Abstract
The increase in obesity prevalence highlights the need for a more comprehensive understanding of the neural systems controlling food intake; one that extends beyond food intake driven by metabolic need and considers that driven by higher-order cognitive factors. The hippocampus, a brain structure involved in learning and memory function, has recently been linked with food intake control. Here we examine whether administration of the adiposity hormone leptin to the dorsal and ventral sub-regions of the hippocampus influences food intake and memory for food. Leptin (0.1 μg) delivered bilaterally to the ventral hippocampus suppressed food intake and body weight measured 24 h after administration; a higher dose (0.4 μg) was needed to suppress intake following dorsal hippocampal delivery. Leptin administration to the ventral but not dorsal hippocampus blocked the expression of a conditioned place preference for food and increased the latency to run for food in an operant runway paradigm. Additionally, ventral but not dorsal hippocampal leptin delivery suppressed memory consolidation for the spatial location of food, whereas hippocampal leptin delivery had no effect on memory consolidation in a non-spatial appetitive response paradigm. Collectively these findings indicate that ventral hippocampal leptin signaling contributes to the inhibition of food-related memories elicited by contextual stimuli. To conclude, the results support a role for hippocampal leptin signaling in the control of food intake and food-related memory processing.
Keywords: obesity, food intake, learning, memory, place preference, ventral hippocampus
INTRODUCTION
Leptin, a hormone predominantly secreted from white adipocytes, is an important contributor to energy balance regulation as it informs the brain about energy stored in peripheral fat depots (Zhang et al, 1994; Maffei et al, 1995; Schwartz et al, 1996; Elias et al, 1999; Cowley et al, 2001). The importance of leptin signaling in the hypothalamus (Balthasar et al, 2004; Dhillon et al, 2006; Leinninger et al, 2009) and the caudal brainstem (Grill et al, 2002; Huo et al, 2007; Hayes et al, 2010) is established for the homeostatic or need-based control of food intake. However, given that the excessive food intake that contributes to human obesity is generally not driven by metabolic need, it is critical to examine and better define the neural basis of non-homeostatic controls on food intake. As leptin receptors (LepRb) are also expressed in brain regions associated with reward and cognitive processes (eg Scott et al, 2009), determining the extent to which leptin signaling in these extra-hypothalamic and extra-hindbrain regions contributes to the non-homeostatic control of feeding is a priority and the subject of this paper.
LepRb are expressed in the hippocampus (Huang et al, 1996; Scott et al, 2009), a brain structure involved with learning and memory function that has more recently been linked with food intake control (see references Davidson et al (2005) (2007) and Kanoski and Davidson (2011) for reviews). Previous research establishes a role for hippocampal LepRb signaling in learning and memory function. Leptin administered in vitro to hippocampal neurons facilitates neuronal processes that are presumed to underlie hippocampal-dependent memory formation, including long-term potentiation (LTP) (Shanley et al, 2001) and long-term depression (LTD) (Durakoglugil et al, 2005). Systemic leptin administration improves performance in a hippocampal-dependent Morris water maze task in rats (Oomura et al, 2006) that requires learning and remembering the spatial location of an escape platform. Further, direct administration of leptin to the hippocampus improves memory consolidation in mice on tasks that involve remembering the location of an aversive event (ie, foot shock) (Farr et al, 2006). Surprisingly, however, the role of hippocampal LepRb signaling in appetitive learning and memory processes, including those directed at learning about the spatial location or context where food was acquired or consumed, or other motivational behaviors related to food consumption, is not well studied.
Two sub-regions of the hippocampus are distinguished anatomically and functionally, with the dorsal region (DHPC) most strongly associated with spatial memory and the ventral region (VHPC) linked with memory involving an emotional or motivational component (see references de Hoz et al (2003); Bannerman et al (2004) and Fanselow and Dong (2010) for reviews). Whether or not leptin signaling in the dorsal and ventral sub-regions of the hippocampus differentially influences appetitive behavior is unknown. Previous studies examining the effects of hippocampal leptin signaling on learning and memory function have focused on the DHPC and not the VHPC. Given that prior neuro-anatomical (Cenquizca and Swanson, 2006) and behavioral work (Davidson et al, 2009) establishes a role for the VHPC in food intake control, we hypothesize that LepRb signaling in this hippocampal region influences energy balance-relevant behaviors by modulating appetitive mnemonic and/or motivational processes.
Conditioned place preference (CPP) is a learning paradigm that requires forming associations between a rewarding event (ie, food or drug delivery) and the location or context where the animal experienced the rewarding effects of the stimulus during training. Leptin administered intra-cerebroventricularly (ICV) to food-restricted rats blocks the expression of a CPP for a location previously paired with a food reward (Figlewicz et al, 2004). This result suggests that energy status signals may modulate CPP for food reward. The relevant CNS LepRb populations mediating the blockade of the appetitive CPP following forebrain leptin administration are unknown given that this mode of delivery makes drug widely available. Hippocampal LepRb signaling may contribute to this effect, as CPP learning for food reward is impaired in rats with neurotoxic lesions to the DHPC, whereas lesions to the VHPC enhance appetitive CPP learning (Ferbinteanu and McDonald, 2001).
The present research used pharmacological and behavioral strategies to examine whether direct hippocampal leptin delivery influences food intake, body weight (BW) gain, and the expression and consolidation of learned appetitive behaviors. Bilateral parenchymal delivery of leptin to either the DHPC or the VHPC was used to examine whether the potential contributions of regionally distinct hippocampal LepRbs to food intake and BW control can be distinguished. To determine whether DHPC or VHPC LepRb signaling contributes to learned appetitive behaviors related to food procurement, several behavioral paradigms were used. First, an appetitive CPP paradigm was used to examine the effects of direct DHPC or VHPC leptin delivery on the expression of a CPP for a location associated with a food reward. Second, the effects of hippocampal leptin administration on operant responding for food reward were examined using an incentive runway paradigm. Third, the effects of DHPC and VHPC leptin administration on memory consolidation were examined in two appetitive elevated maze tasks: (1) a spatial plus-maze task that required learning the location of food in relation to environmental spatial cues and (2) a non-spatial T-maze response task that required making a 90° turn in one specific direction to obtain food. The results show that leptin administered to the hippocampus, particularly the VHPC, suppressed food intake, BW gain, and the expression and consolidation of learned appetitive behaviors, thus providing support for the hypothesis that hippocampal leptin signaling influences behaviors relevant to food procurement and consumption.
