the protein level in the Swiss-Prot database, 309 entries (2.3%) possess a +charge:mw ratio > 0.75, and 104 (0.8%) have a ratio equal to or greater than that of +36 GFP (+1.27/kD). To facilitate expression of the human proteins in E. coli, we performed the same analysis on human proteins within the RSCB Protein Data Bank (PDB) that were expressed in E. coli and chose seven unrelated examples from this list: -defensin 3, HRX, c-Jun bZIP domain, eotaxin 3, HBEGF, N-DEK, and N-HGF (Figure 1A). -defensin 3 is an extracellular antimicrobial protein (Table S1A) (Harder et al., 2001). HRX, c-Jun and N-DEK are nuclear proteins known to interact with DNA (Glover and Harrison, 1995; Hollenbach et al., 2002; Nakamura et al., 2002). HBEGF and N-HGF are extracellular growth factors (Cioce et al., 1996; Elenius et al., 1997) and eotaxin 3 is a chemotactic protein (Shinkai et al., 1999). We purified these proteins as fusions with the red fluorescent protein mCherry. Eotaxin 3-mCherry did not express to appreciable levels. We incubated the remaining fusions with HeLa, 3T3, and BSR cells in vitro for 4 hours and then washed the cells with heparin PBS to remove all membrane-bound protein (Figure S1C). We observed that all six NSHP-mCherry fusions potently penetrated all three cell lines both by live-cell fluorescence microscopy (Figures S1D) and by flow cytometry (Figure 1B). The NSHPs delivered mCherry with up to 40-fold greater efficiency than standard protein transduction domains (PTDs) including Tat, Arg10, and penetratin. Internalization of all NSHP-mCherry fusions tested, but not internalization of transferrin-Alexa568, was inhibited by +36 GFP (Figure 1C) indicating that the NSHPs penetrate cells by a mechanism competitive with that of +36 GFP. We also selected a larger protein (NEIL1 endonuclease, 40.9 kDa) with high net theoretical charge but lower charge to molecular weight ratio and observed that when enzymatically biotinylated, NEIL1 delivers non-covalently associated streptavidin into BSR cells at high nM concentrations (Figure S1H). Taken together, these results suggest that many naturally supercharged human proteins possess the ability to potently penetrate mammalian cells.
Figure 1. Naturally supercharged human proteins (NSHPs) penetrate mammalian cells in vitro.
(A) Plot of human proteins expressed from E. coli within the Protein Data Bank. The blue dots represent proteins with positive charge:molecular weight ratios exceeding +0.75/kDa. (B) Median mCherry fluorescence of HeLa, 3T3 and BSR cells incubated with NSHP-mCherry fusions as measured by flow cytometry. (C) NSHP-mCherry fusions (300 nM) were incubated with HeLa cells for 4 hours with increasing concentrations of +36 GFP. After incubation, cells were washed with heparin PBS and internalized NSHP-mCherry was quantified by flow cytometry. For each fusion protein, the percentage of mCherry fluorescence relative to the mCherry intensity without +36 GFP is shown. The co-incubation experiment was also performed with 10 μg/mLtransferrin-Alexa568 (shown in black), which is internalized through an independent mechanism. See also Figure S1.
Functional Protein Delivery into Mammalian Cells in vitro
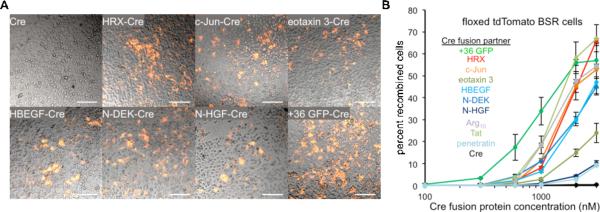
To test the ability of NSHPs to deliver functional protein outside of endosomes we generated Cre recombinase fusions to six NSHPs (HRX, c-Jun bZIP domain, eotaxin 3, HBEGF, N-DEK, and N-HGF) (Figure S2). Functional Cre delivery requires internalization of Cre, localization to the nucleus, tetramerization, and site-specific DNA recombination to activate reporter gene expression. We incubated the six NSHP-Cre fusion proteins with floxed tdTomato BSR cells and again in all six cases observed expression of the tdTomato reporter gene both by live-cell fluorescence microscopy (Figure 2A) and by flow cytometry (Figure 2B). For three of the human proteins (c-Jun, HBEGF, and N-DEK), the efficiency of functional Cre delivery at concentrations below 1 μM was higher than that of Arg10 and penetratin, and comparable to that of Tat. These proteins were not toxic to cultured BSR cells (Figure S2D). Collectively, these results demonstrate that a diverse set of proteins within the human proteome can potently deliver functional protein into mammalian cells, some of which is able to escape endosomes.
