Abstract
We have shown previously that aberrant hippocampal (HPC) output underlies the dopamine (DA) dysfunction observed in the methylazoxymethanol acetate (MAM) developmental model of schizophrenia in the rodent. This alteration of HPC activity was proposed to result from a reduction in parvalbumin (PV)-expressing GABAergic interneurons and consequent destabilization of the output of pyramidal neurons, as well as disrupted activation across a broad neural network. In vivo extracellular recordings were performed in the ventral tegmental area (VTA) and ventral HPC of saline- (SAL) and MAM-treated animals. A novel benzodiazepine-positive allosteric modulator (PAM), selective for the α5 subunit of the GABAA receptor, SH-053-2′F-R-CH3, was tested for its effects on the output of the HPC, leading to dopamine system hyperactivity in MAM-treated animals. In addition, the effect of SH-053-2′F-R-CH3 on the hyperactive locomotor response to amphetamine in MAM animals was examined. We demonstrate that treatment with the α5GABAAR PAM reduced the number of spontaneously active DA neurons in the VTA of MAM animals to levels observed in SAL rats, both when administered systemically and when directly infused into the ventral HPC. Moreover, HPC neurons in both SAL and MAM animals showed diminished cortical-evoked responses following α5GABAAR PAM treatment. In addition, the increased locomotor response to amphetamine observed in MAM rats was reduced following α5GABAAR treatment. This study supports a novel treatment of schizophrenia that targets abnormal HPC output, which in turn normalizes dopaminergic neuronal activity.
Keywords: α5GABAA receptors, hippocampus, dopamine, schizophrenia, positive allosteric modulator
INTRODUCTION
There is mounting evidence supporting a role for hippocampal (HPC) dysfunction in the pathophysiology of schizophrenia. Morphological changes, reduced HPC volume, and GAD67 expression (Benes et al, 2007; Rimol et al, 2010) have been reported post-mortem in the brains of patients with schizophrenia. In addition, imaging studies have demonstrated alterations in both HPC activation and morphology that can precede the presentation of psychotic symptoms or correlate with severity of cognitive deficits (Pantelis et al, 2003; Schobel et al, 2009a, 2009b; Weiss et al, 2006; Wolf et al, 2008), both during baseline and during activation by a cognitive test.
Animal models of schizophrenia have provided insights into network disturbances that contribute to the behavioral pathology. One such model relies on a development disturbance induced by administration of a DNA-methylating agent, methylazoxymethanol acetate (MAM), to pregnant dams on gestational day (GD) 17. MAM-treated offspring demonstrate both structural and behavioral abnormalities, consistent with those observed in patients with schizophrenia. Briefly, MAM animals are characterized by reduced limbic cortical and HPC volumes with increased cell packing density (Moore et al, 2006), increased sensitivity to psychostimulants (Flagstad et al, 2004; Lodge and Grace, 2007; Moore et al, 2006), reduced prepulse inhibition to startle, and deficits in latent inhibition (Flagstad et al, 2004). In addition, MAM animals demonstrate a pathological increase in spontaneous dopamine (DA) activity by the ventral tegmental area (VTA) that can be attributed to aberrant activation within the ventral HPC (Lodge and Grace, 2007). The hyperactivation of the HPC and disruption of normal oscillatory activity in the HPC and cortex of MAM animals is the likely result from reductions in parvalbumin (PV)-stained interneurons (Lodge et al, 2009).
Oscillatory activity within the HPC has been implicated in normal cognitive processes and relies on intact GABAergic activity, via GABAA receptors, of HPC interneurons (Mann and Paulsen, 2007; Whittington et al, 1995). Indeed, fast-spiking, PV-expressing, soma-targeting interneurons are one subtype of interneuron present in the HPC and are known to be crucial in the generation of gamma oscillations (20–80 Hz) (Bartos et al, 2007). The GABAA receptor is a pentameric anion-selective ion channel that is composed of different classes of subunits (α1–6, β1–3, γ1–3, δ, θ, ρ, and ɛ) (McKernan and Whiting, 1996). The α5GABAA receptor subunit is unique in its relatively confined distribution in the HPC, and to a lesser extent in the cortex and thalamus (Heldt and Ressler, 2007; Ramos et al, 2004; Serwanski et al, 2006). In addition, within CA1 and CA3 regions of the HPC, α5GABAA receptors are located extrasynaptically to pyramidal neurons (Fritschy and Mohler, 1995; Serwanski et al, 2006). The tonic inhibition provided by α5-containing GABAA receptors (α5GABAAR) is likely important for coordinating spike timing of pyramidal neurons and balancing excitation (Semyanov et al, 2004). Genetic deletion of α5GABAAR blocks tonic inhibitory currents recorded from pyramidal neurons in the CA1 region of HPC slices (Caraiscos et al, 2004). Moreover, pyramidal neurons from α5−/− mice show reduced depolarizing current thresholds for action potential generation in comparison with wild-type controls (Bonin et al, 2007). In addition, α5GABAAR is also important for regulating gamma oscillatory activity in HPC slice preparations (Towers et al, 2004).