MATERIALS AND METHODS
Animals and Drugs
Adult male Sprague–Dawley rats (Charles River Laboratories), housed individually in hanging metal cages under a 12-h reverse light/dark cycle (lights out at 0900 hours), had ad libitum access to rodent chow (Purina 5001; St Louis, MO) and water except where noted. All procedures conformed to the institutional standards of The University of Pennsylvania Animal Care and Use committee.
Leptin (National Hormone & Peptide Program) was dissolved in sodium bicarbonate. Leptin (and vehicle) was injected intra-parenchymally (volume, 100 nl) using a 33-gauge injector and microsyringe (Hamilton) attached to an infusion pump (Harvard Apparatus).
Surgeries
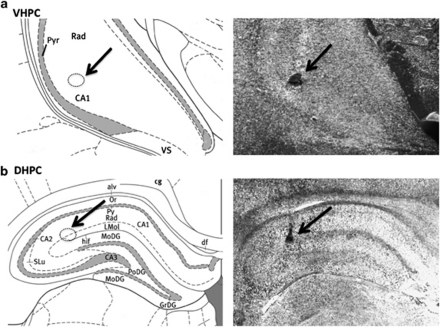
Under ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) anesthesia and analgesia (Metacam; 2 mg/kg), guide cannulae (Plastics One; 26-gauge) cemented to the skull using four jewelers screws were implanted bilaterally at the following coordinates: Dorsal hippocampal placement: 4.0 mm caudal to the bregma, ±3.6 mm from the midline, 3.0 mm below the skull surface. The injectors for drug administration to the dorsal hippocampus project 1 mm beyond the guide cannulae. Ventral hippocampal placement: 4.9 mm caudal to the bregma, ±4.8 mm from the midline, 6.1 mm below the skull surface. The injectors for drug administration to the ventral hippocampus project 2 mm beyond the guide cannulae. Rats were allowed to recover from surgery for a minimum of 7 days prior to behavioral procedures. Intra-parenchymal injection sites were anatomically confirmed through postmortem verification of the position of 100-nl pontamine sky blue injections. Only animals passing histological verifications were included in the final analyses (see Figure 1 for a representative injection site).
Figure 1.
Representative injection sites. Representative injection sites (indicated by arrow) for the ventral (a) and dorsal (b) hippocampus are shown as localization of pontamine sky blue ink (right) and on comparable coronal plane from rat brain atlas (modified from Paxinos and Watson, 6th Ed.). CA1, CA2, CA3, CA fields of the hippocampus; Pyr, pyramidal cell layer hippocampus; Rad, radiatum layer hippocampus; VS, ventral subiculum; MoDG, molecular layer dentate gyrus (refer to the atlas for additional abbreviations).
Experiment-1: feeding behavior
The effects of intra-hippocampal leptin administration on food intake and BW were assessed in ad libitum fed rats using a within-subjects design. Approximately 15 min prior to the onset of the dark cycle, rats with dorsal (n=11) or ventral (n=12) hippocampal cannulae received bilateral drug injections: vehicle, 0.1 μg leptin, 0.2 μg leptin, or 0.4 μg leptin per hemisphere (eg 0, 0.2, 0.4, or 0.8 μg leptin total). Previous research shows that these doses are sub-threshold for intake and BW suppression when given ICV to male Sprague–Dawley rats (Air et al, 2002). Treatment days were separated by 3–4 intervening days and were counterbalanced using a Latin square design. Cumulative chow intake was recorded at 1, 3, 6, and 24 h after dark onset (accounting for spillage). BW s were recorded before and 24 h subsequent to injections.
Experiment-2: appetitive CPP
Following Experiment-1 a subset of the same rats with dorsal (n=10) or ventral (n=10) hippocampal cannulae were food-restricted with daily chow rations aimed at maintaining BW s that approximated 85% of a free feeding BW established after Experiment-1 was completed (85% BW). The procedures for appetitive CPP were modified from Figlewicz et al (2004). All CPP training and testing procedures were conducted in a dimly lit room during the rats' dark cycle. The procedures were conducted in two identical plexiglass CPP chambers (74 cm long, 57.4 cm wide, 24.7 cm wall height) with a removable divider wall in the center. The two sides (henceforth referred to as contexts) of the CPP chamber were made distinguishable by varying wall color (white vs black), floor texture (plexiglass vs ridged rubber), and orientation of stripes (vertical vs horizontal) applied to the walls. After rats achieved 85% BW, they were allowed to freely explore the CPP chamber during one 10-min habituation situation in which the divider wall was removed and the time spent in each of the two contexts was recorded by an experimenter. For each rat, the context that was least preferred during this habituation session was assigned as the food-paired context for subsequent training, whereas the more preferred side was not paired with food. CPP training consisted of six, 20-min sessions (one session per day): three sessions where the rat was isolated in the food-paired context and three sessions isolated in the non-food-paired context. During food-paired sessions, 5 g of a high-fat diet (Research Diets; D12492) were placed in the context with the rat, whereas no food was given during non-food-paired sessions. The training order was randomized and pair-matched across groups. The first three and last three sessions occurred on continuous days; sessions 1–3 and 4–6 were separated by two intervening days.
Testing occurred 2 days after the sixth training session. Rats were assigned to groups matched for baseline context preference (n=5/group): Group Ventral HPC Vehicle, Group Ventral HPC Leptin, Group Dorsal HPC Vehicle, and Group Dorsal HPC Leptin. For each rat, bilateral intra-hippocampal injections of either vehicle or leptin (0.4 μg; a dose that suppressed food intake and BW when injected in either sub-region) were given 3 h before a 15-min test session in which the center divider was removed, no food was given, and the time spent in each context was recorded by an experimenter blind to the group assignments. The timing for leptin injection relative to the test session was based on previous research showing reduced CPP following ICV leptin (Figlewicz et al, 2004). The dependent variable used for analysis was a preference score for the food-paired context (no. of seconds in the food-paired context/total seconds) during the 10-min baseline session and during the 15-min CPP test session.
