Abstract
3,4-Methylenedioxymethamphetamine (MDMA) or ‘ecstasy' has been associated with memory deficits during abstinence and intoxication. The human neuropharmacology of MDMA-induced memory impairment is unknown. This study investigated the role of 5-HT2A and 5-HT1A receptors in MDMA-induced memory impairment. Ketanserin is a 5-HT2A receptor blocker and pindolol a 5-HT1A receptor blocker. It was hypothesized that pretreatment with ketanserin and pindolol would protect against MDMA-induced memory impairment. Subjects (N=17) participated in a double-blind, placebo-controlled, within-subject design involving six experimental conditions consisting of pretreatment (T1) and treatment (T2). T1 preceded T2 by 30 min. T1–T2 combinations were: placebo–placebo, pindolol 20 mg–placebo, ketanserin 50 mg–placebo, placebo–MDMA 75 mg, pindolol 20 mg–MDMA 75 mg, and ketanserin 50 mg–MDMA 75 mg. Memory function was assessed at Tmax of MDMA by means of a word-learning task (WLT), a spatial memory task and a prospective memory task. MDMA significantly impaired performance in all memory tasks. Pretreatment with a 5-HT2A receptor blocker selectively interacted with subsequent MDMA treatment and prevented MDMA-induced impairment in the WLT, but not in the spatial and prospective memory task. Pretreatment with a 5-HT1A blocker did not affect MDMA-induced memory impairment in any of the tasks. Together, the results demonstrate that MDMA-induced impairment of verbal memory as measured in the WLT is mediated by 5-HT2A receptor stimulation.
Keywords: MDMA, ketanserin, pindolol, 5-HT2, 5-HT1, serotonin
INTRODUCTION
3,4-Methylenedioxymethamphetamine (MDMA) is the primary psychoactive constituent in the popular party drug ecstasy. Use of MDMA has consistently been associated with learning and verbal memory deficits in recreational and abstinent users (Cole and Sumnall, 2003; Morgan, 2000; Parrott, 2001); the prototypical example being a reduction in performance on immediate and delayed word-recall tasks. Although the existence of these persistent memory deficits in MDMA users has been well established, their neuropharmacology has been a matter of continuous debate. There is some evidence that these deficits are caused by MDMA-induced neurotoxicity as indicated by depleted serotonin (5-HT) levels in MDMA users and by dose-response relationships between the extent of exposure to MDMA and the magnitude of memory impairment. Yet, imaging studies demonstrated that memory impairment in ecstasy users was not associated with binding to cortical 5-HT transporters or duration of abstinence, which suggests that memory deficits in MDMA users occur independent of serotonergic neurotoxicity (Reneman et al, 2001).
A similar conclusion was drawn by Kuypers and Ramaekers in their 2005 and 2007 papers. They set out to inquire whether memory performance is directly related to 5-HT synaptic availability. They treated 18 recreational MDMA users with single doses of MDMA 75 mg in a double-blind, placebo-controlled study, and conducted both a verbal memory test and a spatial memory test during intoxication (ie, 1–2 h post drug) and during withdrawal (ie, 25–26 h post drug). Subjects displayed transient memory impairment during intoxication while they performed at placebo level in the withdrawal phase, when serotonin levels were depleted. These results suggest that memory performance during MDMA intoxication is not directly affected by changes in the availability of 5-HT in the synaptic cleft.
The apparent dissociation between general 5-HT levels and MDMA-induced memory impairment raises the question whether MDMA exerts its detrimental effect on memory through other mechanisms than synaptic 5-HT-availability alone. Two interesting possibilities include direct and indirect activation of postsynaptic 5-HT2A and 5-HT1A receptors, respectively. It is clear from animal studies that 5-HT2A receptors mediate learning and memory processes (Galizio et al, 2009; Kay et al, 2010; Trigo et al, 2008); however, it is not completely obvious whether 5-HT2A receptor drugs achieve their facilitating and impairing effects through agonism, antagonism or inverse agonism (Meneses, 2002). Still, there seems to be some consensus that a 5-HT2A blockade not only improves learning, but reverses poor memory consolidation conditions associated with, among others, dysfunctional serotonergic neurotransmission (Meneses, 2007a, 2007b) as well. The 5-HT2A receptor agonists such as mCPP have been shown to decrease learning and memory in rats (Meneses, 1999). The same mechanism might be responsible for the decrement in learning and delayed recall following MDMA administration, as it has been previously demonstrated that MDMA possesses a moderate affinity for activating the 5-HT2A receptor (Sadzot et al, 1989).
A second candidate mechanism by which MDMA could cause its disadvantageous effects on memory is indirect activation of the post-synaptic 5-HT1A receptor. The latter has also been shown to modulate memory performance in animals and humans (Liechti et al, 2000; Meneses, 1999; Yasuno et al, 2003). PET studies have shown a negative correlation between memory function and 5-HT1A receptor agonists binding in the hippocampus. More specifically, the 5-HT1A agonist tandospirone dose dependently impaired performance in an immediate and delayed word-recall task (Yasuno et al, 2003). The memory impairing effects of 5-HT1A agonists thus seem identical to those produced by MDMA. It is not known whether MDMA also exerts an effect as a direct 5-HT1A receptor agonist. However, it may achieve the same net result by indirectly stimulating postsynaptic 5-HT1A receptors during the short-term increase in availability of synaptic 5-HT for the period of intoxication.
