SUMMARY
Maturation in hooded seals is characterized by the rapid development of their physiological diving capacity and is accompanied by increases in oxidant production but not oxidative damage. To test the hypothesis that the antioxidant system of hooded seals develops as they transition from a terrestrial to an aquatic environment, we obtained the complete cDNA sequence that encodes the NF-E2-related factor 2 (Nrf2), a central regulator of the antioxidant response, and compared Nrf2 mRNA and protein expression levels in muscle samples from neonate, weaned pups and adult hooded seals, along with glutathione (GSH) levels and the activity/protein content of the antioxidant enzymes catalase, glutathione peroxidase (GPx), peroxyredoxin VI (PrxVI), thioredoxin 1 (Trx1), thioredoxin reductase (TrxR), glutaredoxin 1 (Glrx1), glutathione disulphide reductase, glutathione S-transferase and glutamate-cysteine ligase. The Nrf2 of the hooded seal is 1822 bp long and encodes a protein of 606 amino acids with a leucine zipper domain and Keap1-mediated proteosomal degradation residues, which are key for Nrf2 function and regulation. Although neither Nrf2 mRNA nor Nrf2 nuclear protein content are higher in adults than in pups, GSH levels along with GPx, PrxVI, Trx1, TrxR and Glrx1 activity/protein content increase with maturation, suggesting that the potential for peroxide removal increases with development in hooded seals, and that these enzymes contribute to the regulation of the intracellular redox state and the prevention of oxidative damage in these deep-diving mammals.
KEY WORDS: antioxidant, ischemia, reperfusion, maturation, seal
INTRODUCTION
While diving, seals experience bradycardia and peripheral vasoconstriction to maximize the use of their oxygen stores and are consequently exposed to prolonged ischemia and hypoxemia (Elsner, 1999; Kooyman and Ponganis, 1998; Meir et al., 2009). Ischemia/reperfusion and blood reoxygenation after hypoxemia increase oxidant production and oxidative stress because ATP degradation during ischemia and hypoxemia results in the accumulation of hypoxanthine (HX) and in the proteolytic conversion of xanthine dehydrogenase to xanthine oxidase (XO). Upon reperfusion/reoxygenation, XO is able to reduce HX, generating superoxide radical (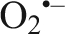 ) and hydrogen peroxide (H2O2) (Kuppusamy and Zweier, 1989; McCord, 1985; Thompson-Gorman and Zweier, 1990).
) and hydrogen peroxide (H2O2) (Kuppusamy and Zweier, 1989; McCord, 1985; Thompson-Gorman and Zweier, 1990).
Although they are routinely exposed to diving-induced hypoxemia and ischemia/reperfusion, seals do not show higher basal levels of oxidative damage than terrestrial mammals (Vázquez-Medina et al., 2007; Wilhelm Filho et al., 2002; Zenteno-Savín et al., 2002). Interestingly, seal tissues do accumulate HX after in vitro exposure to ischemia (Elsner et al., 1998). Moreover, the basal capacity of tissue to produce 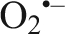 is higher in seals than in non-diving mammals, and
is higher in seals than in non-diving mammals, and 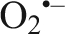 production, but not oxidative damage, is higher in seal than in terrestrial mammal tissues when exposed to an oxidant-generating system (Zenteno-Savín et al., 2002). The latter suggests that seals, as well as other diving vertebrates, are constantly exposed to diving-induced oxidant production (Furtado-Filho et al., 2007; Valdivia et al., 2007; Zenteno-Savín et al., 2010). Seals, however, have higher activities of several antioxidant enzymes and higher glutathione (GSH) content than terrestrial mammals, which likely helps them to cope with the potentially damaging effects of diving-induced hypoxemia and ischemia/reperfusion (Elsner et al., 1998; Murphy and Hochachka, 1981; Vázquez-Medina et al., 2006; Vázquez-Medina et al., 2007; Wilhelm Filho et al., 2002).
production, but not oxidative damage, is higher in seal than in terrestrial mammal tissues when exposed to an oxidant-generating system (Zenteno-Savín et al., 2002). The latter suggests that seals, as well as other diving vertebrates, are constantly exposed to diving-induced oxidant production (Furtado-Filho et al., 2007; Valdivia et al., 2007; Zenteno-Savín et al., 2010). Seals, however, have higher activities of several antioxidant enzymes and higher glutathione (GSH) content than terrestrial mammals, which likely helps them to cope with the potentially damaging effects of diving-induced hypoxemia and ischemia/reperfusion (Elsner et al., 1998; Murphy and Hochachka, 1981; Vázquez-Medina et al., 2006; Vázquez-Medina et al., 2007; Wilhelm Filho et al., 2002).