Antagonism or genetic deletion of α5GABAA R has behavioral consequences that resemble some of the behavioral abnormalities seen in schizophrenia, including reduced prepulse inhibition to startle (Hauser et al, 2005) and impaired latent inhibition (Gerdjikov et al, 2008). In the present study, we use the MAM model to demonstrate the effectiveness of a novel compound, SH-053-2′F-R-CH3, in restoring normal HPC activity and secondary spontaneous DA activity. SH-053-2′F-R-CH3 is unique as a benzodiazepine-positive allosteric modulator (PAM) selective for the α5 subunit of the GABAAR (Cook et al, 2009; Fischer et al, 2010; Savic et al, 2010). Furthermore, the efficacy of α5GABAAR PAM treatment in reducing the hyperlocomotor response of MAM animals to amphetamine is explored. These data have been presented previously in abstract form (Gill et al, 2010).
MATERIALS AND METHODS
Animals
Experiments were conducted according to the guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. All electrophysiological recordings and behavioral experiments were conducted in adult male offspring of MAM- and SAL-treated rats.
Methylazoxymethanol Treatment
MAM treatments were performed as described previously (Moore et al, 2006). In brief, timed pregnant female Sprague–Dawley rats (Hilltop) were obtained on GD 15 and individually housed in ventilated plastic breeding tubs. MAM (20 mg/kg, i.p.) was administered on GD 17. Control dams received injections of SAL (1 ml/kg, i.p.). Male pups were weaned-off on day 21 and pair-housed with littermates, until approximately 3–4 months of age, at which time they were used for physiological or behavioral experiments. Multiple litters of MAM- and SAL-treated rats were used for the completion of this study.
Electrophysiological Recording
Animals were anesthetized with an initial dose of chloral hydrate (Sigma, 400 mg kg, i.p.) and were supplemented periodically (i.v.) to maintain a suppression of the hindlimb withdrawal reflex. After being placed in a stereotaxic frame (Kopf), rats were implanted with a catheter in the lateral tail vein to allow for intravenous injections. Body temperature was maintained at 37°C with a temperature-controlled heating pad (Fintronics). In vivo extracellular recordings were conducted using single glass microelectrodes (WPI; impedance 6–8 MΩ) filled with a 2% Chicago Sky Blue (Sigma) solution in 2 M NaCl. Electrodes were placed in the VTA (AP,−5.3 mm; ML, +0.6 mm from bregma, and −6.5 to −9.0 mm ventral of brain surface) or ventral HPC (AP, −5.3 mm; ML, +5.3 mm from bregma, and −5.5 to −8.5 mm ventral of brain surface) using a hydraulic microdrive (Kopf). The population activity of DA neurons was determined by counting the number of spontaneously firing DA neurons encountered while making 6–9 vertical passes (each track separated by 200 μm). Spontaneous neural activity was monitored in each track with open filter settings (low pass=50 Hz; high pass=16 kHz), until an individual neuron was encountered that met the electrophysiological criteria of DA neurons established previously (Grace and Bunney, 1983). The activity of each DA neuron was recorded for 5 min. Three parameters of activity were measured: (1) population activity (defined as the number of spontaneously active DA neurons recorded per electrode track), (2) basal firing rate, and (3) the proportion of action potentials occurring in bursts (bursts defined as the occurrence of two spikes with an interspike interval of 80 ms, and the termination of the burst defined as the occurrence of an interspike interval of 160 ms) (Grace and Bunney, 1983).
For recordings in the ventral HPC, neurons were selected, based on short-latency (<10 ms) evoked responses to stimulation of entorhinal cortex (0.5 Hz). A concentric bipolar electrode (NEX-100X; Rhodes Medical Instruments) was implanted in the entorhinal cortex (30° angle; AP, −6.6 mm; ML, +1.6 mm from bregma, and −8.5 mm ventral to top of skull). A dual output stimulator (S8800; Grass Technologies) was used to generate single current pulses (duration, 0.20 ms; intensity 300 μA) in the entorhinal cortex, while the recording microelectrode was advanced slowly into the ventral HPC.
SH-053-2′F-R-CH3 Preparation and Treatment
Complete methods regarding synthesis of the novel α5GABAAR PAM, SH-053-2′F-R-CH3 have been described previously (Cook et al, 2009). Briefly, the R stereoisomer of 8-ethynyl-6-(2-fluorophenyl)-4-methyl-4H-2,5,10b-triaza-benzo[e]azulene-3-carboxylic acid ethyl ester was synthesized at the Department of Chemistry and Biochemistry, University of Wisconsin—Milwaukee. SH-053-2′F-R-CH3 has been demonstrated to have a greater relative affinity for α5 GABAAR (Ki=95.2) as well as a lower affinity for α1, α2, and α3 GABAAR (Ki=759.1, 948.2, and 768.8, respectively) (Fischer et al, 2010).
For electrophysiological recordings, SH-053-2′F-R-CH3 was initially dissolved in 50% propylene glycol and 50% deinonized water with 10 μl EtOH. The drug was then brought to a final concentration of 0.1 mg/kg in 50% propylene glycol and 50% deionized water for systemic administration (i.v.). The 1 μM concentration of SH-053-2′F-R-CH3, used for local infusion into the ventral HPC, has been demonstrated in competition binding assays to provide optimal binding to the α5 subunit of the GABAA receptor with little nonspecific binding at the other alpha (1–3) subunits (Savic et al, 2010). Local infusions were performed in anesthetized animals before electrophysiological recordings. Guide cannula (26 gauge) was placed stereotaxically above the ventral HPC (AP, −5.8 mm; ML, +5.0 mm from bregma, and −4.5 mm ventral of brain surface). Subsequently, infusion cannula (33 gauge) was inserted into the guide cannula, extending 1 mm beyond the tip of the guide cannula. Animals were infused bilaterally with either vehicle or SH-053-2′F-R-CH3 (0.5 μl/side; rate of 0.25 μl/min), and infusion cannula remained in place for 2 min after infusion to allow complete diffusion of the compound.