This study was designed to investigate the role of 5-HT2A and 5-HT1A receptors in MDMA-induced memory impairment. Recreational MDMA users performed a number of learning and memory tasks after receiving single doses of MDMA in a placebo-controlled study. In line with previous findings and assumptions, it is hypothesized that (1) an acute dose of MDMA will produce impairments on laboratory measures of learning and memory; (2) MDMA-induced memory impairment will be reversed by pre-treatment with ketanserin, a 5-HT2A receptor antagonist, if impairment is related to direct or indirect stimulation of 5-HT2A receptors; and (3) MDMA-induced memory impairment will be reversed by pre-treatment with pindolol, a 5-HT1A partial agonist, if impairment is related to direct or indirect stimulation of 5-HT1A receptors.
MATERIALS AND METHODS
Participants
A total of 17 healthy MDMA-users (9 male, 8 female), aged between 19 and 27 (mean (SD) 22.76 (2.75)) participated in the study. They were, with the exception of two, all mild to moderate users of MDMA who reported to have taken the drug on 1–65 separate occasions (mean 10.94) in the previous year. Overall, subjects reported to have taken MDMA 3 to 780 times in their lifetime (mean 72.4 times). When the heavy users were excluded from the analysis, the mean lifetime use of MDMA dropped to 13 times. Subject demographics are shown in Table 1.
Table 1. Subject Demographics—Drug History (Lifetime Use in Number of Times Used).
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| Age (years) | 22.76 | 2.75 | 18.6 | 27.2 |
| Height (cm) | 179.6 | 12.3 | 160.0 | 200.0 |
| Weight (kg) | 72.9 | 14.7 | 56.0 | 110.0 |
| Alcohol | 1168.5 | 794.1 | 260.0 | 2600.0 |
| Cannabis | 554.4 | 1104.8 | 0.0 | 3650.0 |
| Ecstasy | 72.4 | 194.7 | 3.0 | 780.0 |
| Amphetamines | 4.1 | 12.3 | 0.0 | 49.0 |
| Cocaine | 4.7 | 7.3 | 0.0 | 25.0 |
| LSD | 4.4 | 12.0 | 0.0 | 50.0 |
| Mushrooms | 0.2 | 0.5 | 0.0 | 2.0 |
| Other | 0.5 | 1.7 | 0.0 | 7.0 |
Participants were recruited through advertisements in local newspapers and by word of mouth. Subjects were examined by the medical supervisor, who checked vital signs, inclusion criteria (written informed consent; age 18–35 years; history of MDMA use; free from psychotropic medication; good physical health as assessed by a medical doctor; normal weight as determined by BMI 18–28) and exclusion criteria (addiction according to DSMIV criteria, presence or history of psychiatric or neurological disorder as assessed during a clinical interview; pregnancy; cardiovascular abnormalities; excessive drinking or smoking, that is, defined as more than 20 standard units of alcohol per week and more than 10 cigarettes per day, hypertension) and took blood samples for standard blood chemistry and hematology.
This study was conducted according to the code of ethics on human experimentation established by the declaration of Helsinki (1964) and amended in Seoul (2008) and approved by the Medical Ethics Committee of the Academic Hospital of Maastricht and Maastricht University. A permit for obtaining, storing, and administering MDMA was obtained from the Dutch drug enforcement administration. Subjects were paid for their participation in the study.
Treatments and Procedures
Subjects participated in a double-blind, placebo-controlled, within-subject design involving six experimental conditions consisting of pretreatment (T1) and treatment (T2). T1 preceded T2 by 30 min. T1–T2 combinations were: placebo–placebo, pindolol 20 mg–placebo, ketanserin 50 mg–placebo, placebo–MDMA 75 mg, pindolol 20 mg–MDMA 75 mg, and ketanserin 50 mg–MDMA 75 mg. Conditions were separated by minimum washout period of 7 days to avoid cross-condition contamination. The 75 mg dose of MDMA was selected because it falls within the normal range of recreational use (Gouzoulis-Mayfrank and Daumann, 2009), and has been consistently shown to impair memory performance and produce robust subjective mood changes in a number of previous studies from our lab (Kuypers and Ramaekers, 2005, 2007; Kuypers et al, 2006; Ramaekers et al, 2009). Doses of pindolol 20 mg and ketanserin 50 mg represent regular therapeutic doses that block ∼40% of 5HT1A receptors and 91% of 5HT2 receptors, respectively (Brogden and Sorkin, 1990; Rabiner et al, 2000; Sharpley et al, 1994).