Figure 2. Naturally supercharged human proteins (NSHPs) deliver active protein into mammalian cells in vitro.
(A) Live-cell fluorescence microscopy of floxed tdTomato BSR cells two days after incubation for 4 hours with 2 μM NSHP-Cre fusions. The scale bar is 200 μm. (B) Percent recombined cells among floxed tdTomato BSR cells incubated with NSHP-Cre fusions as measured by flow cytometry. See also Figure S2.
Functional Protein Delivery into Mammalian Cells in vivo
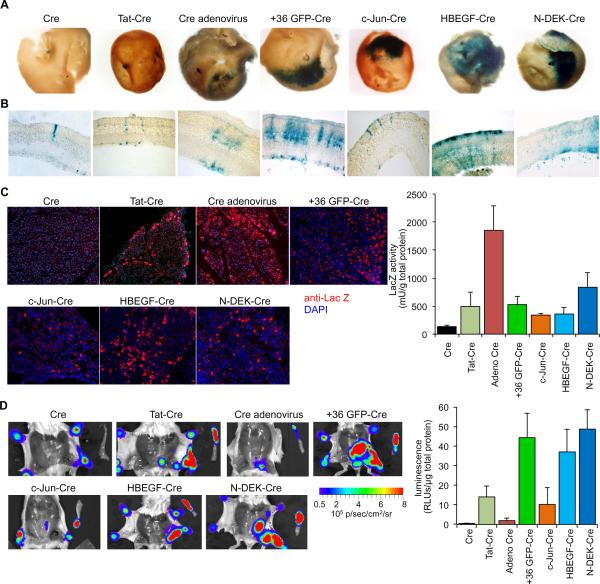
We next sought to determine if this class of proteins could serve as a natural platform for the delivery of macromolecular cargo in vivo. The direct delivery of functional protein into cells in vivo is of special interest because of the rarity of successful in vivo protein transduction examples (Caron et al., 2001; Jarver et al., 2010), the clinical need for protein-delivery agents (Chen and Cepko, 2009; Seale et al., 2007; Zhou et al., 2008), and the drawbacks associated with alternative methods such as viral infection (Atkinson and Chalmers, 2010). We therefore tested the ability of NSHPs to deliver functional protein in vivo in adult mice in three tissues of current therapeutic interest: the retina (Chen and Cepko, 2009), pancreas (Zhou et al., 2008), and white adipose tissues (Seale et al., 2007). Cre fusions of three NSHPs (c-Jun, HBEGF, N-DEK) along with Cre, Tat-Cre, +36 GFP-Cre, Cre expressing adenovirus were injected subretinally into floxed LacZ mice (n = 4). Retinae were harvested three days post-injection and the NSHP-Cre injected retinae were found to possess large patches of recombined cells. In contrast, retinae injected with wild-type Cre did not possess significant populations of recombined cells (Figures 3A and S3A). Cryosections of these retinae indicated labeling of Müller glia cells, as well as other cell types, including cells of hematopoetic origin (Figure 3B). The same set of three NHSP-Cre fusions were individually injected directly into the pancreas of floxed LacZ mice (n = 3 for quantification, n = 1 for imaging). The pancreases were harvested five days post-injection and the NSHP-Cre injected pancreases were found to possess up to 6-fold more LacZ+ cells than wild-type Cre injected pancreases by immunostaining and enzymatic assay (Figure 3C). Finally, the same suite of Cre proteins were injected subcutaneously into the abdomens of floxed luciferase mice (n = 4). The white adipose tissues of these mice were harvested three days post-injection, imaged, assayed for luciferase activity, and the NSHP-Cre injected mice were found to possess up to 125-fold more luminescence in their white adipose tissue over the white adipose tissues of mice injected with wild-type Cre (Figures 3D and S3B). To our knowledge, these results represent the first functional protein delivery into cells of white adipose tissue in vivo. Although these three examples involve local injections of supercharged proteins, we note that +36 GFP is able to circulate intravenously (Figure S3C) and five of the eight human proteins tested in this study are known to be naturally secreted proteins (Table S1A), suggesting that they may represent vectors compatible with systemic protein delivery in vivo. These results collectively establish that NSHPs can deliver functional protein into cells of a variety of mammalian tissues in vivo. For delivery to retinal cells and white adipose tissue, at least one NSHP was more effective at delivering active Cre than two of the most widely used current protein delivery methods, fusion with Tat peptide or infection with adenovirus (Figures 3A and 3D).