In animal cells, enzymatic and non-enzymatic antioxidants scavenge 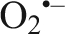 , H2O2 and lipid hydroperoxides, preventing oxidative damage. Superoxide dismutases (SODs) transform
, H2O2 and lipid hydroperoxides, preventing oxidative damage. Superoxide dismutases (SODs) transform 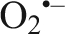 into H2O2 whereas catalase, glutathione peroxidases (GPxs), glutathione-S-transferases (GSTs) and peroxiredoxins (Prxs) remove H2O2 and lipid hydroperoxides. GPx, GST and Prx use GSH in their catalytic reactions, yielding glutathione disulphide (GSSG). GSH is a non-enzymatic antioxidant thiol synthesized in a two-step enzymatic process by glutamate-cysteine ligase (GCL) and glutathione synthase. GSSG is reduced to GSH by glutathione disulphide reductase (GR) but can also react with protein thiol groups causing protein S-glutathionylation, a process that is reversed by glutaredoxins (Glrxs). Thioredoxins (Trxs) can also help to reduce protein disulphide bridges by oxidizing themselves. Thioredoxin reductase (TrxR) then reduces oxidized Trx, maintaining intracellular balance (Halliwell and Gutteridge, 2007). The GSH, Glrx and Trx systems are thus key modulators of the intracellular redox state, which controls the activation of the NF-E2-related factor 2 (Nrf2), a central regulator of the antioxidant response (Jaiswal, 2004).
into H2O2 whereas catalase, glutathione peroxidases (GPxs), glutathione-S-transferases (GSTs) and peroxiredoxins (Prxs) remove H2O2 and lipid hydroperoxides. GPx, GST and Prx use GSH in their catalytic reactions, yielding glutathione disulphide (GSSG). GSH is a non-enzymatic antioxidant thiol synthesized in a two-step enzymatic process by glutamate-cysteine ligase (GCL) and glutathione synthase. GSSG is reduced to GSH by glutathione disulphide reductase (GR) but can also react with protein thiol groups causing protein S-glutathionylation, a process that is reversed by glutaredoxins (Glrxs). Thioredoxins (Trxs) can also help to reduce protein disulphide bridges by oxidizing themselves. Thioredoxin reductase (TrxR) then reduces oxidized Trx, maintaining intracellular balance (Halliwell and Gutteridge, 2007). The GSH, Glrx and Trx systems are thus key modulators of the intracellular redox state, which controls the activation of the NF-E2-related factor 2 (Nrf2), a central regulator of the antioxidant response (Jaiswal, 2004).
Nrf2 induces the transcription of genes involved in antioxidant defense, such as GST, GCL, catalase and heme oxygenase (Immenschuh and Baumgart-Vogt, 2005; Jaiswal, 2004). Nrf2 is, under unaltered conditions, bound to its repressor protein Keap1, which targets its ubiquitin conjugation and, thus, its proteosomal degradation (Itoh et al., 1997). Oxidative/electrophilic stress causes the modification of the Cys273 and Cys288 residues in Keap1, inhibiting Nrf2 ubiquitination and promoting its translocation, nuclear accumulation and binding to the antioxidant response element (Bloom and Jaiswal, 2003; Kobayashi et al., 2004; Kobayashi et al., 2006; Zhang and Hannink, 2003).
It is well established that the diving capacity of hooded seals [Cystophora cristata (Erxleben 1777)] develops with maturation (Burns et al., 2007; Burns et al., 2010; Folkow et al., 2010; Lestyk et al., 2009). It is not known, however, whether their antioxidant system develops as they transition from a terrestrial to an aquatic environment. We have previously shown that maturation increases 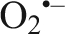 production without increasing oxidative damage to lipids, proteins or DNA in hooded seals (Vázquez-Medina et al., 2011a). In the present study, we compared muscle GSH levels and antioxidant enzyme activities and/or protein content among three age groups of hooded seals to test the hypothesis that the post-natal development of the hooded seal is accompanied by increases in GSH and upregulation of antioxidant enzymes. We also cloned the full-length cDNA sequence of hooded seal Nrf2 and quantified its expression, at transcript and protein levels, along with Nrf2 nuclear content in muscle samples from newborn, weaned and adult hooded seals, in an effort to elucidate its potential role in the development of the seal's antioxidant system.
