Abstract
Postpartum depression (PPD) affects up to 19% of all women after parturition. The non-apeptide oxytocin (OXT) is involved in adjustment to pregnancy, maternal behavior, and bonding. Our aim was to examine the possible association between plasma OXT during pregnancy and the development of PPD symptoms. A total of 74 healthy, pregnant women were included in this prospective study. During the third trimester of pregnancy and within 2 weeks after parturition, PPD symptoms were assessed using the Edinburgh Postnatal Depression Scale (EPDS). Blood samples for plasma OXT assessment were collected in the third trimester. Following the literature, participants with postpartum EPDS scores of 10 or more were regarded as being at risk for PPD development (rPPD group). In a logistic regression analysis, plasma OXT was included as a potential predictor for being at risk for PPD. Results were controlled for prepartal EPDS score, sociodemographic and birth-outcome variables. Plasma OXT concentration in mid-pregnancy significantly predicted PPD symptoms at 2 weeks postpartum. Compared with the no-risk-for-PPD group, the rPPD group was characterized by lower plasma OXT concentrations. To our knowledge, this is the first study to show an association between prepartal plasma OXT concentration and postpartal symptoms of PPD in humans. Assuming a causal relationship, enhancing OXT release during pregnancy could serve as a potential target in prepartum PPD prevention, and help to minimize adverse effects of PPD on the mother–child relationship.
Keywords: postpartum depression, oxytocin, pregnancy, EPDS, adaptation to motherhood
INTRODUCTION
Postpartum depression (PPD) affects up to 19% of all mothers and adversely influences maternal adaptation to motherhood (Gavin et al, 2005) with negative effects on child development, as children of depressive mothers are more vulnerable to develop mental disorders in later life (Grace et al, 2003).
The etiology of PPD is closely related to psychological determinants and experiences during pregnancy (O'Hara and Swain, 1996). Identification of psychological risk factors for PPD has been an important issue in the recent years. Major identified risk factors are a history of previous PPD or affective disorders in general, stressful life events, lack of social support, and low self-esteem (Beck, 2006; Robertson et al, 2004). Additionally, the early postpartum period is seen as a time of increased emotional vulnerability, partly caused by dysregulations of the endocrinological homeostasis (Wisner and Stowe, 1997). Because of the challenging reorganization of physiological processes that comes along with pregnancy and parturition, research started to address endocrine factors as potential determinants in the etiology of PPD. From animal models of PPD, we know that withdrawal from high doses of estradiol and progesterone, comparable to the respective amounts available during pregnancy, is followed by depression-like symptoms (Green et al, 2009). Addtionally, the regulation of the hypothalamic–pituitary–adrenal (HPA) axis seems to be disturbed in women with PPD (Brummelte and Galea, 2010) or short periods of Postpartum Blues (Ehlert et al, 1990). However, existing findings are not consistent (Bloch et al, 2003). One biological parameter that has not yet been considered in PPD etiology, is the non-apeptide oxytocin (OXT). OXT is synthesized in the paraventricular nucleus (PVN) and the supraoptic nucleus (SON) of the hypothalamus and released peripherally into the blood and centrally into different brain regions (Gimpl and Fahrenholz, 2001). In context of pregnancy, OXT is known for its involvement in the process of delivery (Russell et al, 2003) and its physiological role in the onset and maintenance of lactation (Sala and Althabe, 1968).
Beyond its physiological functions in the periphery, animal studies provide evidence for a major role of OXT in behavioral adaptation to pregnancy and motherhood. Characteristic maternal behaviors (pup-grooming, hover over offspring and respond latency) are impaired, if OXT availability is diminished (Higuchi and Kaba, 1997; Olazabal and Young, 2005; Pedersen et al, 2006). Furthermore, maternal OXT functioning influences reciprocal affective behaviors between mother and offspring in mammalian species (Nelson and Panksepp, 1998). Recently, this association was also shown in humans. Parents showing more affectionate and stimulatory behaviors in interactions with their children were characterized by higher plasma OXT concentrations (Gordon et al, 2010). Further, Feldman et al. (2007) reported associations between prepartum-assessed OXT concentrations and postpartum maternal adaptation. The maternal plasma OXT level, measured during early and late pregnancy, as well as in the first month postpartum, predicted maternal behavior (mother's gaze at infant, motherese vocalizations and affectionate touch) in interaction with the child. A study assessing plasma OXT twice during pregnancy showed higher postpartum maternal–fetal attachment-scores in women with a OXT rise between the first and third trimester compared with women with stable or decreasing patterns of OXT (Levine et al, 2007). In non-pregnant women, OXT is known to promote interpersonal relationships and enhance feelings of love and trust (Heinrichs and Domes, 2008).