Order of conditions was balanced over subjects and sessions. All subjects received a training session before onset of the experimental sessions in order to familiarize them with the tests and procedures. Treatments at T1 and T2 were administered using a double dummy technique to synchronize time of maximal drug concentrations (Tmax). Memory function was assessed by means of a number of memory tasks between 1.5–2 h after T2 (at Tmax), during which time subjects were allowed to watch TV or read a book. In addition, blood pressure and body temperature were assessed as safety measures. A summary of the test day is given in Table 2.
Table 2. Schematic Representation of a Testing day in Six Treatment Conditions.
| 0900 hours | 0930 hours, T1 | 01000 hours, T2 | 01130 hours | 01130–01300 hours |
|---|---|---|---|---|
| Arrival | PLA | PLA | Vital-signs measurements and blood-sample collection | Memory tests |
| Ketanserin | PLA | |||
| Pindolol | PLA | |||
| PLA | MDMA | |||
| Ketanserin | MDMA | |||
| Pindolol | MDMA |
During pre-treatment (T1) subjects received either placebo, ketanserin 50 mg or pindolol 20 mg. Treatment (T2) consisted of either MDMA 75 mg or placebo.
Subjects were asked to refrain from drugs at least a week before the start of the experiment and during the study. Subjects were not allowed to use alcohol on the day before an experimental session and were requested to arrive at experimental sessions well rested. Drug and alcohol screens were carried out before experimental sessions upon arrival of the subject. Drug screens assessed for the presence of benzodiazepines, opiates, cocaine, marijuana, MDMA, and (meth)amphetamine. Women were given a pregnancy test. Study treatments were only administered, if subjects were tested negative for drugs and pregnancy.
Memory Tests
Three tests with demonstrated sensitivity (Kuypers and Ramaekers, 2005; Ramaekers et al, 2009) to the impairing effects of MDMA on memory were included in the memory-task battery.
Word-Learning Task
The Word-learning task (WLT) is the Dutch language version of the standardized, clinically validated test for verbal memory (Rey, 1958). The test begins with the sequential presentation of 30 monosyllabic common nouns. Each word is shown on the computer display for 2 s and the subject has to read them out loud. When the series ends, the subject is required to recall as many words as possible. The number correct is scored as the 1st trial score. Thereafter the same list is presented in the same manner on two successive occasions. Numbers correct are scored as before. The main parameter is the sum score of correctly recalled words over three trials. After a 30-min delay, the subject is shown a series of 30 words on the computer display, comprising of 15 words of the original set and 15 new words in random order. He/she was asked to respond as fast as possible to indicate whether the given word was one of the original set. The number of correct recognitions and the average speed (ms) of correct recognitions are recorded as the main dependent parameters (Deelman et al, 1980; Schmitt et al, 2001).
Spatial Memory Task
The spatial memory task is based on the spatial localization task (Vermeeren et al, 1995). It assesses short-term memory for non-verbal information. The subject is briefly shown a fixation point in the center of the computer display. Shortly thereafter, a target appears at a random location for 500 ms. The subject has to memorize the position of the target, and using a computer mouse, relocate the cursor as accurately as possible over that position. The cursor appears either immediately upon target offset or after a delay of 2 or 4 s. The subject presses a button to indicate that the cursor is at the recalled position of the target. The test consists of 75 trials, divided equally among three response delays. The sequence of delays is random. Mean localization error (mm) and average reaction time (ms) are the main parameters.
Prospective Memory Task
The prospective memory task is a new paradigm (Ramaekers et al, 2009) that was developed to examine prospective memory performance in an event-based memory task. The foreground task consists of 240 successive presentations of a letter (A or B) in the center of a computer screen. Subjects are required to respond to each letter as quickly as possible by pressing one of two response buttons. One button is pressed to indicate that the letter ‘A' appeared and the other to indicate the letter ‘B'. Both letters are presented equally often. Subjects are informed about the trial number by means of a trial counter that is always present in the left top corner of the screen. In addition, subjects are presented at irregular times with a future trial number in the right top corner of the display. Subjects are instructed to remember this future trial number and withhold from responding to the foreground task during the actual occurrence of the future trial. The memory set of trial numbers is dynamic and contains up to three future trial numbers. A trial number in the memory set is replaced by a novel future trial number, whenever the actual trial number matches a future trail number in the set. Trials during which subjects are expected to respond are classified as Go trials. Trials during which subjects are instructed to withhold a response are classified as No Go trials (prospective memory trials). Time between presentation of a future trial number and the actual occurrence of the trial (ie, memory delay) varies between 1, 2 and 3 min, equally divided over all No Go trials. In total, the prospective memory task consists of 216 Go trials and 24 No Go trials. Number of correct prospective memory recalls (ie, number of correct response inhibitions) in No Go trials is the primary dependent performance parameter.
Pharmacokinetics
Blood samples were collected before the start of the memory tasks at 1.5 h post T2. Blood samples were centrifuged immediately and the serum was subsequently frozen at −20°C until analyses for pharmacokinetic assessments. MDMA, pindolol, and ketanserin concentrations were determined using solid-phase extraction and gas chromatography with mass spectrometric detection.