Before DA neuron sampling or following a 20-min baseline recording period for HPC neurons, animals were administered either vehicle or SH-053-2′F-R-CH3 (0.1 mg/kg, i.v. or 1 μM/0.5 μl/side, HPC infusion). For behavioral experiments, SH-053-2′F-R-CH3 was dissolved with the aid of sonication in a solvent containing 85% deionized water, 14% propylene glycol, and 1% Tween 80, and was administered intraperitoneally (10 mg/kg) in a volume of 2 ml/kg.
Amphetamine-Induced Locomotion
Rats used for behavior were housed in a reverse light/dark cycle (lights on from 1900 to 0700 h) for at least 10 days before the start of behavioral experiments. Rats were administered the α5GABAAR PAM, SH-053-2′F-R-CH3 (10 mg/kg, i.p.), or SAL (2 ml/kg) 20 min before being placed in an open-field arena (Coulbourn Instruments, Allentown, PA) in which spontaneous locomotor activity in the x–y plane was determined for 30 min by beam breaks and recorded with TruScan software (Coulbourn Instruments). Rats were then injected with -amphetamine sulfate (0.5 mg/kg, i.p.) and locomotor activity recorded for an additional 90 min.
Histology
At the completion of the electrophysiological experiments, the recording location was marked via electrophoretic ejection of Chicago sky blue from the tip of the recording electrode (−20 μA constant current, 30 min). Rats used for electrophysiological recordings were killed with an overdose of anesthetic (chloral hydrate, additional 400 mg/kg, i.v.), whereas rats used for behavioral experiments were deeply anesthetized with isoflurane before decapitation. All rats used for electrophysiological recordings were decapitated and their brains removed, fixed for at least 48 h (8% w/v paraformaldehyde in PBS), and cryoprotected (25% w/v sucrose in PBS) until saturated. Brains were sectioned (60 μm coronal sections), mounted onto gelatin-chrom alum-coated slides, and stained with a mixture of cresyl violet and neutral red for histochemical verification of electrode sites. All histology was performed with reference to a stereotaxic atlas (Paxinos and Watson, 1996).
Analysis
Electrophysiological analysis of DA and ventral HPC neuronal activity was performed using custom-designed computer software (Neuroscope), whereas locomotor behavior was recorded using TruScan software. All data are represented as the mean±SEM, unless otherwise stated. All statistics were calculated using the SigmaStat software program (Systat Software, San Jose, CA).
RESULTS
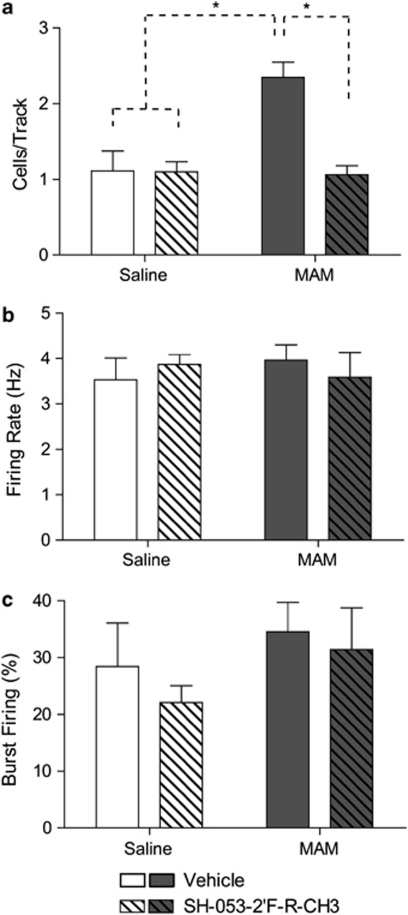
Rats that were administered SAL on GD17 (SAL; n=5, 47 neurons) demonstrated an average of 1.11±0.27 spontaneously active DA neurons per electrode track. Consistent with what has been reported previously for untreated rats, DA neurons recorded in SAL rats (Figures 1a–c) fired at an average rate of 3.53±0.48 Hz with 28.4±7.7% of action potentials fired in bursts (Floresco et al, 2003; Lodge and Grace, 2006a, 2006b; Lodge and Grace, 2007). In addition, adult rats that received injections of MAM at GD17 (n=7, 106 neurons) showed significantly greater DA neuron population activity (2.35±0.20 cells/track; F=15.99, p<0.001) without significant differences in average firing rate (3.96±0.34 Hz; F=0.39, p>0.05) or average burst firing (34.5±5.2% F=2.99, p>0.05) with relative to controls, as described previously (Lodge and Grace, 2007). There was a significant interaction effect between MAM offspring and α5GABAAR PAM injections on spontaneous DA activity (F=17.97, p<0.001). In control animals, treatment with the selective α5GABAAR PAM (n=7, 69 neurons), SH-053-2′F-R-CH3, had no effect on DA neuron population activity (1.10±0.13 cells/track; t=0.05, p>0.05), average firing rate (3.87±0.22 Hz), or average burst firing (22.07±3.02%). In contrast, MAM animals treated with the α5GABAAR PAM (n=7, 67 neurons) displayed a significant reduction in DA population activity compared with MAM rats treated with vehicle (1.06±0.12 cells/track; t=5.82, p<0.001), without a significant change in average firing rate (3.59±0.54 Hz) or average burst firing (31.46±7.29%).