On the basis of current evidence, lower OXT levels in pregnancy could result in impaired emotional adaptation to motherhood, which is a major risk factor for PPD development and subsequently affects the quality of maternal behavior (Murray et al, 1993; Stein et al, 2010). Therefore, the aim of this study was to assess a potential association between OXT during pregnancy and postpartum PPD symptoms in a sample of healthy pregnant women. We expected, that lower plasma OXT levels during the third trimester of pregnancy would result in an increased risk for PPD, as assessed postpartum. Results could help to elucidate the etiopathology of PPD and provide new targets for prepartal prevention of PPD.
METHODS
Subjects
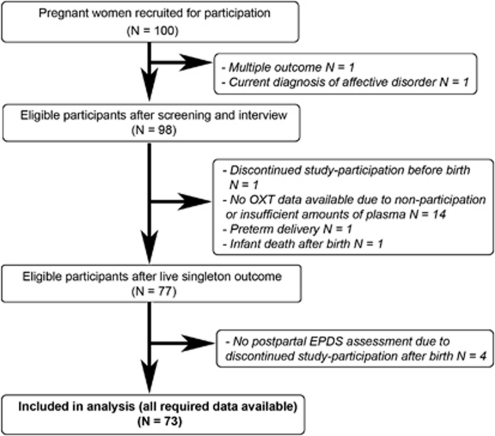
Data were collected within a larger longitudinal study conducted with 100 pregnant women in the area of Basel, Switzerland. All participants were recruited between their 21st and 32nd week of gestation. Recruitment methods included local newspaper announcements, promotion of the study at local hospitals and a call for participants on local TV. A detailed study description was given to all interested women and, if any arised, questions were answered. All participants were screened for the following inclusion criteria: (a) no current mental illness, (b) no severe medical complications (acute or chronic physical diseases, such as gestational diabetes, metabolic diseases, hypertension and thyroid dysfunction), (c) no signs of fetal malformation, (d) a pre-pregnancy BMI below 32, (e) no smoking beyond the 10th week of gestation and (f) good knowledge of German language. Data for analyses of the present paper were available for 73 participants, of which 16 were characterized by at least one lifetime depressive episode. All lifetime episodes of depression occured more than 2 years before participation in the study. A flowchart of study participants is displayed in Figure 1. Comparisons between the 73 women providing complete data and the 27 excluded women indicated no significant differences on age, parity, socioeconomic status and PPD symptoms.
Figure 1.
Flowchart of study participants.
Informed written consent was obtained from all participants. The study protocol was approved by the local ethics committee and is consistent with the revised Helsinki Declaration of 1975.
Blood Sampling and OXT Measurement
All blood samples were obtained between the 30th and 34th week of gestation. Participants visited the study centre for an experimental session, which included blood sampling and other physiological assessments. The samples for the OXT assessment were collected at the beginning of the session, starting between 1300 hours and 1500 hours. Participants were seated on a examination couch and a study nurse sampled 2.7 ml of blood into vacutainer tubes containing lithium heparin and 108 μl of Aprotinin (BioChemica, Germany). Tubes were kept on ice and centrifuged within 10 min at 6 °C at 3000 g for 10 min. Supernatants were pipetted into safe-lock devices and stored at −80 °C until analysis.
Samples were analyzed at the Department of Behavioural Neuroendocrinology, Max Plank Institute of Psychiatry, Munich, Germany, using a radioimmunoassay, as described elsewhere (Landgraf et al, 1995). This assay was reported to have an antiserum cross-reactivity of less than 0.7%, with a detection limit of 0.1 pg per sample. All samples were analyzed in duplicates. The intra-assay and inter-assay coefficients of variability were 6–8 and 8–10%, respectively.
Assessment of Demographic and Psychological Characteristics
After inclusion, participants were interviewed for assessing possible present, recent or life-time depression and anxiety disorders using the German translation of corresponding sections of the Computer Assisted Personal Interview (CAPI) version of the Composite International Diagnostic Interview (Wittchen et al, 1998; Wittchen and Pfister, 1997; World Health Organization, 1990) and general socioeconomic data.
Depressive symptoms were assessed within 2 weeks after delivery using the Edinburgh Postnatal Depression Scale (EPDS), a scale originally developed as a screening measure for depression, showing good reliability (split-half: 0.82; standardized α=0.81) (Bergant et al, 1998). A total of 10 items, dealing with typical PPD symptoms are answered on a 4-point scale. As a control variable, the prepartal EPDS score was assessed between the 32nd and 34th week of gestation.
Information on length of gestation and birth outcome were collected from medical records.