Statistics
The hypotheses that pretreatment with ketanserin or pindolol would interact with MDMA-induced memory impairment was tested in two separate General Linear Model (GLM) analyses. Memory effects of MDMA, Ketanserin and MDMA × Ketanserin were analyzed by means of a GLM-repeated measures ANOVA with MDMA (two levels, ie, present-absent) and Ketanserin (two levels; present-absent) as the main factors (GLM 1 see Table 2). Memory effects of MDMA, pindolol, and MDMA × pindolol were analyzed by means of a GLM-repeated measures ANOVA with MDMA (two levels, ie, present-absent) and pindolol (two levels: present-absent) as the main factors (GLM 2, see Table 2). In case of a significant interaction between MDMA and ketanserin or MDMA and pindolol, additional drug-placebo contrasts were conducted in order to further elucidate the nature of the interaction. The alpha criterion significance level was set at P=0.05. Safety measures were analyzed in the same manner as measures of memory performance. All statistical tests were conducted with SPSS version 15.0.
RESULTS
In all, 17 complete data sets entered statistical analysis. Statistical analysis of memory data is described in detail below. A summary of main effects on measures of memory performance is given in Table 3.
Table 3. Summary of Main Effects and Interactions Following Two Major GLM Analyses for all Dependent Variables in the Word Learning Task (WLT), the Prospective Memory Task (PMT), and the Spatial Memory Task (SMT).
| GLM 1: Factors MDMA ( present-absent) and Ketanserin (present-absent) Treatments included PLA–PLA, PLA–MDMA, KET–PLA, and KET–MDMA | ||||
|---|---|---|---|---|
| WLT immediate recall | PMT prospective recall | SMT localization error | SMT reaction time | |
| Main effects | ||||
| MDMA | 0.003 | 0.013 | 0.001 | 0.015 |
| Ketanserin | — | — | 0.005 | — |
| MDMA × Ketanserin | 0.004 | — | — | — |
|
GLM 2: Factors MDMA (present-absent) and Pindolol (present-absent) Treatments included PLA–PLA, PLA–MDMA, PIN–PLA, and PIN–MDMA | ||||
| Main effects | ||||
| MDMA | 0.001 | — | 0.005 | 0.001 |
| Pindolol | — | — | — | — |
| MDMA × Pindolol | — | — | — | — |
Significance (P<0.05) and nonsignificance (—) of main effects is shown.
WLT
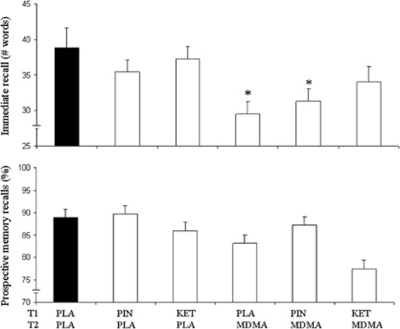
MDMA significantly decreased immediate recall in both the ketanserin and pindolol comparisons (F1,16=12.1; p<0.003 and F1,16=69.1; p<0.001, respectively). Ketanserin did not affect memory but significantly interacted with MDMA to prevent impairment of immediate recall (F1,16=11.7; p=0.004). Pindolol neither affected immediate recall nor interacted with the effect of MDMA on memory. Separate drug-placebo contrasts supported ANOVA and revealed memory impairment only after treatment with MDMA (p=0.001) and the combination of pindolol with MDMA (p=0.024). Mean (SE) immediate recall scores in each treatment condition are given in Figure 1. Recognition scores were not affected by MDMA, nor by pindolol or ketanserin.
Figure 1.
Mean (SE) immediate recall and prospective memory recall in the word-learning task and the prospective memory task for every treatment condition. Each treatment condition consisted of a pre-treatment (T1) and a treatment (T2). (PLA, placebo; PIN, pindolol; KET, ketanserin; MDMA, 3,4-methylenedioxymethamphetamine, *p<0.05 as indicated by drug-placebo contrasts following a significant main interaction effect of MDMA × ketanserin in the Word-learning task).
Prospective Memory Task
MDMA significantly increased the number of prospective memory failures in the No Go trials in one of the GLM comparisons (F1,16=7.8; p=0.013) and showed a trend in the other (F1,16=4; p=0.06). The factors ketanserin and pindolol or their interaction with MDMA did not affect prospective memory. Mean (SE) prospective memory recalls as a percentage of the total prospective memory trials in each treatment condition are given in Figure 1.
Spatial Memory Task
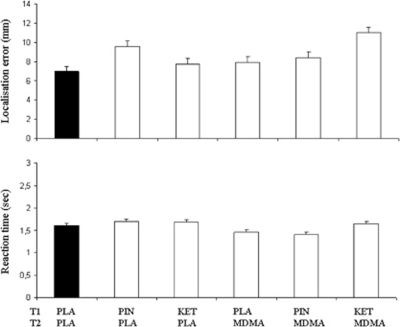
MDMA significantly increased localization error in both GLM comparisons (F1,16=19.26; p<0.001 and F1,16=10.4; p=0.005). The factor ketanserin also significantly increased localization error (F1,16=10.5; p=0.005). MDMA did not interact with any of the pretreatments. In addition, MDMA significantly decreased reaction time in both GLM comparisons (F1,16=7.48; p=0.015 and F1,16=32.42; p<0.001). The factors pindolol, ketanserin or their interaction with MDMA did not affect reaction time. Mean localization error and reaction time in all treatment conditions are shown in Figure 2.