Experiment-3: operant incentive runway
A separate group of rats with dorsal (n=12) or ventral (n=12) hippocampal cannulae were maintained at 85% BW using the procedures described above. The runway apparatus (117 cm length, 14.2 cm width, 9.5 cm wall height) had a start box (21.7 cm length, 14.2 cm width, 9.5 cm wall height) at one end of the runway. Training was conducted during the rats' dark cycle in a dimly lit room. The rats received six training sessions (one per day) that consisted of 10 trials per session. For each trial, a rat was placed in an enclosed start box for 8 s; the start box door was then removed and the rat was allowed to traverse the runway. On the first two trials of the first two training sessions, four food reinforcements (0.2 g of high-fat, high-sucrose diet) were placed along the length of the runway. For all other trials, only one food reinforcement was placed in a recessed food cup located 2.54 cm from the end wall at the end of the runway opposite the start box (∼93 cm from the start box). After each trial the rat was placed in a separate holding box located outside of the runway apparatus for approximately 30 s. The training sessions were separated by 1–2 intervening days. On the sixth training session, the rats received a mock intra-hippocampal injection 3 h before training to habituate them to the injection procedures.
Runway testing was conducted on two days (separated by one intervening day) using a within-subjects design. The injection parameters (leptin dose and injection timing) were the same as that used in the CPP experiment. Rats received bilateral intra-hippocampal injections of leptin (0.4 μg) or vehicle (counterbalanced treatment order) 3 h before a 10-trial testing session; the timing of the injection relative to runway testing was based on the procedures used in the CPP paradigm. Runway performance was video-recorded and later scored by two experimenters blind to the treatment conditions (inter-rater reliability verified). The following dependent variables were recorded across each trial: (1) latency to start (ie, time from removal of start door to initiation of movement toward the goal), (2) the number of distractions (defined as the occurrence of cessation of movement toward the goal after leaving the start line), (3) total distraction time, and (4) net runway speed (m/s), which was calculated by subtracting the total distraction time from the total runway time (time from when the rat left the start line until crossing a finish line located 2.54 cm in front of the recessed food cup).
Experiment-4: spatial memory consolidation
The spatial task required learning which one of four arms in an elevated maze was consistently baited with food, based on environmental spatial cues located outside the maze. A separate group of rats with cannulae aimed at the ventral (n=10) or dorsal (n=10) hippocampus were maintained at 85% BW as described above. Food reinforcers (Froot Loops) were placed in the home cage several days before maze habituation to reduce neophobia. The elevated plus-shaped maze was located in a lit room and had the following dimensions: 100 cm from floor; each arm width, 14.2 cm; length, 60 cm; central platform diameter, 30 cm. Sidewalls (9.5 cm height) extended around the exterior of the maze. A recessed food well (1 inch diameter) was located 2.54 cm from the end of each arm. Distinct visual cues (eg posters and other objects with different shapes, colors, and patterns) were placed on the walls of the room ∼1.5 m from the ends of each arm. Rats were habituated to the plus maze on two sessions that occurred on separate days. In the first session 15 Froot Loops were scattered around the surface floor of the maze. The rats were allowed to freely explore and consume the reinforcers for 5 min. On the second habituation session, one Froot Loop was placed in a recessed food well located at the end of each of the four arms. Additional habituation sessions were conducted for rats that did not consume all four of the Froot Loops during the second habituation session.
The procedures for training were modified from Chang and Gold (2003). Each rat was assigned a goal arm (baited with food) that was in the same position relative to the extra-maze room cues throughout the entire training session. The other three arms were randomly selected as the start arm for each trial. Each rat received one training session. Before each trial, one Froot Loop was placed in a recessed food well at the end of the goal arm. The rat was allowed to freely explore until either the Froot Loop was consumed or 3 min lapsed. An experimenter remained in a fixed location and recorded arm entries. Incorrect trials were defined as the goal arm not being the first arm entered. After each trial the rat was placed in a holding cell for a 30-s inter-trial interval. To avoid possible influences of intra-maze cues, the maze was rotated 90° clockwise and cleaned with soap and water every third trial. Training ended when rats reached a criterion of 9/10 correct trials in a row. Immediately after the criterion was achieved, the rats were given intra-ventral or dorsal hippocampal bilateral injections of either vehicle or leptin (0.4 μg/100 nl). Animals that did not achieve the criterion were not injected and were removed from the experiment.
A test session occurred 7 days after the training session. The procedures for testing were identical to those of training, with the exception that no injections were given after criterion was achieved.
Experiment-5: response memory consolidation
To determine whether leptin's effects on memory consolidation for the spatial task (Experiment-4) were based on alterations in spatial/contextual-based memory as opposed to more general effects on consolidation of food-related memory, a control experiment was conducted that used a non-spatial response memory paradigm of comparable difficulty. For the response task, spatial cues were not informative about the location of food reinforcement. Instead, obtaining food required learning to make a particular response (making a 90° right turn) instead of another (turning left).
A separate group of rats with either ventral (n=16) or dorsal (n=12) bilateral hippocampal cannulae were maintained at 85% BW as previously described. The elevated T-maze was identical to the elevated plus-shaped maze used in Experiment-4, except that one of the four arms was obstructed from entry resulting in a T-shape. A curtain was placed around the exterior of the maze to minimize the saliency of extra-maze cues. The general procedures for habituation, training, and testing were the same for response training as those for spatial training, with the following exceptions: The start arm for each trial was the arm located at the bottom of the ‘T', and the goal arm was the arm located to the right of the start arm. Trials ended when one of the following occurred: (1) the reinforcer was obtained and consumed from the right arm; (2) the subject entered the incorrect (left) arm (entries defined as traversing to the food well at the end of the left arm); or (3) 3 min lapsed without an arm entry. The maze was rotated 90° and cleaned with soap and water every third trial to eliminate the use of spatial or olfactory strategies, respectively. Testing occurred 7 days after the training session.