Data Analyses
All variables were checked for normal distribution, missing data and outliers (defined as more than two standard deviations below or above the mean) by the Kolmogorov–Smirnof test and visual inspection. Outliers were checked for validity and excluded if reasonable. If necessary, variables were subjected to transformation by natural logarithm before further analyses. Differences on demographic, biological and psychological characteristics between included and excluded study participants were tested by t and χ2 tests. Participants were divided into a risk-for PPD group (rPPD) and a no-risk-for PPD group (nPPD), according to the respective postpartum EPDS score. On the basis of the proposals of Bergant et al. (1998) and Jardri et al. (2006), the chosen cut-off score for being at risk for PPD was 10 or more within the first 2 weeks postpartum. T and χ2 tests were computed between the groups, to identify possible confounders among the demographical and medical variables. Descriptive statistics of EPDS scores and OXT values are reported. The postpartal EPDS scores of nursing and not nursing mothers were compared using the t-test. The bivariate correlation was computed between the pre- and postpartal EPDS score. The association between OXT and PPD symptoms was tested by conducting a binary logistic regression analysis with the group variable as outcome variable and OXT concentration as the potential predictor in the first run. In a second run the prepartal EPDS score was added, to control for potential confounding by previous depressive symptoms. Further analyses were computed including OXT concentration as the first predictor and other potential predictors, identified through previous group comparisons. Because of the expected, unequal group sizes, every logistic regression analysis was conducted with not more than two predictors, of which the first one was always OXT concentration. Because accurate classification of participants is difficult when groups are not evenly split, primary emphasis was placed on prediction rather than classification of being at risk for PPD. Data were analyzed using SPSS 16.0.2 for Mac OS X. The level of significance for all analyses was set at α=0.05.
RESULTS
Sample and Group Characteristics
Demographic and pregnancy-related sample and group characteristics are displayed in Table 1. A group variable was introduced according to the postpartum EPDS score. In all, 14 participants were identified as having a postpartum EPDS score of 10 or more and were assigned to the rPPD group, representing a higher risk for the development of PPD. The remaining 59 participants were assigned to the nPPD group. Groups were tested for significant differences regarding sociodemographic and birth characteristics. Groups differed only in length of gestation. Participants in the rPPD group had a significantly shorter length of gestation (M=39.02 weeks) compared with participants in the nPPD group (M=39.73 weeks) (T (71)=2.049; P<0.05). Therefore length of gestation was included as a potential mediator in further analysis of the relationship between OXT and PPD symptoms.
Table 1. Sample and Group Characteristics and Tests for Group Differences.
| Total sample M (SD)/% (N=73) | nPPD group M (SD)/% (n=59) | rPPD group M (SD)/% (n=14) | Test between nPPD and rPPD P-value | |
|---|---|---|---|---|
| Maternal age (years) | 31.05 (4.70) | 31.22 (4.69) | 30.36 (4.88) | 0.541a |
| Income category | 0.928b | |||
| Low | 8.70 | 8.90 | 7.70 | |
| Average/high | 73.90 | 73.20 | 76.90 | |
| Very high | 17.40 | 17.90 | 15.40 | |
| Pre-pregnancy BMI | 22.31 (3.47) | 22.10 (3.26) | 23.32 (4.40) | 0.292a |
| Parity | ||||
| Primiparae | 74.0 | 71.20 | 85.70 | |
| Multiparae | 26.0 | 28.80 | 14.30 | |
| Length of gestation (weeks) | 39.6 (1.2) | 39.7 (1.1) | 39.1 (1.4) | 0.044a |
| Birth mode | ||||
| Cesarean section | 23.3 | 23.70 | 21.40 | |
| Mother is nursing | 90.0 | 89.7 | 92.3 | |
| Infant birth weight (g) | 3338.56 (378.44) | 3345.93 (359.21) | 3307.50 (465.02) | 0.735a |
| Infant sex | ||||
| Female | 46.6 | 50.8 | 28.6 | |
| Male | 53.4 | 49.2 | 71.4 | |
Monthly income categories low=0–3750 swiss franks, average/high=3750–11250 swiss franks, very high=above 11250 swiss franks; nPPD=no risk for postpartum depression; rPPD=at risk for postpartum depression; BMI=body mass-index.
t-test.
χ2-test
Pre and Postpartal EPDS Scores
Postpartal EPDS scores did not differ between nursing and not nursing mothers (T (69)=0.025; P=0.98). Prepartal EPDS scores (Range 0–17) were significantly correlated with postpartal EDPS scores (Range 0–22) (r=0.232; p=0.048). Mean prepartal and postpartal total EPDS scores were 4.77 and 5.85 respectively.
Plasma OXT Concentrations
Plasma OXT concentrations had a range of 14.39–245.71 pg/ml and mean OXT concentration for the overall sample was 80.81 pg/ml (SD=48.81 pg/ml). Three outliers with OXT values above 200 pg/ml were identified. Information on these three subjects did not provide a clear reason for exclusion of these cases or any indication for invalidity of assessments. Therefore, they were retained in the analyses. All further analyses were conducted with the log-transformed OXT concentrations to assure normal distribution. The bivariate correlation between prepartal EPDS scores and OXT concentrations was not significant (r=−0.086; p=0.467).