Figure 2.
Mean (SE) localization error and reaction time in the spatial memory task for every treatment condition. Each treatment condition consisted of a pre-treatment (T1) and a treatment (T2). (PLA, placebo; PIN, pindolol; KET, ketanserin; MDMA, 3,4-methylenedioxymethamphetamine).
Safety Data
Safety data revealed a significant main effect of MDMA (F1,16=24.5; p<0.001), pindolol (F1,16=26.7; p<0.001 and ketanserin (F1,16=4.48; p<0.05) on blood pressure. Overall, mean blood pressure ranged from 73.6 to 87.3 mmHg over all treatments. MDMA increased mean blood pressure by 7 mmHg relative to placebo, whereas ketanserin and pindolol mildly reduced mean blood pressure relative to placebo. However, ketanserin and pindolol did not interact with the effect of MDMA on blood pressure. Mean body temperature ranged from 36.2–36.8°C over all treatment conditions. Overall, MDMA increased body temperature (F1,16=4.4; p<0.05). Relative to placebo, body temperature increased by 0.2°C after MDMA alone. Pretreatments did not affect body temperature or the effect of MDMA on body temperature.
Pharmacokinetics
Mean drug concentrations in all treatment conditions are shown in Table 4.
Table 4. Dose and Mean (SD) Drug Concentrations in Each of the Six Treatment Conditions.
| T1 and T2 | PLA–PLA | PIN–PLA | KET–PLA | PLA–MDMA | PIN–MDMA | KET–MDMA |
|---|---|---|---|---|---|---|
| Dose (mg) | — | 20 | 50 | — | 20 | 50 |
| — | — | — | 75 | 75 | 75 | |
| Cmean (ng/ml) | — | 133 (80) | 86 (42) | — | 130 (53) | 104 (41) |
| — | — | — | 157 (48) | 156 (56) | 164 (62) |
Abbreviations: KET, ketanserin; MDMA, 3,4-methylenedioxymethamphetamine; PIN, pindolol; PLA, placebo.
During pre-treatment subjects received either placebo, ketanserin 50 mg or pindolol 20 mg. Treatment consisted of either MDMA 75 mg or placebo. Each treatment condition consisted of a pre-treatment (T1) and a treatment (T2).
DISCUSSION
The goal of this study was to investigate the role of 5-HT2A and 5-HT1A receptors in MDMA-induced memory impairment. Subjects were given single doses of MDMA combined with placebo, ketanserin or pindolol, after which several learning and memory tasks were carried out.
Single doses of MDMA produced memory impairments in all memory tasks. GLM analyses revealed that overall, MDMA decreased immediate recall in the WLT, increased the number of prospective memory failures in the prospective memory task and increased localization error in the spatial memory task. These results are fully in line with previous studies that have reported identical memory impairments during intoxication with MDMA (Kuypers and Ramaekers, 2005, 2007; Ramaekers et al, 2009), as assessed in identical memory paradigms. These results basically indicate that the current memory paradigms were sensitive to the impairing effects of MDMA and should serve as reliable models to assess changes in MDMA-induced memory impairments after pretreatments with 5-HT1A and 5-HT2A receptor blockers, the main goal of this study.
The hypotheses that pretreatment with ketanserin or pindolol would interact with MDMA-induced memory impairment was tested in two separate GLM analyses that assessed main effects of MDMA, ketanserin and MDMA × Ketanserin and main effects of MDMA, pindolol and MDMA × pindolol, respectively. The GLM models were chosen because they offer a robust and direct assessment of the interaction between pretreatments and MDMA in only two major analyses. This procedure was much preferred over the alternative approach of multiple comparisons between drug–drug and drug–placebo that can only provide indirect evidence of pretreatment–treatment interactions. Consequently, this study only conducted drug-placebo contrasts as supportive, secondary tests following a significant interaction between pretreatment and MDMA as evinced by GLM ANOVA.