production without increasing oxidative damage to lipids, proteins or DNA in hooded seals (Vázquez-Medina et al., 2011a). In the present study, we compared muscle GSH levels and antioxidant enzyme activities and/or protein content among three age groups of hooded seals to test the hypothesis that the post-natal development of the hooded seal is accompanied by increases in GSH and upregulation of antioxidant enzymes. We also cloned the full-length cDNA sequence of hooded seal Nrf2 and quantified its expression, at transcript and protein levels, along with Nrf2 nuclear content in muscle samples from newborn, weaned and adult hooded seals, in an effort to elucidate its potential role in the development of the seal's antioxidant system.
MATERIALS AND METHODS
Animal handling and sample collection
Animal capture and handling protocols were reviewed and approved by the Canadian Department of Fisheries and Oceans (DFO) and the University of Alaska Anchorage IACUC Committees. Samples were imported into the United States under Marine Mammal permits 782-1399 and 782-1694-02.
Fifteen healthy hooded seals were captured on the pack ice near the Magdalen Islands, Gulf of St Lawrence, Canada (∼47°23′N, 61°52′W). Animals were categorized visually as adults (five reproductive females, mean body mass: 249.3±25.2 kg) or pups. Pups were categorized as neonates (three males and two females, mean body mass: 21.6±3.3 kg, age=1 day) or weaners (three females and two males, mean body mass: 48.1±4.5 kg, age=5–14 days) following Bowen et al. (Bowen et al., 1987). Seals were physically restrained, drugged with diazepam (0.2–0.3 mg kg–1) or tiletamine-zolazepam (0.7–1.0 mg kg–1) and killed using methods approved for scientific collection by DFO. Tissue sample collection was performed in parallel with other studies to maximize the use of the samples obtained (Burns et al., 2007; Burns et al., 2010; Lestyk et al., 2009). Samples of longisimus dorsi muscle (ca. 2 g) were placed in cryogenic vials, fast-frozen in liquid nitrogen and stored at –80°C until analyzed.
Western blot
Frozen tissue samples were homogenized 1:10 (w/v) in 50 mmol l–1 potassium phosphate buffer containing 1 mmol l–1 EDTA, 1% Triton X-100, 1% phenylmethylsulfonyl fluoride (PMSF) and 1% protease inhibitor cocktail (Sigma, St Louis, MO, USA) (crude extracts), or using the NE-PER nuclear protein extraction kit (Pierce, Rockford, IL, USA) following the manufacturer's instructions (nuclear fractions). Total protein content in crude extracts and nuclear fractions was measured using the Bio-Rad Bradford protein assay (Bio-Rad Laboratories, Hercules, CA, USA). One independent crude extract and one nuclear fraction were prepared from each of the individual samples available and used for the western blot experiments (N=5 per group). Either 20 μg of total or 10 μg of nuclear protein were mixed with sodium dodecyl sulfate Laemmli sample buffer, boiled and resolved in either 4–15% or 10–20% (for Glrx1 and Trx1 analyses) Tris-HCl gradient gels under denaturizing conditions. Electrophoresis was performed for 65 min at 100–120 V. Proteins were electroblotted for 25 min at 25 V using the Bio-Rad Trans Blot SD semi-dry cell onto 0.45 μm nitrocellulose membranes. Membranes were blocked for 1 h at room temperature with 3% bovine serum albumin (BSA) in phosphate buffered saline (PBS) containing 0.05% of Tween 20 (PBS-T), and incubated overnight with primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) against mammalian GCLm (sc-22754), Glrx1 (sc-32943), Trx1 (sc-18215), PrxVI (sc-134478) or Nrf2 (H-300) (sc-13032) diluted 1:500 – 1:3000 in PBS-T with 1% BSA. Membranes were washed, incubated for 1 h at room temperature with IgG horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology) diluted 1:5000, re-washed and developed using the Immun-Star Western C kit (Bio-Rad). Blots were visualized using a Chemi-Doc XRS system (Bio-Rad) and quantified using Bio-Rad's Quantity One software. Results were expressed as percent change from neonates. Actin and lamin were used as loading controls for crude extracts and Nrf2 nuclear protein content, respectively. An immunizing peptide (Santa Cruz Biotechnology, sc-18215P) was used to validate Trx1 measurements as multiple bands were detected in the proximity of the predicted molecular weight of the protein of interest.