The Association between OXT Concentration in Pregnancy and Postpartum PPD Symptoms
The test statistics of the logistic regression analyses, with OXT predicting PPD symptoms are displayed in Table 2. Plasma OXT level significantly predicted PPD symptoms (Exp (b)=0.290; p<0.05). The coefficient of association between OXT concentration and PPD was below one, indicating lower OXT levels in the rPPD group and higher OXT levels in the nPPD group. The addition of the prepartal EPDS score as a further covariate in a second analysis did not improve the model fit (Δχ2 (1)=0.302; P>0.05). According to the results of descriptive statistics, length of gestation was tested as a potential mediator in a third analysis. Length of gestation did not predict PPD symptoms (Exp (b)=0.931; p>0.05) and the model fit did not improve either (Δχ2 (1)=3.507; P>0.05).
Table 2. Binary Logistic Regression Analysis Fort he Prediction of Being at Risk for Postpartal Depression.
|
Model statistics |
Predictor statistics |
|||||||
|---|---|---|---|---|---|---|---|---|
| χ2 (df) | P | R2NK | −2 LL | Wald's χ2 (df) | p | Exp(b) | 95% CI for Exp(b) | |
| First analysis | 6.195 (1) | 0.013 | 0.130 | 65.169 | ||||
| Plasma oxytocin | 5.555 (1) | 0.018 | 0.290 | 0.103–0.812 | ||||
| Second analysis | 6.497 (2) | 0.039 | 0.137 | 64.867 | ||||
| Plasma oxytocin | 5.366 (1) | 0.021 | 0.294 | 0.105–0.828 | ||||
| Prepartal EPDS score | 0.299 (1) | 0.584 | 1.254 | 0.557–2.828 | ||||
| Change of model fit compared with first analysis: Δχ2 (1)=0.302; p=0.583 | ||||||||
| Third analysis | 9.702 (2) | 0.008 | 0.200 | 61.662 | ||||
| Plasma oxytocin | 5.250 (1) | 0.022 | 0.294 | 0.103–0.838 | ||||
| Length of gestation | 3.447 (1) | 0.063 | 0.931 | 0.864–1.004 | ||||
| Change of model fit compared with first analysis: Δχ2 (1)=3.507; p=0.061 | ||||||||
R2NK=Nagelkerke R2; −2 LL=−2 log likelihood (deviance); EPDS=Edinburgh Postnatal Depression Scale.
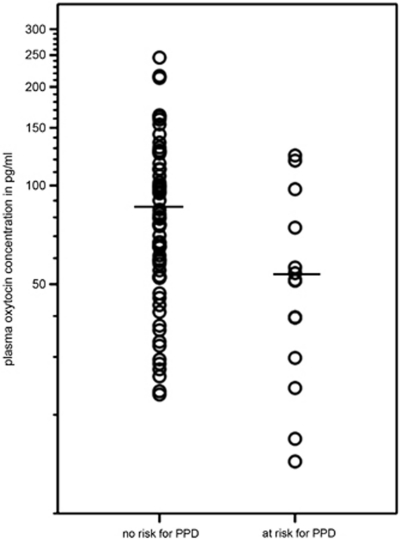
With OXT as predictor of PPD symptoms, 83.6% of the sample was classified correctly into the nPPD and rPPD group. To visualize the difference in OXT values between the groups, mean OXT concentrations are displayed in Figure 2.
Figure 2.
Graph shows individual oxytocin concentrations in the two groups and group means. Oxytocin values are shown on a logarithmic scale.
Repeating the analyses, excluding the three cases with outlying OXT concentrations, did not change the results.
DISCUSSION
In line with our hypothesis, we could show that OXT during pregnancy was negatively associated with a positive screen on the EPDS at greater than or equal to 10, indicating a higher risk for the development of PPD. This suggests an increased occurrence of depressive symptoms in the first 2 weeks after delivery in individuals with low plasma OXT concentrations during pregnancy. The relationship persisted after controlling for prepartal EPDS scores.
Our findings are in agreement with the only human study addressing the link between plasma OXT during pregnancy and postpartal maternal behavior. Plasma OXT concentrations during pregnancy were found to be positively associated with a set of maternal bonding behaviors, such as positive affect and gaze in interactions, as well as cognitive attachment representations towards the newborn in the early postpartum period (Feldman et al, 2007). In women suffering from PPD the same behaviors are impaired, accompanied by feelings of overload and difficulties in emotional attachment development towards their child (Beck, 2006; Martins and Gaffan, 2000). Correspondingly studies with rodents report deficits in maternal behavior, such as less protective behavior and less pup-licking, and longer latencies in postpartal onset of maternal behavior in animals with decreased central OXT availability (Pedersen et al, 2006; van Leengoed et al, 1987). Non-human primate mothers show increased maternal affiliation towards offspring when central OXT is enhanced (Holman and Goy, 1995). The present findings are also in agreement with human studies reporting relationships between plasma OXT, assessed in the 2nd and 6th month postpartum and affectionate maternal behavior during mother–child interactions (Gordon et al, 2010). Again, mothers' OXT concentrations were positively correlated with the behavioral indicators of attachment, such as motherese vocalization, affectionate touch and positive affect. As anxiety and excessive preoccupation are other important symptoms of PPD, our results also match reports of OXT acting as anxiolytic and enhancing positive emotional affiliation in non-pregnant humans (Uvnas-Moberg, 1998). There is also evidence for decreased plasma OXT concentrations in individuals suffering from major depression or reporting increased depressive symptoms (Frasch et al, 1995; Ozsoy et al, 2009; Scantamburlo et al, 2007).