Figure 3. Naturally supercharged human proteins (NSHPs) deliver active proteins into three adult murine tissues in vivo.
(A) Adult floxed LacZ mice were injected subretinally with Cre fusion proteins. Recombination results in LacZ activity, which was visualized with X-gal stain (blue) three days after injection. (B) Sections of recombined retinae from the samples in (A) reveal that most of the recombination signal arises from Müller glial cells. (C) Adult floxed LacZ mice injected in the pancreas with Cre fusion proteins exhibit recombination in the exocrine tissues as indicated by LacZ immunostaining (red) and LacZ activity assay five days post-injection. (D) Adult floxed luciferase mice injected subcutaneously with Cre fusion proteins exhibit recombination in the white adipose tissue as visualized by luminescence and luciferase activity assay three days post-injection. For (A) and (D), representative results are shown; see Figure S3 for data from additional replicates.
DISCUSSION
Our previous work with engineered supercharged proteins led us in the present study to identify a class of hundreds of naturally occurring supercharged human proteins within the human proteome that possess net theoretical charge:molecular weight ratios similar to that of +36 GFP. All eight tested NSHPs selected from the PDB were observed to penetrate mammalian cells and deliver covalently or non-covalently associated protein, despite their diverse structures and known functions. It is generally accepted that positively charged peptides, polymers, and liposomes are able to penetrate mammalian cells (Nakase et al., 2008). Cationic peptides, however, have been reported to possess maximal cell-penetration potency with 8–15 positively charge amino acids and are inhibited by additional positively charged amino acids (Futaki et al., 2001; Mitchell et al., 2000). Our results suggest that even small proteins (5.2–15.1 kDa) that possess up to 31 positively charged amino acids behave differently than cationic peptides and exhibit cell-penetration and macromolecule-delivery capabilities that are not inhibited by their high charge magnitude. The unusual potency of NSHPs compared with cationic peptides may arise from differences in charge density, structure, or surface area. These results also suggest that the potent macromolecule delivery properties of supercharged GFPs are not limited to a small number of engineered proteins but instead are present among many different protein structures and folds, including proteins of much lower net theoretical charge magnitude (+10 to +19).
Previous bioinformatic methods have identified minimal cell-penetrating peptides within human proteins by searching for densely cationic regions of the protein sequence (Futaki et al., 2003; Hansen et al., 2008). In contrast, the proteins examined in the current study possess positively charged amino acids widely dispersed throughout their sequence, and would not have been identified using these previously reported methods. Moreover, the NSHPs we have identified were able to mediate internalization of fused proteins with up to 40-fold higher potency than commonly used cell-penetrating peptides. Thus, NSHPs are not only structurally distinct from most peptide-based protein delivery reagents, but are also functionally distinct, mediating internalization of fused protein with significantly greater potency for some applications. A 28-amino acid peptide from the bZIP domain of c-Jun has previously been identified as cell-penetrating based upon its known biological role of binding to DNA (Futaki et al., 2001). HBEGF has also been used to deliver protein into mammalian cells based upon its known affinity for epidermal growth factor receptors (Chandler et al., 1998). Our method was able to positively identify these cell-penetrating proteins based solely on their amino acid sequence.
Our findings also results raise the possibility that NSHPs may play biological roles that arise from their ability to penetrate cells but are currently unknown. For example, DEK was recently reported to be secreted by macrophage upon stimulation with interleukin-8 (Mor-Vaknin et al., 2006), a curious finding for a nuclear protein thought to be involved in chromatin remodeling. Our discovery that N-DEK can potently penetrate cells and escape endosomes as a fusion protein in vivo suggests that DEK may possess an intracellular function within target cells. Considering the large number of proteins within the NSHP class, as well as known cases in which secreted proteins have been shown to exhibit intracellular activity (Brunet et al., 2005; Frankel and Pabo, 1988; Green and Loewenstein, 1988; Joliot et al., 1991; Löfgren et al., 2008; Mallery et al., 2010), the possibility that some NSHPs may enter cells as part of their native functions merits further study.