Figure 1.
Treatment with SH-053-2′F-R-CH3 (0.1 mg/kg, i.v.; patterned bars) normalizes the aberrant increase in the number of spontaneously firing dopamine neurons (expressed as cells/track) in methylazoxymethanol acetate (MAM)-treated animals (a). There was no effect of SH-053-2′F-R-CH3 treatment in control animals (open bars, a–c) or on firing rate and burst activity in MAM animals (dark bars; b–c). (*p<0.05, two-way ANOVA, Holm–Sidak post hoc; N=5–7 rats/group).
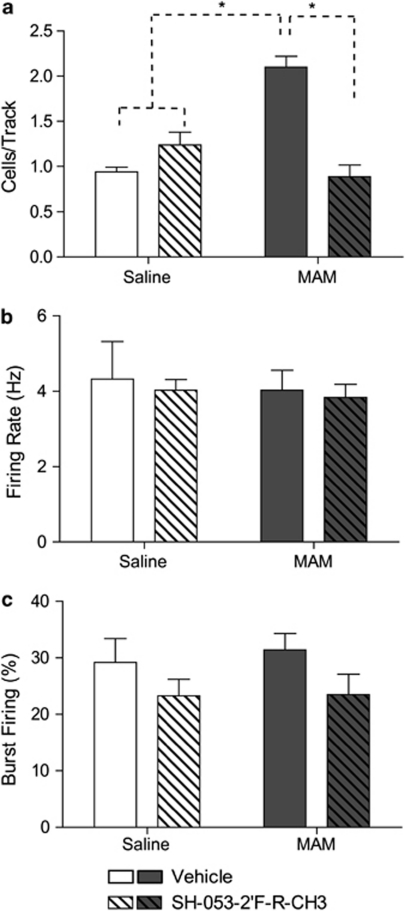
To verify the site of action of the α5 GABAAR PAM in the ventral HPC, SH-053-2′F-R-CH3 or vehicle was microinfused directly into the ventral HPC of a separate group of MAM- and SAL-treated rats before sampling spontaneous DA neuron activity. Consistent with the results reported above, in control animals infused bilaterally with vehicle in the ventral HPC (Figures 2a–c), DA neurons (0.94±0.10 cells/track) fired at an average rate of 4.23±0.41 Hz, with 29.20±4.20% of action potentials fired in bursts. In comparison, MAM animals infused with vehicle demonstrated significantly greater DA neuron population activity (2.10±0.12 cells/track, F=4.70, p<0.05) without significant differences in firing rate (4.18±0.28 Hz; F=0.13, p>0.05) or average burst firing (31.41±2.89% F=0.12, p>0.05) relative to controls. There was a significant interaction between MAM and α5GABAAR PAM infusions on spontaneous DA activity (F=20.59, p<0.001). In control animals (N=7, 70 neurons) bilateral infusion of the α5GABAAR PAM directly into the ventral HPC had no effect on DA neuron population activity (1.24±0.14 cells/track; t=0.61, p>0.05) or average firing rate (4.03±0.28 Hz). Similar to what was observed following intravenous administration of the compound, MAM animals infused with the α5GABAAR PAM in the ventral HPC (n=7, 47 neurons) displayed a significant reduction in DA neuron population activity compared with MAM rats treated with vehicle (0.89±0.14 cells/track; t=5.80, p<0.001), without a significant change in average firing rate (3.84±0.34 Hz). Unlike systemic treatment, HPC infusion of the α5GABAAR PAM did significantly reduce burst firing in comparison with vehicle infusion (F=3.96, p<0.05). There was not a significant interaction effect of MAM and α5GABAAR PAM (F=0.08, p>05) on burst firing, indicating the decrease was present in both control (23.25±2.97%) and MAM animals (23.49±3.62%) following α5GABAAR PAM infusions.
Figure 2.
Hippocampal (HPC) infusion of SH-053-2′F-R-CH3 (1 μM/side; patterned bars) reduces the increase in the number of spontaneously firing dopamine neurons (cells/track) in methylazoxymethanol acetate (MAM)-treated animals (a). There was no effect of SH-053-2′F-R-CH3 treatment in control animals (open bars, a–c) or on firing rate in MAM animals (dark bars; b). Hippocampal (HPC) infusion of SH-053-2′F-R-CH3 significantly reduced the percentage of spikes occurring in bursts of dopamine (DA) neurons in MAM and control animals (c). (*p<0.05, two-way ANOVA, Holm–Sidak post hoc; N=7 rats/group).