Statistical Analysis
Food intake and BW following intra-hippocampal leptin administration were analyzed separately at each time point recorded by repeated-measures analysis of variance (ANOVA) using Drug (0, 0.1, 0.2, 0.4 μg of leptin) as a within-subjects variable. Planned comparisons to vehicle treatment were made for each dose of leptin when significant overall Drug effects were obtained. CPP expression and maze task performance were analyzed by one-way ANOVA using Drug (0, 0.4 μg leptin) as a between-subjects variable. The operant runway performance variables (latency to start, number of distractions, total distraction time, and net runway speed) were analyzed by repeated-measures ANOVA using Drug (0, 0.4 μg leptin) and Trial (1–10) as within-subjects variables.
RESULTS
Feeding Behavior
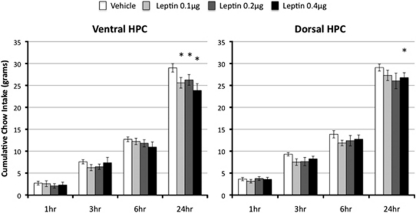
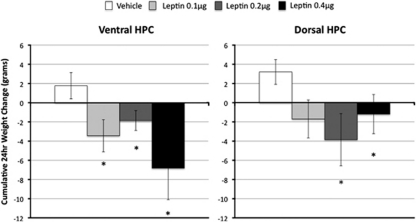
All doses of leptin (0.1–0.4 μg) delivered directly to the ventral hippocampus in ad libitum fed rats suppressed chow intake 24 h after administration (Figure 2, left). This was supported by a significant overall main effect of Drug (F(3,30)=4.36, p<0.05). Group comparisons to vehicle treatment confirmed that each dose significantly suppressed 24-h chow intake (Fs(1,10) >9.18, p-values <0.05). Similar results were obtained for delta BW recorded 24 h after administration (overall F(3,30)=3.20, p<0.05): each dose of leptin delivered to the ventral hippocampus significantly suppressed 24-h BW relative to vehicle treatment (Fs(1,10) >11.96, p-values <0.01) (Figure 3, left). Despite trends at 3 and 6 h, food intake was not significantly altered by ventral hippocampal leptin administration at any other time point (overall Fs(3,30) <1.84).
Figure 2.
Food intake. Leptin administration to the ventral and dorsal hippocampus reduced food intake in rats. Chow intake is shown at 1, 3, 6, and 24 h following bilateral intra-hippocampal leptin injections at dark onset in ad libitum fed rats (*p<0.05).
Figure 3.
Delta BW. Leptin administration to the ventral and dorsal hippocampus reduced BW in rats. Delta BW is shown 24 h following bilateral intra-hippocampal leptin injections at dark onset in ad libitum fed rats (*p<0.05).
Only the highest dose of leptin (0.4 μg bilateral) delivered to the dorsal hippocampus significantly suppressed 24-h food intake relative to vehicle administration (F(1,10)=6.1, p<0.05) (Figure 2, right). Both 0.2 and 0.4 μg of leptin delivered bilaterally to the dorsal hippocampus significantly suppressed 24-h BW relative to vehicle treatment (Fs(1,10) >5.56, p-values <0.05) (Figure 3, right). As with ventral delivery, non-significant trends toward intake suppression were observed at 3 and 6 h after administration of leptin (overall Fs(3,30) <2.12).
Appetitive CPP
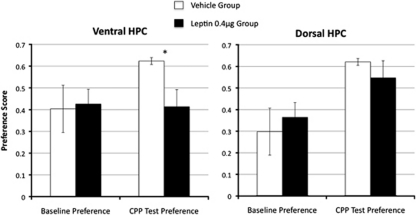
Leptin (0.4 μg bilateral) delivered to the ventral hippocampus 3 h before testing significantly blocked the expression of appetitive CPP relative to vehicle-treated rats (Figure 4). This conclusion was supported by a significant main effect of Drug for the ventral group (F(1,8)=6.83, p<0.05) for preference score during the test session. Leptin administered to the dorsal hippocampus prior to CPP testing, however, did not influence the expression of CPP during the test session relative to vehicle (F(1,8)=0.50).
Figure 4.
Appetitive CPP. Leptin administration to the ventral hippocampus, in contrast to dorsal injection, blocked the expression of CPP for food. The preference score is shown at baseline and during place preference testing, which occurred 3 h following bilateral intra-hippocampal administration of leptin (0.4 μg) or its vehicle (*p<0.05).
Operant Runway
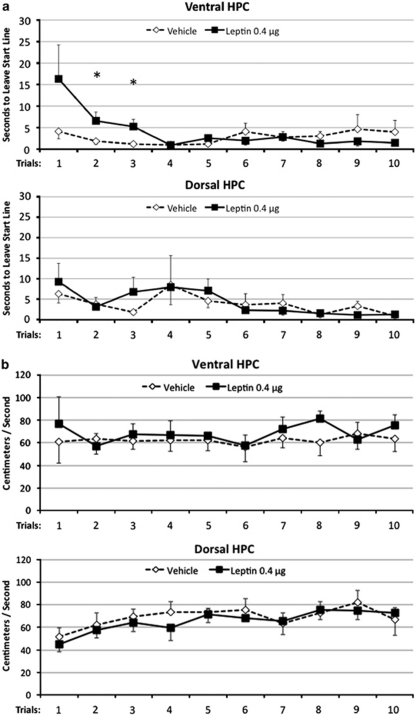
Ventral hippocampal leptin delivery increased the overall runway time through a specific increased latency to leave the starting point on early trials (trial no. 2 and 3) but not on later trials (Figure 5a). On the other hand, rats treated with leptin in the ventral hippocampus did not differ from vehicle-treated rats in net runway speed (Figure 5b) or total distraction time (data not shown) after leaving the start line. These conclusions were supported by ANOVA; an overall ANOVA for latency to leave the starting point showed that the overall main effect of Drug was not significant (F(1,6)=0.62); however, the interaction between Drug and Trial was significant (F(9,54)=2.17, p<0.05). As seen in Figure 5a, this significant interaction results from an increased latency to leave the starting point in leptin-treated rats on early trials but not on later trials (significant effect of Drug on Trial no. 2 and no. 3, p-values <0.05). For net runway speed (Figure 5b), neither the main effect of Drug (F(1,6)=1.49), nor the Drug × Trial interaction (F(9,54)=0.44) was significant. Furthermore, the main effects of Drug (Fs(1,6) <1.24) and the Drug × Trial interactions (Fs(9,54) <0.64) were not significant for the no. of distractions and the total distraction time.