GLM analyses revealed only one significant interaction between pretreatment–treatment in the three memory tasks. In the WLT, pretreatment with ketanserin prevented impairment of word learning during treatment with MDMA. Separate drug-placebo contrasts also provided indirect and supportive evidence of the interaction between ketanserin and MDMA. T1–T2 combinations of placebo-MDMA and pindolol-MDMA both produced significant reductions in immediate recall. Drug-placebo contrasts also showed that performance in the word-learning task after combination of ketanserin and MDMA did not differ from that during the placebo–placebo combination. Together these data strongly suggest that MDMA-induced impairments on verbal memory are in large part caused by direct or indirect stimulation of the 5-HT2A receptor. This finding appears in line with a previous report on 5-HT2A receptors densities in recent MDMA and ex-MDMA users (Reneman et al, 2002). That study showed that 5-HT2A receptor densities were significantly lower in recent MDMA users and significantly higher in ex-MDMA users. The authors speculated that the latter was evidence of compensatory upregulation of post-synaptic 5-HT2A receptors due to low synaptic 5-HT levels. This study suggests that downregulation of 5-HT2A receptors in recent MDMA users may result from high synaptic levels of 5-HT,and 5-HT2A receptor stimulation during MDMA intoxication. Studies showing involvement of 5-HT2 receptors in acute MDMA intoxication, as well as in persistent memory impairments in chronic MDMA users indicate that these phenomena may share related underlying mechanisms. Any definitive conclusion in that direction, however, remains premature as we do not know at present whether acute and chronic memory effects of MDMA are truly interconnected.
Pretreatment with pindolol did not affect memory impairments in any of the memory tasks after treatment with MDMA. The interaction between pindolol and MDMA never reached statistical significance in any of the GLM analyses. These data suggest that 5-HT1A receptors do not have a crucial role in the neuropharmacology of MDMA-induced memory impairment. These finding confirm recent results from a study assessing the role of 5-HT1A receptors on MDMA effects on visual-spatial working memory as measured with a CANTAB test battery (Hasler et al, 2009) in a placebo-controlled study. A single dose of MDMA significantly impaired visual-spatial memory as compared with placebo. However, pretreatment with pindolol did not significantly alter MDMA-induced impairment of memory. It should be noted, however, that Pindolol is not a very selective drug. It is basically a beta-blocker that also blocks ∼40% of 5HT1A receptors in the brain (Rabiner et al, 2000). Unfortunately, 5HT1A ligands that selectively and fully block 5HT1A receptors are presently not available. Nevertheless, recent molecular imaging studies also suggested that 5-HT1A receptors may not be involved in human cognition at all (Borg, 2008; Borg et al, 2006).These studies failed to find any correlation between regional [11C]WAY 100635 binding to 5-HT1A receptors and cognitive performance in humans as measured with standard neuropsychological tests batteries (Borg, 2008; Borg et al, 2006). Together, these results question the role of 5-HT1A receptors in human memory, as it cannot explain the general memory deficit in MDMA users.
This study did not provide clues to neuropharmacological mechanisms of MDMA-induced impairments in every memory domain. The preventive effect of ketanserin on MDMA-induced memory impairment was selective for verbal memory performance in the WLT. Ketanserin did not affect spatial memory and prospective memory impairments following MDMA. The selectivity of the interaction between ketanserin and MDMA on verbal memory was also supported by the lack of any interactions between pretreatment and MDMA on safety measures such as blood pressure and body temperature. The latter finding is in line with a previous study that demonstrated that co-administration of ketanserin did not alter MDMA effects on physiological measures (ie, blood pressure, heart rate, and body temperature) and subjective measures (eg, well-being and positive mood) (Herin et al, 2005; Liechti et al, 2000).
The selective interaction between ketanserin and MDMA effects on verbal memory may indicate differences in neural memory networks and differences in distributions of 5-HT1A and 5-HT2A receptors within these networks. It is clear from the functional neuroimaging literature that the frontal, parietal, and occipitotemporal cortices are generally involved in a large variety of memory tasks, but that the pattern of brain recruitments within these networks may differ as a function of memory task or memory process (Linden, 2007). Autoradiographic mapping of 5-HT1 and 5-HT2 receptors in Rhesus monkeys demonstrated complementary patterns of distribution of 5-HT1 and 5-HT2 receptors in cortical areas of frontal, parietal, and occipital lobes. The 5-HT1 receptors were concentrated layers I–III, whereas 5-HT2 receptors were primarily concentrated throughout layers III and IV (Lidow et al, 1989). Involvement of 5-HT2A receptors in MDMA-induced memory impairment thus may depend on task-related network activations that occur within these layers.
Recent studies have also shown that a functional genetic polymorphism of the 5-HT2A receptor (His452Tyr) has been associated with variations in memory performance, more specifically in hippocampus-dependent memory tasks (Schott et al, 2011; Sigmund et al, 2008). Future studies may focus on whether genetic variation of the 5-HT2A receptors affects the magnitude of MDMA-induced verbal memory impairment or determines the sensitivity of MDMA users for MDMA-induced impairment of verbal memory.
In sum, this study demonstrated that single doses of MDMA impaired memory in a range of tasks, and that pretreatment with ketanserin offered selective protection against MDMA-induced verbal memory impairment. This demonstrates that MDMA-induced verbal memory impairment is mediated by 5-HT2A receptor stimulation.
Acknowledgments
We thank Dr S Toennes and Dr G Kauert (Institute of Forensic Toxicology, Goethe University Frankfurt) for establishing drug-serum concentrations and MSc Uschi Poesmans for her assistance in data collection.
This research was funded by the Netherlands Organization for Scientific Research (NWO), Grant number: 400-05-096, awarded to JR. The authors declare that over the past 3 years they have also received funding for research not related to this paper. JR, ET, and KK have received grants from pharmaceutical industries, the European Committee, and the Netherlands Organization for Scientific Research (NWO).