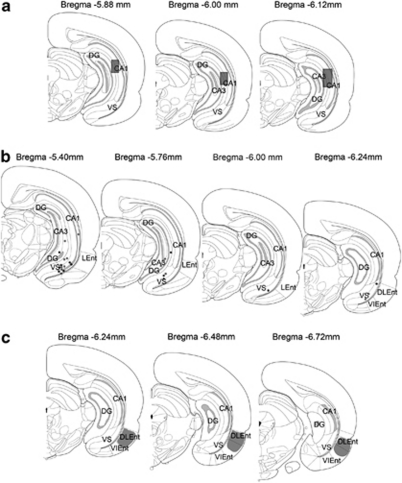
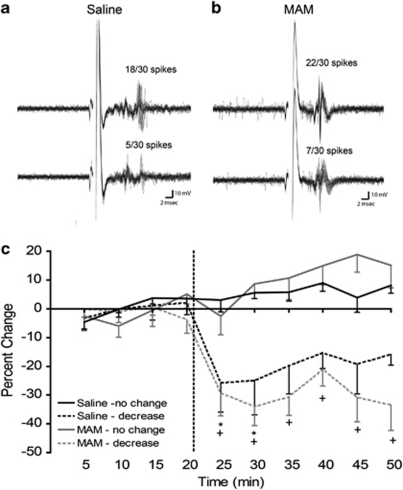
Given previous evidence that alterations in HPC activity underlies the hyperactivity in the DA system in MAM rats (Lodge and Grace, 2007), changes in the response of ventral HPC neurons following α5 GABAAR PAM treatment was explored. Illustrated in Figure 3 is a representation of the localization of recording sites within the ventral HPC. In SAL rats, neurons (n=15) showed a short-latency (10.21±0.66 ms; Figure 4c) excitatory response to stimulation of the neighboring entorhinal cortex. A subset of these neurons (50%) displayed a transient (<15 min) reduction in evoked responses to entorhinal cortex stimulation following α5 GABAAR treatment (Figure 4a; F=25.21, p< 0.001). Short-latency (9.96±0.72 ms; n=16) excitatory responses to entorhinal cortex stimulation were also observed in the HPC of MAM-treated animals (Figure 4b). Following α5 GABAAR treatment, 62.5% of neurons showed a longer lasting (up to 30 min) decrease in evoked responses to entorhinal cortex stimulation in comparison with controls (Figure 4a; F=27.08, p< 0.05). Therefore, the α5 GABAAR PAM appeared to attenuate the excitation evoked by entorhinal cortex stimulation within the ventral HPC. Moreover, this appears to be selective for regions such as the hippocampus in which the α5 GABAAR is localized, as this drug failed to attenuate thalamic-evoked activation of the cingulate cortex at 1 × and 10 × the dose effective in the hippocampus (N=2). The sustained reduction in HPC excitability and corresponding decrease in population activity in the VTA of MAM animals is parallel to the impact of inactivation of the ventral HPC with tetrodotoxin (TTX) on spontaneous DA activity in MAM rats (Lodge and Grace, 2007).
Figure 3.
Schematic illustrates infusion cannula tip locations in ventral hippocampal (HPC) (a; shaded rectangle), electrophysiological recording sites in ventral HPC (b; grey=saline, black=methylazoxymethanol acetate (MAM)), and target stimulation sites (c; shaded area) in dorsolateral entorhinal cortex. Adapted from Paxinos and Watson, 1996.
Figure 4.
Extracellular recording traces illustrate the reduction in evoked responses in the ventral hippocampal (HPC) to entorhinal cortex stimulation in both methylazoxymethanol acetate (MAM) and saline-treated animals (a, b). Treatment with SH-053-2′F-R-CH3 (0.1 mg/kg, i.v.) decreases the evoked excitatatory response (dashed lines) of ventral HPC neurons to entorhinal cortex stimulation in both MAM- and saline- treated animals (c). (*p<0.05 for saline and +p<0.05, two-way repeated measures ANOVA, Holm–Sidak post-hoc).
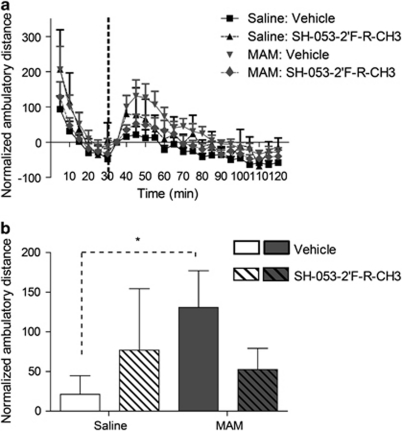
Given that rats treated on GD17 with MAM show a heightened response to psychostimulants that can be reduced following inactivation of the ventral HPC, it was tested whether treatment with a GABAAR PAM can have a similar effect on behavior (Flagstad et al, 2004; Lodge and Grace, 2007; Moore et al, 2006). A repeated measures ANOVA on the locomotor response to a low dose (0.5 mg/kg, i.p.) of -amphetamine showed both a significant difference between MAM and control animals (F=9.42, p<0.05), as well as a significant interaction between MAM and α5GABAAR PAM injections (Figure 5a; F=30.36, p<0.05). MAM rats (n=11) displayed a significantly enhanced peak locomotor response (∼10.5-fold increase above peak response observed in controls, n=12; p<0.05) to -amphetamine (F=6.42, p< 0.05; Figure 5b). In control rats, the locomotor response to amphetamine was not significantly altered by α5GABAAR treatment (n=11; F=0.79, p>0.05). In contrast, α5GABAAR PAM was able to reduce the psychostimulant-induced locomotor response in MAM rats (n=12), such that the response was not significantly different from control rats (F=0.89, p>0.05). These data suggest that, by attenuating HPC activity and secondary DA neuron population activity in MAM rats, α5GABAAR treatment can attenuate the behavioral hyperresponsivity to amphetamine.
Figure 5.
Treatment with SH-053-2′F-R-CH3 (10 mg/kg, i.p.) reduces the aberrant increased locomotor response to -amphetamine (0.5 mg/kg i.p.) observed in methylazoxymethanol acetate (MAM) rats (a). MAM animals demonstrated a significantly larger peak locomotor response than both saline-treated animals and MAM animals pre-treated with the alpha-5 PAM (b). (There was a significant difference between MAM-vehicle and all other groups, p<0.05, two-way repeated measures ANOVA, Holm–Sidak post-hoc).