The herein observed prevalence of subjects above cutoff resembles to those of other studies using the same screening instrument. Here, we identified 19.18% of the sample having an EPDS score of 10 or more. Other comparable studies found a rate of 20% at 5 days postpartum (Bergant et al, 1998) and 16% at 2 months postpartum (Yim et al, 2009).
Group comparisons revealed a significantly shorter gestation among women within the rPPD group. In women with lower OXT availability in pregnancy, the oxytocinergic inhibition of the HPA axis could be decreased. Decreased HPA axis inhibition would enhance the exponential increase of placental corticotropin-releasing factor that promotes the onset of labor (Smith, 1998). However, the length of gestation variable did not reach significance in the following prediction of PPD symptoms. It remains to be elucidated whether length of gestation has a mediating role in the relationship between OXT and PPD in samples including premature deliveries.
The range of OXT concentrations found in the present sample, is in line with those of previous studies (Dawood et al, 1979; De Geest et al, 1985). One often-mentioned issue concerning OXT assessment in human samples is that central OXT release is not necessarily related to peripheral OXT release, and therefore associations between centrally regulated psychological variables and peripheral measured OXT should be handled with caution (Jones et al, 1983; Landgraf and Neumann, 2004). However, in animals, there are also studies accounting for joint control mechanisms of central and peripheral OXT release in context of fear-related stress responses (Wotjak et al, 1998), and for autostimulatory effects at the level of the hypothalamus, in terms of peripheral OXT release activation by centrally released OXT (McKenzie et al, 1995; Neumann et al, 1994). Given the current evidence from animal studies and the difficulties in the determination of central OXT release in humans, it seems justifiable to revert to peripheral OXT assessment. This is supported by several findings reporting relations between peripheral OXT in humans and various psychological constructs, all representing aspects of human affiliation and attachment (Feldman et al, 2007; Light et al, 2004; Tops et al, 2007; Wismer Fries et al, 2005).
The mechanisms behind the observed association between OXT and PPD symptoms remain to be elucidated. Although we cannot rule out residual confounding by unknown factors, the prospective design and the inclusion of pre-pregnancy EPDS scores as a covariate make a causal relationship imaginable. There already exists evidence that OXT concentrations are lower in mothers characterized by depressive symptoms and negative affect during pregnancy, when assessed postpartum (Light et al, 2004). Further, OXT is known to reduce psychological and physiological stress responses (Heinrichs et al, 2003) and to inhibit hyperactive fear-responses of the amygdala (Labuschagne et al, 2010). There is also evidence that the properties of endocrine systems during pregnancy have programming functions for the postpartal period (Meinlschmidt et al, 2010; Pop et al, 1993). In our study, an interplay between low OXT and effects on amygdala reactivity and the HPA axis in pregnancy could indicate an increased reactivity to stressful stimuli at that time and promote the development of depressive symptoms after birth, when mothers are challenged by a bulk of potentially stressful new conditions. Additionally, expectations of the social environment and the growing demands of the child may promote feelings of fear and insecurity. Notably, a study comparing the symptomatology of postpartum and non-postpartum depression found more anxious features among the investigated PPD group (Hendrick et al, 2000). As we know from animal studies, besides the general importance of OXT in the formation of social bonds in females (Insel, 1997), the positive feedback mechanism of the oxytocinergic system is supposed to provide long-lasting stimulation of maternal behaviors after parturition (DaCosta et al, 1996). It may be less efficient, if OXT availability is diminished. Adopted to human mothers, this would be reflected by the difficulties depressed mothers have in implementing maternal behaviors and forming a relationship with their child (Beck, 1995; Cooper and Murray, 1998). Considering the profound physiological challenge caused by endocrinological changes over the course of gestation and the following abrupt shift after parturition, it is not possible to form a biological model for PPD development, that accounts for all contributing factors yet. Future studies should try to experimentally modify OXT concentrations in mid-pregnancy, to verify, whether OXT during pregnancy contributes to the generation of depressive symptoms during the postpartum period.