Nederlands Trial Register
http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2352
References
- Borg J. Molecular imaging of the 5-HT1A receptor in relation to human cognition. Behav Brain Res. 2008;195:103–111. doi: 10.1016/j.bbr.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Borg J, Andree B, Lundberg J, Halldin C, Farde L. Search for correlations between serotonin 5-HT1A receptor expression and cognitive functions--a strategy in translational psychopharmacology. Psychopharmacology (Berl) 2006;185:389–394. doi: 10.1007/s00213-006-0329-z. [DOI] [PubMed] [Google Scholar]
- Brogden RN, Sorkin EM. Ketanserin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in hypertension and peripheral vascular disease. Drugs. 1990;40:903–949. doi: 10.2165/00003495-199040060-00010. [DOI] [PubMed] [Google Scholar]
- Cole JC, Sumnall H. The pre-clinical behavioural pharmacology of 3,4-methylenedioxymethamphetamine (MDMA) Neurosci Biobehav Rev. 2003;27:199–217. doi: 10.1016/s0149-7634(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Deelman BG, Liebrand WB, Koning-Haanstra M, van den Burg W. Measurements of aphasic disorders. A brief description of the SAN-battery. Gerontologie. 1980;11:17–21. [PubMed] [Google Scholar]
- Galizio M, McKinney P, Cerutti DT, Pitts RC. Effects of MDMA, methamphetamine and methylphenidate on repeated acquisition and performance in rats. Pharmacol Biochem Behav. 2009;94:305–311. doi: 10.1016/j.pbb.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Daumann J. Neurotoxicity of drugs of abuse—the case of methylenedioxyamphetamines (MDMA, ecstasy), and amphetamines. Dialogues Clin Neurosci. 2009;11:305–317. doi: 10.31887/DCNS.2009.11.3/egmayfrank. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler F, Studerus E, Lindner K, Ludewig S, Vollenweider FX. Investigation of serotonin-1A receptor function in the human psychopharmacology of MDMA. J Psychopharmacol. 2009;23:923–935. doi: 10.1177/0269881108094650. [DOI] [PubMed] [Google Scholar]
- Herin DV, Liu S, Ullrich T, Rice KC, Cunningham KA. Role of the serotonin 5-HT2A receptor in the hyperlocomotive and hyperthermic effects of (+)-3,4-methylenedioxymethamphetamine. Psychopharmacology (Berl) 2005;178:505–513. doi: 10.1007/s00213-004-2030-4. [DOI] [PubMed] [Google Scholar]
- Kay C, Harper DN, Hunt M. Differential effects of MDMA and scopolamine on working versus reference memory in the radial arm maze task. Neurobiol Learn Mem. 2010;93:151–156. doi: 10.1016/j.nlm.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Kuypers KP, Ramaekers JG. Transient memory impairment after acute dose of 75 mg 3.4-Methylene-dioxymethamphetamine. J Psychopharmacol. 2005;19:633–639. doi: 10.1177/0269881105056670. [DOI] [PubMed] [Google Scholar]
- Kuypers KPC, Samyn N, Ramaekers JG. MDMA and alcohol effects, combined and alone, on objective and subjective measures of actual driving performance and psychomotor function. Psychopharmacology (Berl) 2006;187:467–475. doi: 10.1007/s00213-006-0434-z. [DOI] [PubMed] [Google Scholar]
- Kuypers KP, Ramaekers JG. Acute dose of MDMA (75 mg) impairs spatial memory for location but leaves contextual processing of visuospatial information unaffected. Psychopharmacology (Berl) 2007;189:557–563. doi: 10.1007/s00213-006-0321-7. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Quantitative autoradiographic mapping of serotonin 5-HT1 and 5-HT2 receptors and uptake sites in the neocortex of the rhesus monkey. J Comp Neurol. 1989;280:27–42. doi: 10.1002/cne.902800104. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Saur MR, Gamma A, Hell D, Vollenweider FX. Psychological and physiological effects of MDMA (‘ecstasy') after pretreatment with the 5-HT2 antagonist Ketanserin in healthy humans. Neuropsychopharmacology. 2000;23:396–404. doi: 10.1016/S0893-133X(00)00126-3. [DOI] [PubMed] [Google Scholar]
- Linden DE. The working memory networks of the human brain. Neuroscientist. 2007;13:257–267. doi: 10.1177/1073858406298480. [DOI] [PubMed] [Google Scholar]
- Meneses A. Are 5-HT(1B/1D) and 5-HT(2A/2B/2C) receptors involved in learning and memory processes. IDrugs. 1999;2:796–801. [PubMed] [Google Scholar]
- Meneses A. Involvement of 5-HT(2A/2B/2C) receptors on memory formation: simple agonism, antagonism, or inverse agonism. Cell Mol Biol. 2002;22:675–688. doi: 10.1023/a:1021800822997. [DOI] [PubMed] [Google Scholar]