SIGNIFICANCE
Our results reveal that potent vehicles for macromolecule delivery into mammalian cells in vivo already exist within the human body in the form of naturally supercharged proteins. The fact that the set of NSHPs tested include several secreted, circulating human proteins (Table S1) suggests that they could serve as a new set of biologic delivery agents with a diversity of important properties such as charge, structure, molecular weight, immunogenicity, stability, and in vivo half-life. Moreover, the ability of virtually all NHSPs to penetrate cells has not been previously reported (Chandler et al., 1998; Futaki et al., 2003; Kaouass et al., 2006; Rosenbluh et al., 2005). Our findings are consistent with the intriguing possibility that members of this substantial class of natural proteins may play undiscovered biological roles that arise from their ability to penetrate cells.
EXPERIMENTAL PROCEDURES
Identification of Naturally Supercharged Human Proteins
To identify naturally supercharged human proteins amenable to recombinant expression in E. coli, the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB, http://www.rcsb.org) was filtered for PDB IDs of proteins with source organism = Homo sapiens and expression organism = Escherichia coli. Approximately 10,000 unique PDB IDs matched both criteria and were downloaded as FASTA files. The primary sequence of each chain within each PDB ID was used to calculate net theoretical charge (lysine and arginine = +1 and aspartate and glutamate = −1) and molecular weight. The resulting data was compiled into one text file using a Python script.
To estimate the prevalence of naturally supercharged human proteins within the entire human proteome, the same analysis was performed on human proteins with evidence of existence at the protein level within the Swiss-Prot database (http://www.uniprot.org/).
Additional experimental procedures can be found in the Supplemental Experimental Procedures.
Supplementary Material
HIGHLIGHTS
Human proteins with unusually high net positive charge potently penetrate mammalian cells.
Naturally supercharged human proteins deliver fused protein in functional form to mammalian cells in vitro and in vivo in adult mice.
Naturally supercharged human proteins provide a diverse new class of non-viral vectors for the delivery of macromolecular cargo into mammalian cells.
ACKNOWLEDGEMENTS
We thank Paul Cohen and Bruce Spiegelman for assistance with white adipose tissue delivery experiments. We are grateful to Juliana Brown and Laurie Jackson-Grusby for assistance with pancreas injections. This work was supported by the National Institutes of Health/NIGMS (R01GM065400 and R01 GM095501) and by the Howard Hughes Medical Institute. Q.Z. is supported by NIDDK U01 DK089536 and the Harvard Stem Cell Institute (HSCI). D.R.L. is a consultant for Permeon Biologics, a company that has licensed Harvard's intellectual property on macromolecule delivery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION Supplemental Information includes Supplemental Experimental Procedures, three figures and one table and can be found online.
REFERENCES
- Atkinson H, Chalmers R. Delivering the goods: Viral and non-viral gene therapy systems and the inherent limits on cargo DNA and internal sequences. Genetica. 2010;138:485–498. doi: 10.1007/s10709-009-9434-3. [DOI] [PubMed] [Google Scholar]
- Brunet I, Weinl C, Piper M, Trembleau A, Volovitch M, Harris W, Prochiantz A, Holt C. The transcription factor engrailed-2 guides retinal axons. Nature. 2005;438:94–98. doi: 10.1038/nature04110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron NJ, Torrente Y, Camirand G, Bujold M, Chapdelaine P, Leriche K, Bresolin N, Tremblay JP. Intracellular delivery of a tat-egfp fusion protein into muscle cells. Mol Ther. 2001;3:310–318. doi: 10.1006/mthe.2001.0279. [DOI] [PubMed] [Google Scholar]
- Chandler LA, Sosnowski BA, McDonald JR, Price JE, Aukerman SL, Baird A, Pierce GF, Houston LL. Targeting tumor cells via egf receptors: Selective toxicity of an hbegf-toxin fusion protein. Int. J. Cancer. 1998;78:106–111. doi: 10.1002/(sici)1097-0215(19980925)78:1<106::aid-ijc17>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Chen B, Cepko CL. Hdac4 regulates neuronal survival in normal and diseased retinas. Science. 2009;323:256–259. doi: 10.1126/science.1166226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioce V, Csaky KG, Chan AM, Bottaro DP, Taylor WG, Jensen R, Aaronson SA, Rubin JS. Hepatocyte growth factor (hgf)/nk1 is a naturally occurring hgf/scatter factor variant with partial agonist/antagonist activity. J Biol. Chem. 1996;271:13110–13115. doi: 10.1074/jbc.271.22.13110. [DOI] [PubMed] [Google Scholar]