Figure 5.
Operant runway performance. Leptin administration to the ventral hippocampus increased the latency to leave the start line on early trials in an operant runway task (a), while having no effect on the net runway speed (b). The latency to leave the start line and the net runway speed recorded after leaving the start line are shown across trials in a test session that occurred 3 h following bilateral hippocampal leptin administration (*p<0.05).
Dorsal hippocampal leptin administration did not influence operant runway performance relative to vehicle (Figure 5a and b). The main effect of Drug (Fs(1,6) <1.8) and the Drug × Trial interaction (Fs(9,54) <1.0) were not significant for latency to leave the starting point, net runway speed, no. of distractions, or total distraction time. Overall, these results show that ventral but not dorsal hippocampal leptin delivery influenced runway time by increasing latency to leave the starting point on early trials.
Spatial Memory Consolidation
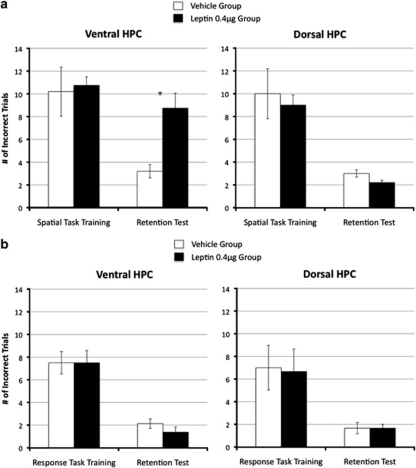
Leptin delivered to the VHPC after training suppressed memory consolidation for the spatial location of food reinforcement, whereas DHPC leptin did not significantly influence memory retention performance relative to vehicle (see Figure 6a). ANOVA for the retention test (dependent variable=number of incorrect trials) that occurred 1 week after training yielded a significant main effect of Drug for the ventral group (F(1,8)=17.39, p<0.01). A marginal improvement in memory retention test performance was observed for the DHPC leptin group compared with the DHPC vehicle group; however, this difference did not achieve significance (F(1,8)=4.57, p=0.065).
Figure 6.
Spatial and non-spatial memory consolidation. Ventral but not dorsal hippocampal leptin delivery (0.4 μg) after training suppressed memory consolidation for the spatial location of food relative to vehicle treatment (a). Leptin did not influence the consolidation of an appetitive non-spatial response task (b) (*p<0.05).
Response Memory Consolidation
Memory retention performance for the T-maze response task was not influenced by post-training leptin delivery to either the VHPC or DHPC (see Figure 6b). ANOVA for the retention test showed non-significant main effects of Drug for both the ventral and dorsal groups (Fs <1.0).
DISCUSSION
Brain leptin signaling is an essential contributor to energy balance control (Cohen et al, 2001). Most studies focus on hypothalamic LepRb signaling to explain leptin's effects on food intake and BW regulation (eg arcuate nucleus, LH, VMH) (Balthasar et al, 2004; Dhillon et al, 2006; Leinninger et al, 2009). However, recent findings strongly suggest that leptin's contribution to energy balance control is mediated by endogenous signaling that is anatomically distributed (Grill, 2010) across multiple brain regions that include the hindbrain (eg nucleus tractus solitarius (NTS) (Hayes et al, 2010)) and midbrain (eg ventral tegmental area (VTA) (Fulton et al, 2006; Hommel et al, 2006; Morton et al, 2009)) nuclei. Here, we identify the hippocampus, a forebrain structure traditionally associated with learning and memory function, as a novel CNS site of relevance to leptin-mediated control of food intake. The present results show that direct administration of leptin to the ventral (VHPC) or dorsal (DHPC) sub-regions of the hippocampus suppressed food intake and BW in ad libitum fed rats 24 h after administration, with a larger magnitude of intake suppression observed following ventral (∼11–15%) compared with dorsal hippocampal (∼8%) administration. Leptin delivery to the VHPC but not DHPC also blocked the expression of appetitive CPP, increased latency to respond for food in an operant runway paradigm, and suppressed memory consolidation for the spatial location of food. These findings support a differential regional effect of hippocampal leptin signaling on feeding-related behavior.
Previous research shows that forebrain ICV leptin reduces CPP for food (Figlewicz et al, 2004). Here we identify a specific neural target for leptin-mediated CPP reduction by showing that leptin administered to the VHPC but not DHPC blocked preference for a location previously paired with food. One interpretation of these data is that VHPC leptin receptor activation influenced memory retrieval by inhibiting the activation of food-related memory by contextual cues. Other data are consistent with this interpretation. VHPC lesions that eliminate leptin signaling in this brain region enhance CPP learning relative to intact controls (Ferbinteanu and McDonald, 2001), which suggests that blockade of CPP expression by VHPC leptin delivery can be characterized as gain of function (facilitating memory inhibition), a notion consistent with the role of the hippocampus in certain types of appetitive memory inhibition (Chan et al, 2001; Davidson and Jarrard, 2004). Taken together these findings are consistent with the hypothesis that the VHPC contributes to neural processes related to learning and remembering locations where food was consumed, and that this type of memory is modulated by signals, such as leptin, that provide energy status information to the brain.