DISCUSSION
We have demonstrated that the pathological increase in dopaminergic activity observed in the MAM model of schizophrenia can be resolved by treatment with a selective α5GABAAR PAM, SH-053-2′F-R-CH3. Moreover, the decrease in DA population activity following α5GABAA R treatment is likely the result of reduced activation of the ventral HPC. These alterations in ventral HPC output and secondary reductions in spontaneous DA activity are also reflected behaviorally. The heightened locomotor response to a low dose of -amphetamine, observed in MAM animals, was also reduced by treatment with the α5GABAA R PAM.
Changes in normal HPC function likely contribute to the psychotic features of schizophrenia. There is substantial evidence for a DA involvement in psychosis: (1) DA agonists are known to exacerbate psychosis in schizophrenia (Curran et al, 2004; Yui et al, 2002), (2) DA antagonist antipsychotic drugs are more effective at treating the positive psychotic symptoms of this disorder (Seeman et al, 1976; Strange, 2001), (3) and PET imaging studies show higher amphetamine-induced raclopride displacement in schizophrenia subjects, indicative of increased DA release that correlates with a worsening of psychosis (Breier et al, 1997; Laruelle et al, 1999). It has already been established that the HPC regulates DA population activity in the VTA via a multisynaptic pathway (vHPC—nucleus accumbens—ventral pallidum—VTA) (Floresco et al, 2001; Floresco et al, 2003). In the MAM rodent model of schizophrenia, increased activity of this multisynaptic pathway, originating in the ventral HPC, underlies the pathological increase in DA population activity (Lodge et al, 2009; Lodge and Grace, 2007). It has been demonstrated that in addition to simultaneous increases in population activity in both the ventral HPC and VTA, inactivation of the HPC with TTX can abolish the increase in population activity in the VTA seen in MAM animals (Lodge and Grace, 2007). There are also distinct morphological changes in the MAM HPC, decreases in structure volume, as well as decreased expression of PV, indicative of diminished interneuron functionality (Lodge et al, 2009; Moore et al, 2006). Ultimately, the loss of functionality of this subset of GABAergic interneurons projecting to pyramidal cell neurons likely accounts for the HPC hyperactivity and secondary increases in DA population response in the VTA. This is also consistent with clinical data showing that patients with schizophrenia demonstrate abnormal resting state activation in the HPC (Heckers et al, 1999; Nordahl et al, 1996) that correlates with psychosis (Grace, 2010). Therefore, abnormally increased ventral HPC excitability appears to drive the increased DA neuron population activity that we propose correlates with psychosis in schizophrenia.
The non-principal neurons in the HPC are a heterogeneous population of GABAergic neurons. The four main categories of interneurons are characterized by their expression of nitric oxide synthase, calretinin, somatostatin, and PV (Nomura et al, 1997a). Parvalbumin expression is observed in interneurons located mainly in the pyramidal cell layers in CA1, CA3, and the dentate gyrus. Three different neuron types express PV; basket cells, axo-axonic cells, and bistratified cells, and form synapses on somata, axon initial segments, and proximal dendrites of pyramidal and granule cells (Nomura et al, 1997a, 1997b). On the basis of their connectivity with pyramidal neurons, as well as with each other, PV-expressing interneurons are effectively positioned to participate in network oscillatory activity within the HPC. In patients with schizophrenia, as well as in animal models of the disease, a significant reduction in PV has been reported in cortical as well as HPC areas (Hashimoto et al, 2003; Lewis et al, 2001; Lodge et al, 2009; Tseng et al, 2008). The loss of PV corresponds with a pathological alteration in oscillatory activity with broad consequences on network dynamics between brain regions. Gamma oscillations in the HPC require GABAA receptor activity (Whittington et al, 1995), and there is a loss of evoked gamma oscillatory activity, both in MAM rats and in humans, in regions showing PV neuron decreases (Gonzalez-Burgos et al, 2010; Lodge et al, 2009; Minzenberg et al, 2010).
GABAA R are pentameric and most of them contain a combination of at least 1α, 1β, and 1γ subunit (McKernan and Whiting, 1996; Pritchett et al, 1989; Quirk et al, 1996). The α5GABAA subunit shows a high affinity for GABA as well as a restricted pattern of expression, predominately in the HPC, with less expression in the cortex and thalamus (Heldt and Ressler, 2007; Ramos et al, 2004; Sur et al, 1999). In the adult HPC, α5GABAAR are located extrasynaptically, primarily in the dendritic fields of CA1, CA3, dentate gyrus, and subiculum (Sur et al, 1999). In addition, α5GABAAR expression and binding is greater in the ventral HPC, mainly stratum lacunosum-moleculare, in comparison with the dorsal HPC (Sarantis et al, 2008; Sotiriou et al, 2005), suggesting that targeted agonism of this GABA subunit would alter ventral HPC activity selectively. It has been shown that extrasynaptic GABAA receptors mediate tonic inhibitory conductances, at both pyramidal and dentate granule cells (Bai et al, 2001; Nusser and Mody, 2002). We demonstrated that treatment with the α5GABAAR PAM, SH-053-2′F-R-CH3 was able to reduce the responsiveness of a subset of HPC neurons to entorhinal cortex stimulation. In MAM-treated animals, the reduction in HPC activity was also accompanied by a reduction in spontaneous DA neuron activity in the VTA.