There are some limitations of the study. First, our finding needs to be confirmed in future studies with more than one OXT assessment over the course of pregnancy to clarify, if the relationship is specific to OXT concentrations between the 30th and 34th week of gestation. Studies assessing OXT at different stages of gestation suggest individually different patterns of OXT fluctuations over time, indicating that there might be a functional difference between women with stable OXT levels and those with rising ones (Dawood et al, 1979; Levine et al, 2007). But high intra-individual stability of values has also been reported (Feldman et al, 2007; Leake et al, 1981; van der Post et al, 1997). From our point of view, the present results refer to plasma OXT levels between the 30th and 34th week of gestation only, as we did not assess potential alterations in peripheral OXT release over the course of gestation. Second, the sample consisted of women with mostly medium to high socioeconomic status. Consequently, results need to be replicated with more heterogenous samples. Third, PPD symptoms were assessed by questionnaire (EPDS), which should be complemented in future studies by structured or standardized interviews to verify the presence of a diagnosis of PPD. It should be noted that as yet, estimations of the sensitivity and specificity of the EPDS to detect PPD vary across studies, warranting further attention of this issue (Eberhard-Gran et al, 2001; Gaynes et al, 2005; Gibson et al, 2009). Nevertheless there are validation studies reporting good sensitivity and specificity values for the EPDS within comparable study designs and according to DSM-III and ICD-10 criteria (Harris et al, 1989; Jardri et al, 2006). Important to note, our use of the EPDS within a period of 2 weeks postpartum does not provide information on the diagnosis of PPD, which requires the presence of symptoms for at least 2 weeks. Moreover, heightened EPDS scores in this early postpartum period may still be picking up the tail end of postpartum blues, which itself is a risk factor for the development of PPD. Finally, future studies should clarify, if the association between prepartal OXT and depressive symptoms during the postpartum period remains stable beyond the first 2 weeks up to several months postpartum, and if this relationship holds true for individuals with diagnosed episodes of PPD, as not all women with increased depressive symptoms after delivery develop a full-blown affective disorder.
In summary, our findings suggest that OXT is involved in the etiology of depressive symptoms during the postpartum period, and need to be further elaborated in studies assessing neuroendocrinological aspects of PPD. If replicated, the presented results will have important clinical relevance. Prepartal identification of subjects at risk for PPD could allow for early preventive interventions and minimize adverse effects for the physiological and psychological wellbeing of mother and child.
Acknowledgments
This work is part of the National Centre of Competence in Research (NCCR) Swiss Etiological Study of Adjustment and Mental Health (sesam). The Swiss National Science Foundation (SNF) (project no. 51A240-104890), the University of Basel, the Hoffmann-La Roche Corp. and the Basel Scientific Society provided core support for the NCCR sesam. We are grateful to the Max Planck Institute of Psychiatry, Munich, Germany for the biochemical analyses. Further, we thank Andrea H Meyer, PhD, for his statistical support.
The authors declare no conflict of interest.
References
- Beck CT. The effects of postpartum depression on maternal-infant interaction: A meta-analysis. Nurs Res. 1995;44:298–304. [PubMed] [Google Scholar]
- Beck CT. Postpartum depression. Am J Nurs. 2006;106:40–50. doi: 10.1097/00000446-200605000-00020. [DOI] [PubMed] [Google Scholar]
- Bergant AM, Nguyen T, Heim K, Ulmer H, Dapunt O. German version and validation of the Edinburgh postnatal depression scale (EPDS)) Deutsche Medizinische Wochenschrift. 1998;123:35–40. doi: 10.1055/s-2007-1023895. [DOI] [PubMed] [Google Scholar]
- Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry. 2003;44:234–246. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LAM. Depression during pregnancy and postpartum: Contribution of stress and ovarian hormones. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010;34:766–776. doi: 10.1016/j.pnpbp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Cooper PJ, Murray L. Postnatal depression. Br Med J. 1998;316:1884–1886. doi: 10.1136/bmj.316.7148.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaCosta APC, GuevaraGuzman RG, Ohkura S, Goode JA, Kendrick KM. The role of oxytocin release in the paraventricular nucleus in the control of maternal behaviour in the sheep. J Neuroendocrinol. 1996;8:163–177. doi: 10.1046/j.1365-2826.1996.04411.x. [DOI] [PubMed] [Google Scholar]
- Dawood MY, Ylikorkala O, Trivedi D, Fuchs F. Oxytocin in maternal circulation and amniotic-fluid during pregnancy. J Clin Endocrinol Metabol. 1979;49:429–434. doi: 10.1210/jcem-49-3-429. [DOI] [PubMed] [Google Scholar]
- De Geest K, Thiery M, Piron-Possuyt G, Vanden Driessche R. Plasma oxytocin in human pregnancy and parturition. J Perina Med. 1985;13:3–13. doi: 10.1515/jpme.1985.13.1.3. [DOI] [PubMed] [Google Scholar]