The expression of CPP was blocked by VHPC leptin administration 3 h prior to CPP testing, whereas significant food intake suppression following hippocampal leptin administration did not occur until 24 h after administration. This brings to question whether the same or different mechanisms mediate leptin's effects on food intake suppression and CPP memory. Differences between these paradigms, however, make it difficult to directly compare the effective time course of leptin's effects on food intake and CPP. For instance, food intake analysis was conducted in ad libitum fed rats, whereas analysis of leptin's effects on CPP was conducted in rats maintained on a food restriction regime. Furthermore, food intake was assessed in rats consuming standard lab chow, whereas CPP was based on memory for the location of a limited amount of palatable high-fat food.
An alternative interpretation for the leptin-mediated reduced CPP expression that does not involve memory is that VHPC leptin administration reduced the motivation to seek out and consume food (reduced appetitive motivational drive) rather than influenced memory for the location of food. Consistent with this explanation, ICV leptin reduces breakpoint operant responding in a progressive ratio reinforcement schedule (Figlewicz et al, 2006), a measure used to examine the effect of a treatment on the motivation to work for food reinforcement. Another interpretation of the data that does not involve memory is that VHPC leptin administration indirectly influenced appetitive motivational drive through mechanisms involving altered anxiety levels. It is known that VHPC damage reduces anxiety in rats in the elevated plus maze and other tests of anxiety (Bannerman et al, 2003). Further, recent studies link leptin signaling to processes that modulate anxiety levels (Finger et al, 2010; Liu et al, 2010). The results from the operant runway paradigm help in distinguishing between these alternate interpretations.
The runway data suggest that reduced CPP expression following VHPC leptin administration is more likely attributable to suppressed memory retrieval for food reward than it is to nonspecific behavioral alterations in either anxiolytic or motivational processes. For instance, using the same dose and time frame between injections and testing as that used in the CPP paradigm we found that leptin delivery to the VHPC increased the rats' latency to leave the starting position on early trials but not on later trials relative to vehicle treatment. Runway speed measured after leaving the starting point, however, was not influenced by leptin administration, suggesting that VHPC leptin did not influence locomotor function. If exogenous leptin decreased anxiety levels, which has previously been shown in rodents following peripheral leptin delivery (Liu et al, 2010), then the reduced anxiety would be more likely to decrease the latency to start, which differs from the observed increase in latency in the present results. Furthermore, if general changes in appetitive motivation were responsible for the observed leptin-induced increase in latency to leave the starting position, it is unclear how this type of performance decrement could specifically influence latency on early trials, without also altering latency on later trials throughout the 10-trial runway session. For example, motivation or ‘wanting' for food is linked with opioid- and dopaminergic-mediated signaling within the nucleus accumbens (see reference Berridge et al (2009) for review). Mu-opioid receptor antagonists (eg β-funaltrexamine (BFNA), naloxone) administered either directly into the nucleus accumbens shell (Shin et al, 2010) or peripherally (Wakonigg et al, 2003; Barbano and Cador, 2006) increase the runway time to obtain food. In these studies the increased runway time by opioid receptor blockade either did not differ across trials (Shin et al, 2010) or was enhanced on later trials compared with earlier trials (Wakonigg et al, 2003; Barbano and Cador, 2006). This contrasts with the effect of VHPC leptin on runway performance in the present study, which increased the latency to start on early but not later trials relative to vehicle treatment. Thus, the pattern of runway deficits following VHPC leptin differs from the effects observed by others following opioid antagonist administration, suggesting that the effects of VHPC leptin delivery on runway latency were not based on alterations in opioid-mediated motivational processing.
The increased latency in operant runway responding on early trials observed following VHPC leptin administration is more likely explained by suppressed activation of the memory of food reinforcement by contextual cues (ie, the runway context). On subsequent trials, the active working memory representation of recently obtained food reinforcement from previous trials appears to have overridden the effect of VHPC leptin to inhibit the memory of food reinforcement. Viewed in this way, the increased runway latency effect triggered by VHPC leptin can be explained by a mechanism involving inhibition of memory for food reward rather than by producing a general reduction in motivational drive. We note, however, that the problem of distinguishing between differences in learning and motivation is unfortunately inherent in all reinforcement paradigms. Furthermore, the extent to which reduced memory for food influences the latency to run but not running speed cannot be concluded from the present results. Nevertheless, it is clear that the observed increased latency to respond for food following VHPC leptin administration was only present on early trials, which differs from what has been shown previously following opioid receptor blockade.
Results from the elevated maze paradigms further support the hypothesis that VHPC leptin modulates appetitive memory processing independent of anxiety or motivational effects. Leptin delivery to the VHPC but not DHPC immediately after rats learned an appetitive spatial plus-maze task suppressed memory consolidation for food location as shown by impaired performance relative to vehicle-treated rats in a memory retention test 1 week later. Leptin did not, however, influence memory consolidation for an appetitive non-spatial response task following either DHPC or VHPC delivery. It is unlikely that the effects of VHPC leptin delivery on spatial memory consolidation were based on altered anxiety or motivational states. First, leptin was not administered immediately prior to the training or test session, but was only given after the training session was completed. Thus, responding for food in the maze was not directly influenced by exogenous leptin administration. Second, if an altered physiological state produced by changes in anxiety or appetitive motivational drive was responsible for the suppressed memory consolidation, the effects would likely be observed for both the spatial and the non-spatial response task. Yet, VHPC leptin suppressed memory consolidation only for the spatial task and had no effect on consolidation of a non-spatial response task of comparable difficulty. These results as well as those obtained from the CPP and runway paradigms are consistent with the hypothesis that VHPC leptin signaling suppresses memory based on spatial/contextual cues associated with food. Whether memory consolidation of context–food associations learned in the CPP paradigm, or memory retention of spatial cue–food associations learned in the plus-shaped maze paradigm, are also altered by VHPC leptin administration requires further study.