- Cronican JJ, Thompson DB, Beier KT, McNaughton BR, Cepko CL, Liu DR. Potent delivery of functional proteins into mammalian cells in vitro and in vivo using a supercharged protein. ACS Chem. Biol. 2010;5:747–752. doi: 10.1021/cb1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenius K, Paul S, Allison G, Sun J, Klagsbrun M. Activation of her4 by heparin-binding egf-like growth factor stimulates chemotaxis but not proliferation. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:1268–1278. doi: 10.1093/emboj/16.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Futaki S, Goto S, Suzuki T, Nakase I, Sugiura Y. Structural variety of membrane permeable peptides. Curr. Protein Pept. Sci. 2003;4:87–96. doi: 10.2174/1389203033487261. [DOI] [PubMed] [Google Scholar]
- Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, Sugiura Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 2001;276:5836–5840. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- Glover JN, Harrison SC. Crystal structure of the heterodimeric bzip transcription factor c-fos-c-jun bound to DNA. Nature. 1995;373:257–261. doi: 10.1038/373257a0. [DOI] [PubMed] [Google Scholar]
- Green M, Loewenstein PM. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1988;55:1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- Hansen M, Kilk K, Langel U. Predicting cell-penetrating peptides. Adv. Drug Delivery. Rev. 2008;60:572–579. doi: 10.1016/j.addr.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- Hollenbach AD, McPherson CJ, Mientjes EJ, Iyengar R, Grosveld G. Daxx and histone deacetylase ii associate with chromatin through an interaction with core histones and the chromatin-associated protein dek. J. Cell Sci. 2002;115:3319–3330. doi: 10.1242/jcs.115.16.3319. [DOI] [PubMed] [Google Scholar]
- Jarver P, Mager I, Langel U. In vivo biodistribution and efficacy of peptide mediated delivery. Trends Pharmacol. Sci. 2010;31:528–535. doi: 10.1016/j.tips.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Joliot A, Pernelle C, Deagostini-Bazin H, Prochiantz A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc. Natl. Acad. Sci. USA. 1991;88:1864–1868. doi: 10.1073/pnas.88.5.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaouass M, Beaulieu R, Balicki D. Histonefection: Novel and potent non-viral gene delivery. J Controlled Release. 2006;113:245–254. doi: 10.1016/j.jconrel.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Lawrence MS, Phillips KJ, Liu DR. Supercharging proteins can impart unusual resilience. J. Am. Chem. Soc. 2007;129:10110–10112. doi: 10.1021/ja071641y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfgren K, Wahlström A, Lundberg P, Langel U, Gräslund A, Bedecs K. Antiprion properties of prion protein-derived cell-penetrating peptides. FASEB J. 2008;22:2177–2184. doi: 10.1096/fj.07-099549. [DOI] [PubMed] [Google Scholar]
- Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (trim21) Proc. Natl. Acad. Sci. USA. 2010;107:19985–19990. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BR, Cronican JJ, Thompson DB, Liu DR. Mammalian cell penetration, sirna transfection, and DNA transfection by supercharged proteins. Proc. Natl. Acad. Sci. USA. 2009;106:6111–6116. doi: 10.1073/pnas.0807883106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DJ, Kim DT, Steinman L, Fathman CG, Rothbard JB. Polyarginine enters cells more efficiently than other polycationic homopolymers. J. Pept. Res. 2000;56:318–325. doi: 10.1034/j.1399-3011.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- Mor-Vaknin N, et al. The dek nuclear autoantigen is a secreted chemotactic factor. Mol Cell Biol. 2006;26:9484–9496. doi: 10.1128/MCB.01030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, et al. All-1 is a histone methyltransferase that assembles a supercomplex of proteins involved in transcriptional regulation. Mol. Cell. 2002;10:1119–1128. doi: 10.1016/s1097-2765(02)00740-2. [DOI] [PubMed] [Google Scholar]
- Nakase I, Takeuchi T, Tanaka G, Futaki S. Methodological and cellular aspects that govern the internalization mechanisms of arginine-rich cell-penetrating peptides. Adv. Drug Delivery. Rev. 2008;60:598–607. doi: 10.1016/j.addr.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Rosenbluh J, Hariton-Gazal E, Dagan A, Rottem S, Graessmann A, Loyter A. Translocation of histone proteins across lipid bilayers and mycoplasma membranes. J. Mol. Biol. 2005;345:387–400. doi: 10.1016/j.jmb.2004.10.046. [DOI] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by prdm16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai A, et al. A novel human cc chemokine, eotaxin-3, which is expressed in il-4-stimulated vascular endothelial cells, exhibits potent activity toward eosinophils. J. Immunol. 1999;163:1602–1610. [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.