Targeted deletion of the α5-subunit causes alterations in learning and memory processes, consistent with alterations in normal HPC functions. Mice with mutations of the α5-subunit display impairments in latent inhibition, prepulse inhibition, and extinction of fear behaviors (Gerdjikov et al, 2008; Hauser et al, 2005). Consequently, with otherwise normal HPC activity, loss of the α5-subunit has behavioral consequences in the processing of contextual stimuli. In the MAM animal, in which oscillatory activity is already impaired by a loss of PV, increasing α5GABAAR activation may act to restore normal HPC function, for example, increasing gamma oscillations, and consequently affecting behavior. MAM-treated animals are known to show heightened behavioral responses to psychostimulants (Flagstad et al, 2004; Lodge and Grace, 2007; Moore et al, 2006), which is consistent with the increased amphetamine-induced raclopride displacement seen in human schizophrenia patients (Breier et al, 1997). In the present study, treatment with the specific α5GABAAR PAM, SH-053-2′F-R-CH3 reduced the locomotor response to a low dose of -amphetamine observed in MAM animals, reflecting the reduced activation of DA neurons in the VTA.
By targeting aberrant HPC activity in MAM rats with a selective α5GABAAR PAM, we successfully reversed the pathological increase in tonic DA transmission. In addition, we were able to reduce the behavioral sensitivity to psychostimulants observed in MAM rats. This suggests that this novel agent would be effective in alleviating DA-mediated psychosis. However, if this drug can also restore rhythmicity within HPC-efferent structures, it may also affect other aspects of this disease state such as cognitive disabilities and negative symptoms. Considering that the MAM model provides schizophrenia-like neuroanatomical and behavioral phenotypes, the successful resolution of some of these aberrations with α5GABAAR PAM treatment is suggestive of the possible success of similar treatment in patients. Using a selective α5GABAAR agonist in lieu of the less selective benzodiazepine drugs, such as diazepam, would provide the therapeutic benefit of reducing psychotic symptoms without producing unwanted sedation.
Acknowledgments
This work was supported by the following grants and awards: United States Public Health Service Grants T32-MH18273-23 (KMG); MH090067 (DJL); MH046851 (JMC); DA15408 and MH57440 (AAG); and NARSAD award from the Maltz Family Foundation (DJL). We thank Niki MacMurdo for her technical assistance; Brian Lowry for the production, development, and support with the custom-designed electrophysiology software (Neuroscope); and Pauline Belujon for critical reading and helpful discussions.
AAG: Johnson&Johnson, Lundbeck, Pfizer, GSK, Puretech Ventures, Merck, Takeda, and Dainippon Sumitomo. All other authors declare no conflict of interest.
References
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin RP, Martin LJ, MacDonald JF, Orser BA. Alpha5GABAA receptors regulate the intrinsic excitability of mouse hippocampal pyramidal neurons. J Neurophysiol. 2007;98:2244–2254. doi: 10.1152/jn.00482.2007. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, de Bartolomeis A, Weinberger DR, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JM, Zhou H, Huang S, Sarma PV, et al. United States of America. US Patent 7,618,458; 2009. Stereospecific and anticonvulsant agents with reduced muscle-relaxant, sedative-hypnotic and ataxic effects. [Google Scholar]
- Curran C, Byrappa N, McBride A. Stimulant psychosis: systematic review. Br J Psychiatry. 2004;185:196–204. doi: 10.1192/bjp.185.3.196. [DOI] [PubMed] [Google Scholar]
- Fischer BD, Licata SC, Edwankar RV, Wang ZJ, Huang S, He X, et al. Anxiolytic-like effects of 8-acetylene imidazobenzodiazepines in a rhesus monkey conflict procedure. Neuropharmacology. 2010;59:612–618. doi: 10.1016/j.neuropharm.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagstad P, Mork A, Glenthoj BY, van Beek J, Michael-Titus AT, Didriksen M. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology. 2004;29:2052–2064. doi: 10.1038/sj.npp.1300516. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Gerdjikov TV, Rudolph U, Keist R, Mohler H, Feldon J, Yee BK. Hippocampal alpha 5 subunit-containing GABA A receptors are involved in the development of the latent inhibition effect. Neurobiol Learn Mem. 2008;89:87–94. doi: 10.1016/j.nlm.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Gill K, Lodge DJ, Grace AA. Abstracts of the 2nd Biennial Schizophrenia International Research Conference, Florence, Italy, 10–14 April 2010. Schizophr Res. 2010;117:103–536. doi: 10.1016/j.schres.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Dopamine system dysregulation by the ventral subiculum as the common pathophysiological basis for schizophrenia psychosis, psychostimulant abuse, and stress. Neurotox Res. 2010;18:367–376. doi: 10.1007/s12640-010-9154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser J, Rudolph U, Keist R, Mohler H, Feldon J, Yee BK. Hippocampal alpha5 subunit-containing GABAA receptors modulate the expression of prepulse inhibition. Mol Psychiatry. 2005;10:201–207. doi: 10.1038/sj.mp.4001554. [DOI] [PubMed] [Google Scholar]