- Meneses A. Do serotonin(1−7) receptors modulate short and long-term memory. Neurobiol Learn Mem. 2007a;87:561–572. doi: 10.1016/j.nlm.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Meneses A. Stimulation of 5-HT1A, 5-HT1B, 5-HT2A/2C, 5-HT3 and 5-HT4 receptors or 5-HT uptake inhibition: short- and long-term memory. Neurobiol Learn Mem. 2007b;184:81–90. doi: 10.1016/j.bbr.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Morgan MJ. Ecstasy (MDMA): a review of its possible persistent psychological effects. Psychopharmacology. 2000;152:230–248. doi: 10.1007/s002130000545. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Human psychopharmacology of Ecstasy (MDMA): a review of 15 years of empirical research. Hum Psychopharmacol Clin Exp. 2001;16:557–577. doi: 10.1002/hup.351. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Gunn RN, Wilkins MR, Sargent PA, Mocaer E, Sedman E, et al. Drug action at the 5-HT(1A) receptor in vivo: autoreceptor and postsynaptic receptor occupancy examined with PET and [carbonyl-(11)C]WAY-100635. Nucl Med Biol. 2000;27:509–513. doi: 10.1016/s0969-8051(00)00120-7. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kuypers KP, Wingen M, Heinecke A, Formisano E. Involvement of inferior parietal lobules in prospective memory impairment during acute MDMA (ecstasy) intoxication: an event-related fMRI study. Neuropsychopharmacology. 2009;34:1641–1648. doi: 10.1038/npp.2008.219. [DOI] [PubMed] [Google Scholar]
- Reneman L, Endert E, de Bruin K, Lavalaye J, Feenstra MG, de Wolff FA, et al. The acute and chronic effects of MDMA (″ecstasy″) on cortical 5-HT2A receptors in rat and human brain. Neuropsychopharmacology. 2002;26:387–396. doi: 10.1016/S0893-133X(01)00366-9. [DOI] [PubMed] [Google Scholar]
- Reneman L, Lavalaye J, Schmand B, de Wolff FA, van den Brink W, den Heeten GJ, et al. Cortical serotonin transporter density and verbal memory in individuals who stopped using 3,4-methylenedioxymethamphetamine (MDMA or ‘ecstasy'): preliminary findings. Arch Gen Psychiatry. 2001;58:901–906. doi: 10.1001/archpsyc.58.10.901. [DOI] [PubMed] [Google Scholar]
- Rey A. The clinical examination in psychology/L'examen clinique en psychologie. Presses Universitaires de France: Oxford, England; 1958. [Google Scholar]
- Sadzot B, Baraban JM, Glennon RA, Lyon RA, Leonhardt S, Jan CR, et al. Hallucinogenic drug interactions at human brain 5-HT2 receptors: implications for treating LSD-induced hallucinogenesis. Psychopharmacology. 1989;98:495–499. doi: 10.1007/BF00441948. [DOI] [PubMed] [Google Scholar]
- Schmitt JAJ, Kruizinga MJ, Riedel WJ. Non-serotonergic pharmacological profiles and associated cognitive effects of serotonin reuptake inhibitors. J Psychopharmacol. 2001;15:173–179. doi: 10.1177/026988110101500304. [DOI] [PubMed] [Google Scholar]
- Schott BH, Seidenbecher CI, Richter S, Wustenberg T, Debska-Vielhaber G, Schubert H, et al. Genetic variation of the serotonin 2A receptor affects hippocampal novelty processing in humans. PLoS One. 2011;6:e15984. doi: 10.1371/journal.pone.0015984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpley AL, Elliott JM, Attenburrow MJ, Cowen PJ. Slow wave sleep in humans: role of 5-HT2A and 5-HT2C receptors. Neuropharmacology. 1994;33:467–471. doi: 10.1016/0028-3908(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Sigmund JC, Vogler C, Huynh KD, de Quervain DJ, Papassotiropoulos A. Fine-mapping at the HTR2A locus reveals multiple episodic memory-related variants. Biol Psychol. 2008;79:239–242. doi: 10.1016/j.biopsycho.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Cabrero-Castel A, Berrendero F, Maldonado R, Robledo P. MDMA modifies active avoidance learning and recall in mice. Psychopharmacology. 2008;197:391–400. doi: 10.1007/s00213-007-1045-z. [DOI] [PubMed] [Google Scholar]
- Vermeeren A, Jackson JL, Muntjewerff ND, Quint PJ, Harrison EM, O'Hanlon JF. Comparison of acute alprazolam (0.25, 0.50 and 1.0 mg) effects versus those of lorazepam 2 mg and placebo on memory in healthy volunteers using laboratory and telephone tests. Psychopharmacology. 1995;118:1–9. doi: 10.1007/BF02245243. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Suhara T, Nakayama T, Ichimiya T, Okubo Y, Takano A, et al. Inhibitory effect of hippocampal 5-HT1A receptors on human explicit memory. Am J Psychiatry. 2003;160:334–340. doi: 10.1176/appi.ajp.160.2.334. [DOI] [PubMed] [Google Scholar]