- Eberhard-Gran M, Eskild A, Tambs K, Opjordsmoen S, Samuelsen SO. Review of validation studies of the Edinburgh postnatal depression scale. Acta Psychiatrica Scandinavica. 2001;104:243–249. doi: 10.1034/j.1600-0447.2001.00187.x. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Patalla U, Kirschbaum C, Piedmont E, Hellhammer DH. Postpartum blues: salivary cortisol and psychological factors. J Psychosomat Res. 1990;34:319–325. doi: 10.1016/0022-3999(90)90088-l. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation - Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Frasch A, Zetzsche T, Steiger A, Jirikowski GF.1995Reduction of plasma oxytocin levels in patients suffering from major depressionIn: Ivell R, Russell JA (eds).Oxytocin—Cellular and Molecular Approaches in Medicine and Research Plenum Press Div Plenum Publishing Corp: New York; Vol 395, pp257–258. [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression—A systematic review of prevalence and incidence. Obstetr Gynecol. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ) 2005;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J, McKenzie-McHarg K, Shakespeare J, Price J, Gray R. A systematic review of studies validating the Edinburgh postnatal depression Scale in antepartum and postpartum women. Acta Psychiatrica Scandinavica. 2009;119:350–364. doi: 10.1111/j.1600-0447.2009.01363.x. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The Oxytocin receptor system: Structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biol Psychiatry. 2010;68:377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch Womens Ment Health. 2003;6:263–274. doi: 10.1007/s00737-003-0024-6. [DOI] [PubMed] [Google Scholar]
- Green AD, Barr AM, Galea LAM. Role of estradiol withdrawal in ‘anhedonic' sucrose consumption: A model of postpartum depression. Physiol Behav. 2009;97:259–265. doi: 10.1016/j.physbeh.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Harris B, Huckle P, Thomas R, Johns S, Fung H. The use of rating-scales to identify post-natal depression. Br J Psychiatry. 1989;154:813–817. doi: 10.1192/bjp.154.6.813. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Adv Vasopressin Oxytocin: from Genes to Behav to Disease. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Hendrick V, Altshuler L, Strouse T, Grosser S. Postpartum and nonpostpartum depression: Differences in presentation and response to pharmacologic treatment. Depress Anxiety. 2000;11:66–72. doi: 10.1002/(sici)1520-6394(2000)11:2<66::aid-da3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Higuchi T, Kaba H.1997Neuromodulatory role of oxytocin in the control of maternal behaviorIn: Maeda K-I, Tsukamura H, Yokoyama A (eds).Neural control of reproduction: Physiology and behavior S. Karger AG: New York; 237–248. [Google Scholar]
- Holman SD, Goy RW.1995Experiential and hormonal correlates of care-giving in rhesus macaquesIn: Pryce CR, Martin RD, Skuse D (eds).Motherhood in Human and Nonhuman Primates Karger: Basel; 87–93. [Google Scholar]
- Insel TR. A neurobiological basis of social attachment. Am J Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- Jardri R, Pelta J, Maron M, Thomas P, Delion P, Codaccioni X, et al. Predictive validation study of the Edinburgh postnatal depression scale in the first week after delivery and risk analysis for postnatal depression. J Affect Disord. 2006;93:169–176. doi: 10.1016/j.jad.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Jones PM, Robinson I, Harris MC. Release of oxytocin into blood and cerebrospinal-fluid by electrical-stimulation of the hypothalamus or neural lobe in the rat. Neuroendocrinology. 1983;37:454–458. doi: 10.1159/000123592. [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, et al. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35:2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Kubota M, Holsboer F, Wotjak CT. Release of vasopressin and oxytocin within the brain and into blood: Microdialysis and antisense targeting. Neurohypophysis. 1995;1098:243–256. [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Leake RD, Weitzman RE, Glatz TH, Fisher DA. Plasma oxytocin concentrations in men, non-pregnant women, and pregnant-women before and during spontaneous labor. J Clin Endocrinol Metabol. 1981;53:730–733. doi: 10.1210/jcem-53-4-730. [DOI] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: Individual patterns and maternal-fetal attachment. Peptides. 2007;28:1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA, Boccia M, Brownley KA, Johns JM. Deficits in plasma oxytocin responses and increased negative affect, stress, and blood pressure in mothers with cocaine exposure during pregnancy. Addict Behav. 2004;29:1541–1564. doi: 10.1016/j.addbeh.2004.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins C, Gaffan EA. Effects of early maternal depression on patterns of infant-mother attachment: A meta-analytic investigation. J Child Psychol Psychiatry. 2000;41:737–746. [PubMed] [Google Scholar]
- McKenzie DN, Leng G, Dyball REJ. Electrophysiological evidence for mutual excitation of oxytocin cells in the supraoptic nucleus of the rat hypothalamus. J Physiol London. 1995;485:485–492. doi: 10.1113/jphysiol.1995.sp020744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinlschmidt G, Martin C, Neumann ID, Heinrichs M. Maternal cortisol in late pregnancy and hypothalamic-pituitary-adrenal reactivity to psychosocial stress postpartum in women. Stress-the Int J Biol Stress. 2010;13:163–171. doi: 10.3109/10253890903128632. [DOI] [PubMed] [Google Scholar]