- Heckers S, Goff D, Schacter DL, Savage CR, Fischman AJ, Alpert NM, et al. Functional imaging of memory retrieval in deficit vs nondeficit schizophrenia. Arch Gen Psychiatry. 1999;56:1117–1123. doi: 10.1001/archpsyc.56.12.1117. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Forebrain and midbrain distribution of major benzodiazepine-sensitive GABAA receptor subunits in the adult C57 mouse as assessed with in situ hybridization. Neuroscience. 2007;150:370–385. doi: 10.1016/j.neuroscience.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006a;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci USA. 2006b;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 2007;30:343–349. doi: 10.1016/j.tins.2007.05.003. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain. Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology. 2010;35:2590–2599. doi: 10.1038/npp.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60:253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Fukuda T, Aika Y, Heizmann CW, Emson PC, Kobayashi T, et al. Distribution of nonprincipal neurons in the rat hippocampus, with special reference to their dorsoventral difference. Brain Res. 1997a;751:64–80. doi: 10.1016/s0006-8993(96)01395-9. [DOI] [PubMed] [Google Scholar]
- Nomura T, Fukuda T, Aika Y, Heizmann CW, Emson PC, Kobayashi T, et al. Laminar distribution of non-principal neurons in the rat hippocampus, with special reference to their compositional difference among layers. Brain Res. 1997b;764:197–204. doi: 10.1016/s0006-8993(97)00457-5. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Kusubov N, Carter C, Salamat S, Cummings AM, O'Shora-Celaya L, et al. Temporal lobe metabolic differences in medication-free outpatients with schizophrenia via the PET-600. Neuropsychopharmacology. 1996;15:541–554. doi: 10.1016/S0893-133X(96)00098-X. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.(Eds.) (1996The Rat Brain in Stereotaxic Coordinates. Academic: San Diego [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, et al. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, et al. [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the alpha 5 subunit. Neuropharmacology. 1996;35:1331–1335. doi: 10.1016/s0028-3908(96)00061-5. [DOI] [PubMed] [Google Scholar]
- Ramos B, Lopez-Tellez JF, Vela J, Baglietto-Vargas D, del Rio JC, Ruano D, et al. Expression of alpha 5 GABAA receptor subunit in developing rat hippocampus. Brain Res Dev Brain Res. 2004;151:87–98. doi: 10.1016/j.devbrainres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Hartberg CB, Nesvag R, Fennema-Notestine C, Hagler DJJr, Pung CJ, et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2010;68:41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Sarantis K, Sotiriou E, Papatheodoropoulos C, Matsokis N, Angelatou F. Differential pharmacological properties of GABAA/benzodiazepine receptor complex in dorsal compared to ventral rat hippocampus. Neurochem Int. 2008;52:1019–1029. doi: 10.1016/j.neuint.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Savic MM, Majumder S, Huang S, Edwankar RV, Furtmüller R, imoviæ S, et al. Novel positive allosteric modulators of GABAA receptors: do subtle differences in activity at alpha1 plus alpha5 versus alpha2 plus alpha3 subunits account for dissimilarities in behavioral effects in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:376–386. doi: 10.1016/j.pnpbp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Kelly MA, Corcoran CM, Van Heertum K, Seckinger R, Goetz R, et al. Anterior hippocampal and orbitofrontal cortical structural brain abnormalities in association with cognitive deficits in schizophrenia. Schizophr Res. 2009a;114:110–118. doi: 10.1016/j.schres.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009b;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, De BlasAL. Synaptic and nonsynaptic localization of GABAA receptors containing the alpha5 subunit in the rat brain. J Comp Neurol. 2006;499:458–470. doi: 10.1002/cne.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriou E, Papatheodoropoulos C, Angelatou F. Differential expression of gamma-aminobutyric acid--a receptor subunits in rat dorsal and ventral hippocampus. J Neurosci Res. 2005;82:690–700. doi: 10.1002/jnr.20670. [DOI] [PubMed] [Google Scholar]
- Strange PG. Antipsychotic drugs: importance of dopamine receptors for mechanisms of therapeutic actions and side effects. Pharmacol Rev. 2001;53:119–133. [PubMed] [Google Scholar]
- Sur C, Fresu L, Howell O, McKernan RM, Atack JR. Autoradiographic localization of alpha5 subunit-containing GABAA receptors in rat brain. Brain Res. 1999;822:265–270. doi: 10.1016/s0006-8993(99)01152-x. [DOI] [PubMed] [Google Scholar]
- Towers SK, Gloveli T, Traub RD, Driver JE, Engel D, Fradley R, et al. Alpha 5 subunit-containing GABAA receptors affect the dynamic range of mouse hippocampal kainate-induced gamma frequency oscillations in vitro. J Physiol. 2004;559:721–728. doi: 10.1113/jphysiol.2004.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Hashimoto T, Sesack SR, Kloc M, Lewis DA, et al. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss AP, Goff D, Schacter DL, Ditman T, Freudenreich O, Henderson D, et al. Fronto-hippocampal function during temporal context monitoring in schizophrenia. Biol Psychiatry. 2006;60:1268–1277. doi: 10.1016/j.biopsych.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Hose A, Frasch K, Walter H, Vasic N. Volumetric abnormalities associated with cognitive deficits in patients with schizophrenia. Eur Psychiatry. 2008;23:541–548. doi: 10.1016/j.eurpsy.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Yui K, Ikemoto S, Goto K, Nishijima K, Yoshino T, Ishiguro T. Spontaneous recurrence of methamphetamine-induced paranoid-hallucinatory states in female subjects: susceptibility to psychotic states and implications for relapse of schizophrenia. Pharmacopsychiatry. 2002;35:62–71. doi: 10.1055/s-2002-25067. [DOI] [PubMed] [Google Scholar]