A number of studies support a functional differentiation between the rodent DHPC (posterior hippocampus in primates) and the VHPC (primate anterior hippocampus). While there is debate on assigning function to each of these hippocampal sub-regions, the DHPC is considered by some to be necessary for learning and memory processes that involve the utilization of spatial information, whereas the VHPC is linked with learning processes that involve an emotional or motivational component (de Hoz et al, 2003; Bannerman et al, 2004; Fanselow and Dong, 2010). This differentiation of function, however, is complicated by several findings showing that VHPC lesions or inactivation interferes with spatial memory processing (eg Ogren et al, 1996; Schott et al, 1998; Wilkerson and Levin, 1999; Levin et al, 1999; Ferbinteanu and McDonald, 2001; de Hoz et al, 2003). Here we show that VHPC leptin signaling modulates the consolidation of memory for the spatial location of food, which further challenges the belief that hippocampal spatial memory processing is mediated exclusively by the dorsal region.
In combination with other recent findings our results provide support for the notion that the functional differentiation between the DHPC and VHPC may also extend to the control of food intake and other appetitive behavior, with the VHPC assuming a larger role. First, we observed larger effects on food intake and BW suppression following VHPC compared with DHPC leptin administration. Second, appetitive CPP expression, runway performance, and memory consolidation for food location were suppressed by VHPC but not DHPC leptin. Third, Davidson et al (2009) showed that lesions restricted to the VHPC produced elevations in food intake and BW in rats that were of comparable magnitude to that observed following complete hippocampal lesions. Fourth, the ventral but not the dorsal hippocampal CA1 fields project directly to hypothalamic nuclei that are critical in food intake control, including the LH and the VMH (Cenquizca and Swanson, 2006). Collectively, these findings direct attention to the VHPC as a forebrain structure of importance in the higher-order inhibitory controls of food intake and other appetitive behaviors.
Previous studies show that DHPC leptin delivery influences memory based on aversive but not appetitive reinforcement. For instance, DHPC leptin administration improved memory consolidation in two tasks that involve learning and remembering the location of an aversive event in mice: a T-maze foot shock avoidance and a step down inhibitory avoidance paradigm (Farr et al, 2006). On the other hand, Paulus et al (2005) show that direct DHPC leptin administration did not influence performance relative to vehicle treatment in an appetitive spatial working memory task that requires animals to remember where food was recently obtained. Similarly, we show that DHPC leptin delivery did not significantly influence appetitive memory in CPP, operant runway, or a spatial memory consolidation paradigm, which contrasts to effects following VHPC leptin delivery. Whether VHPC leptin signaling also contributes to learning and memory processes that involve aversive reinforcement is not yet known.
Evidence from both humans and rodents show that the hippocampus is necessary for the detection and utilization of interoceptive energy state cues. For instance, amnesic patients with hippocampal damage will consume a second test meal immediately after consuming a full meal (Hebben et al, 1985; Rozin et al, 1998), which suggests that (1) they do not remember consuming the first meal and/or (2) that they are impaired in detecting and utilizing internal cues (eg meal-related gastric distension and other satiation signals) arising from the previously consumed food. Human fMRI studies show that the hippocampus is significantly activated by consuming food to satiation (DelParigi et al, 2004) as well as following gastric electrical stimulation of the vagus nerve (Wang et al, 2006) which increases subjective feelings of fullness and reduces food intake and BW in obese subjects (Cigaina, 2004). In rats, hippocampal lesions impair performance in a deprivation intensity discrimination paradigm that requires the animals to use interoceptive cues produced from different levels of food deprivation (non-deprived vs 24 h food-deprived) as discriminative cues for food reward (Davidson et al, 2010). In the same paradigm, peripheral leptin administered to 24 h food-deprived rats produces interoceptive cues that generalize to ad libitum feeding conditions (Kanoski et al, 2007). These findings suggest that the hippocampus contributes to the neural processes whereby leptin signaling informs the brain about sufficient energy reserves. Within this framework we posit that when energy reserves are sufficient and endogenous leptin levels are elevated, hippocampal neurons integrate information about energy status (eg levels of peripheral fat depots) through alterations in leptin signaling with correlates of learned information about previous food location and consumption, and use this integrated information to inhibit memory for food location and reduce subsequent food procurement.
In conclusion, experiments show that leptin administered to the DHPC and VHPC reduced food intake and BW in non-deprived rats. CPP expression for food reward was blocked following VHPC but not DHPC leptin delivery. VHPC leptin administration also increased the latency to respond for food on early trials in an appetitive operant runway task and suppressed memory consolidation for the spatial location of food, whereas DHPC leptin did not significantly alter performance in these paradigms. These findings provide support for the hypothesis that ventral hippocampal leptin signaling contributes to the non-homeostatic control of food intake by suppressing the ability of contextual cues to elicit memory for food. The results support the perspective of an anatomically distributed control of energy balance whereby leptin signaling in neurons within multiple CNS regions contributes to different aspects of food intake control.
Acknowledgments
The following individuals are thanked for their constructive comments and other contributions: Ted Abel, Robert Rescorla, Isabel Muzzio, Terry Davidson, Karolina Skibicka, John Andrews-Labenski, Raymond Lee, Laura Rupprecht, Amber Alhadeff, and Theresa Leichner. This work is supported by NIH Grants DK21397 (HJG), DK089752 (SEK), and DK085435 (MRH), and The Obesity Society Early Career Research Grant (SEK).
The authors declare no conflict of interest.