- Murray L, Kempton C, Woolgar M, Hooper R. Depressed Mothers Speech to Their Infants and Its Relation to Infant Gender and Cognitive-Development. J Child Psychol Psychiatry. 1993;34:1083–1101. doi: 10.1111/j.1469-7610.1993.tb01775.x. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: Contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22:437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Neumann I, Koehler E, Landgraf R, Summylong J. An Oxytocin receptor antagonist infused into the supraoptic nucleus attenuates intranuclear and peripheral release of oxytocin during suckling in conscious rats. Endocrinology. 1994;134:141–148. doi: 10.1210/endo.134.1.8275928. [DOI] [PubMed] [Google Scholar]
- O'Hara MW, Swain AM. Rates and risk of postpartum depression—A meta-analysis. Int Rev Psychiatry. 1996;8:37–54. [Google Scholar]
- Olazabal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate ‘spontaneous' maternal behavior in female prairie voles. Hormones Behav. 2005;48:177. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Ozsoy S, Esel E, Kula M. Serum oxytocin levels in patients with depression and the effects of gender and antidepressant treatment. Psychiatry Res. 2009;169:249–252. doi: 10.1016/j.psychres.2008.06.034. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Vadlamudi SV, Boccia ML, Amico JA. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes Brain Behav. 2006;5:274–281. doi: 10.1111/j.1601-183X.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Pop VJM, Derooy HAM, Vader HL, Vanderheide D, Vanson MM, Komproe IH. Microsomal antibodies during gestation in relation to postpartum thyroid-dysfunction and depression. Acta Endocrinologica. 1993;129:26–30. doi: 10.1530/acta.0.1290026. [DOI] [PubMed] [Google Scholar]
- Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289–295. doi: 10.1016/j.genhosppsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Russell JA, Leng G, Douglas AJ. The magnocellular oxytocin system, the fount of maternity: adaptations in pregnancy. Front Neuroendocrinol. 2003;24:27–61. doi: 10.1016/s0091-3022(02)00104-8. [DOI] [PubMed] [Google Scholar]
- Sala NL, Althabe O. The milk ejecting effect induced by oxytocin during human lactation. Acta Physiol Lat Am. 1968;18:96–103. [PubMed] [Google Scholar]
- Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Marechal P, Pequeux C, et al. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32:407–410. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Smith R. Alterations in the hypothalamic pituitary adrenal axis during pregnancy and the placental clock that determines the length of parturition. J Reprod Immunol. 1998;39:215–220. doi: 10.1016/s0165-0378(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Stein A, Arteche A, Lehtonen A, Craske M, Harvey A, Counsell N, et al. Interpretation of infant facial expression in the context of maternal postnatal depression. Infant Behav Dev. 2010;33:273–278. doi: 10.1016/j.infbeh.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tops M, Van Peer JM, Korf J, Wijers AA, Tucker DM. Anxiety, cortisol, and attachment predict plasma oxytocin. Psychophysiology. 2007;44:444–449. doi: 10.1111/j.1469-8986.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- van der Post JAM, van Buul BJA, Hart AAM, van Heerikhuize JJ, Pesman G, Legros JJ, et al. Vasopressin and oxytocin levels during normal pregnancy: Effects of chronic dietary sodium restriction. J Endocrinol. 1997;152:345–354. doi: 10.1677/joe.0.1520345. [DOI] [PubMed] [Google Scholar]
- van Leengoed E, Kerker E, Swanson HH. Inhibition of postpartum maternal-behavior in the rat by injecting an oxytocin antagonist into the cerebral-ventricles. J Endocrinol. 1987;112:275–282. doi: 10.1677/joe.0.1120275. [DOI] [PubMed] [Google Scholar]
- Wismer Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc Natl Acad Sci USA. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KL, Stowe ZN. Psychobiology of postpartum mood disorders. Sem Reprod Endocrinol. 1997;15:77–89. doi: 10.1055/s-2008-1067970. [DOI] [PubMed] [Google Scholar]
- Wittchen H-U, Lachner G, Wunderlich U, Pfister H. Test-restes reliability of the computerized DSM-IV version of the Munich Composite International Diagnostic Interview (M-CIDI) Soc Psychiatry Psychiatric Epidemiol. 1998;33:568–578. doi: 10.1007/s001270050095. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Pfister H. Instruktionsmanual zur Durchführung von DIA-X-Interview) Swets Test Services: Frankfurt; 1997. [Google Scholar]
- World Health Organization . Composite International Diagnostic Interview (CIDI): a) CIDI-interview (version 1.0), b) CIDI-user manual, c) CIDI-training manual, d) CIDI-computer programs. World Health Organization: Geneva; 1990. [Google Scholar]
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: New insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- Yim IS, Glynn LM, Schetter CD, Hobel CJ, Chicz-DeMet A, Sandman CA. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry. 2009;66:162–169. doi: 10.1001/archgenpsychiatry.2008.533. [DOI] [PMC free article] [PubMed] [Google Scholar]