References
- Air EL, Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin combine additively to reduce food intake and body weight in rats. Endocrinology. 2002;143:2449–2452. doi: 10.1210/endo.143.6.8948. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology. 2006;31:1371–1381. doi: 10.1038/sj.npp.1300908. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking', ‘wanting', and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. J Comp Neurol. 2006;497:101–114. doi: 10.1002/cne.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KH, Morell JR, Jarrard LE, Davidson TL. Reconsideration of the role of the hippocampus in learned inhibition. Behav Brain Res. 2001;119:111–130. doi: 10.1016/s0166-4328(00)00363-6. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Intra-hippocampal lidocaine injections impair acquisition of a place task and facilitate acquisition of a response task in rats. Behav Brain Res. 2003;144:19–24. doi: 10.1016/s0166-4328(03)00063-9. [DOI] [PubMed] [Google Scholar]
- Cigaina V. Long-term follow-up of gastric stimulation for obesity: the Mestre 8-year experience. Obes Surg. 2004;14 (Suppl 1:S14–S22. doi: 10.1007/BF03342133. [DOI] [PubMed] [Google Scholar]
- Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, et al. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19:235–252. doi: 10.1002/hipo.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Jarrard LE. The hippocampus and inhibitory learning: a ‘Gray' area. Neurosci Biobehav Rev. 2004;28:261–271. doi: 10.1016/j.neubiorev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Chan K, Clegg DJ, Benoit SC, Jarrard LE. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behav Neurosci. 2010;124:97–105. doi: 10.1037/a0018402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC. A potential role for the hippocampus in energy intake and body weight regulation. Curr Opin Pharmacol. 2007;7:613–616. doi: 10.1016/j.coph.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiol Behav. 2005;86:731–746. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- de Hoz L, Knox J, Morris RG. Longitudinal axis of the hippocampus: both septal and temporal poles of the hippocampus support water maze spatial learning depending on the training protocol. Hippocampus. 2003;13:587–603. doi: 10.1002/hipo.10079. [DOI] [PubMed] [Google Scholar]
- DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, et al. Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obes Relat Metab Disord. 2004;28:370–377. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Durakoglugil M, Irving AJ, Harvey J. Leptin induces a novel form of NMDA receptor-dependent long-term depression. J Neurochem. 2005;95:396–405. doi: 10.1111/j.1471-4159.2005.03375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures. Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420–1425. doi: 10.1016/j.peptides.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, McDonald RJ. Dorsal/ventral hippocampus, fornix, and conditioned place preference. Hippocampus. 2001;11:187–200. doi: 10.1002/hipo.1036. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav Neurosci. 2004;118:479–487. doi: 10.1037/0735-7044.118.3.479. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol Behav. 2006;89:611–616. doi: 10.1016/j.physbeh.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Finger BC, Dinan TG, Cryan JF. Leptin-deficient mice retain normal appetitive spatial learning yet exhibit marked increases in anxiety-related behaviours. Psychopharmacology (Berl) 2010;210:559–568. doi: 10.1007/s00213-010-1858-z. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Grill HJ. Leptin and the systems neuroscience of meal size control. Front Neuroendocrinol. 2010;31:61–78. doi: 10.1016/j.yfrne.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–246. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010;11:77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebben N, Corkin S, Eichenbaum H, Shedlack K. Diminished ability to interpret and report internal states after bilateral medial temporal resection: case H.M. Behav Neurosci. 1985;99:1031–1039. doi: 10.1037//0735-7044.99.6.1031. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Huang XF, Koutcherov I, Lin S, Wang HQ, Storlien L. Localization of leptin receptor mRNA expression in mouse brain. Neuroreport. 1996;7:2635–2638. doi: 10.1097/00001756-199611040-00045. [DOI] [PubMed] [Google Scholar]
- Huo L, Maeng L, Bjorbaek C, Grill HJ. Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology. 2007;148:2189–2197. doi: 10.1210/en.2006-1572. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103:59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Walls EK, Davidson TL. Interoceptive ‘satiety' signals produced by leptin and CCK. Peptides. 2007;28:988–1002. doi: 10.1016/j.peptides.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Christopher NC, Weaver T, Moore J, Brucato F. Ventral hippocampal ibotenic acid lesions block chronic nicotine-induced spatial working memory improvement in rats. Brain Res Cogn Brain Res. 1999;7:405–410. doi: 10.1016/s0926-6410(98)00044-5. [DOI] [PubMed] [Google Scholar]
- Liu J, Garza JC, Bronner J, Kim CS, Zhang W, Lu XY. Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine. Psychopharmacology (Berl) 2010;207:535–545. doi: 10.1007/s00213-009-1684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Blevins JE, Kim F, Matsen M, Figlewicz DP. The action of leptin in the ventral tegmental area to decrease food intake is dependent on Jak-2 signaling. Am J Physiol Endocrinol Metab. 2009;297:E202–E210. doi: 10.1152/ajpendo.90865.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren SO, Kehr J, Schott PA. Effects of ventral hippocampal galanin on spatial learning and on in vivo acetylcholine release in the rat. Neuroscience. 1996;75:1127–1140. doi: 10.1016/0306-4522(96)00215-1. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, et al. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27:2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Paulus K, Schulz C, Lehnert H. Central nervous effects of leptin and insulin on hippocampal leptin and insulin receptor expression following a learning task in Wistar rats. Neuropsychobiology. 2005;51:100–106. doi: 10.1159/000084167. [DOI] [PubMed] [Google Scholar]
- Rozin P, Dow S, Moscovitch M, Rajaram S. What causes humans to begin and end a meal? A role for memory for what has been eaten, as evidenced by a study of multiple meal eating in amnesic patients. Psychol Sci. 1998;9:392–396. [Google Scholar]
- Schott PA, Bjelke B, Ogren SO. Distribution and kinetics of galanin infused into the ventral hippocampus of the rat: relationship to spatial learning. Neuroscience. 1998;83:123–136. doi: 10.1016/s0306-4522(97)00360-6. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, et al. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21:RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin AC, Pistell PJ, Phifer CB, Berthoud HR. Reversible suppression of food reward behavior by chronic mu-opioid receptor antagonism in the nucleus accumbens. Neuroscience. 2010;170:580–588. doi: 10.1016/j.neuroscience.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakonigg G, Sturm K, Saria A, Zernig G. Opioids, cocaine, and food change runtime distribution in a rat runway procedure. Psychopharmacology (Berl) 2003;169:52–59. doi: 10.1007/s00213-003-1488-9. [DOI] [PubMed] [Google Scholar]
- Wang G-J, Yang J, Volkow ND, Telang F, Ma Y, Zhu W, et al. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proc Natl Acad Sci USA. 2006;103:15641–15645. doi: 10.1073/pnas.0601977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson A, Levin ED. Ventral hippocampal dopamine D1 and D2 systems and spatial working memory in rats. Neuroscience. 1999;89:743–749. doi: 10.1016/s0306-4522(98)00346-7. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. (see comment) (erratum appears in Nature 30 March 1995; 374(6521):479. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]