Glutathione assay
Frozen tissue samples (N=5 per group) were independently homogenized (1:10 w/v) in 5% ice-cold sulfosalicylic acid previously bubbled with nitrogen gas for 10 min to prevent artifactual GSH oxidation during the extraction procedure. Tissue extracts were bubbled with nitrogen gas for 10 s and centrifuged at 19,000 g at 4°C for 5 min. Supernatants were used immediately to measure total glutathione content (GSH-Eq=GSH+2GSSG) by following the enzymatic recycling method of Griffith (Griffith, 1980) with minor modifications as previously described (Vázquez-Medina et al., 2007). Results are expressed in nmol GSH-Eq g–1 wet tissue.
Enzyme activities
Frozen tissue samples (50 mg) from each individual were independently homogenized (1:20 w/v) in 50 mmol l–1 potassium phosphate buffer containing 1 mmol l–1 EDTA, 1% Triton X-100, 1% PMSF and 1% protease inhibitor cocktail (Sigma). Homogenates were centrifuged at 2000 g for 20 min at 4°C. Supernatants were taken and used immediately for antioxidant enzyme activity analyses. Catalase, GPx, GST and GR activities were measured as described previously (Vázquez-Medina et al., 2006; Vázquez-Medina et al., 2007). TrxR activity was measured using a commercially available kit (Cayman Chemical, Ann Arbor, MI, USA). Results are expressed as mU mg–1 protein. Total protein content in tissue homogenates was measured with the Bio-Rad Bradford protein assay.
cDNA cloning and mRNA expression of Nrf2
Total RNA was isolated from each frozen muscle sample available (N=5 per group) using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. RNA integrity was confirmed by measuring the ratio of absorbance at 260 nm/280 nm and by 1% agarose gel electrophoresis (Sambrook and Russell, 2001). First-strand cDNAs were reverse-transcribed from total DNA-free RNA (1 μg) using the QuantiTec Reverse Transcription kit (Qiagen, Valencia, CA, USA) and oligo-dT (0.5 μg). Annealing and extension steps were performed at 42°C for 30 min and 95°C for 3 min, respectively.
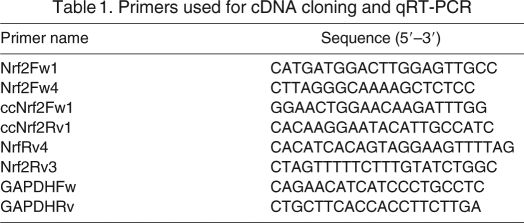
A full-length sequence that encodes for Nrf2 was obtained using primers designed based on mammalian Nrf2 nucleotide sequences. Initial PCR reactions (PCR1) were performed with Nrf2Fw1 + NrfRv4 primers under the following conditions: 26 μl of Platinum PCR SuperMix (Invitrogen), 3 μl of cDNA and 1 μl (20 μmol l–1) of each primer. The Nrf2 5′- and 3′-end extremes were obtained using Nrf2Fw1 + Nrf2Rv3 and Nrf2Fw4 + NrfRv3 primers, respectively (Table 1), under the following conditions: 26 μl of Platinum PCR SuperMix (Invitrogen), 2.5 μl of PCR1 and 1 μl (20 μmol l–1) of each primer. Amplification conditions consisted of one 3 min cycle at 94°C, and 40 cycles at 94°C for 40 s, 55°C for 60 s and 68°C for 120 s, and an overextension step at 68°C for 10 min. PCR fragments of ∼900 bp (Nrf25′) and 1400 bp (Nrf23′) were obtained for each reaction and cloned using the pGEM-T Easy Vector System (Promega Corporation, Fitchburg, WI, USA). Sequences were identified as Nrf2 by comparing them with GenBank data using the Blast algorithm (Altschul et al., 1990). A full-length sequence of 1822 bp (ccNrf2) that encodes for Nrf2 was obtained by overlapping Nrf25′ and Nrf23′ sequences. Predicted amino acid sequences were obtained using a free-access translation program (http://arbl.cvmbs.colostate.edu/molkit/translate/) and aligned with other Nrf2 sequences using Clustal W (Thompson et al., 1994).
Table 1.
Primers used for cDNA cloning and qRT-PCR
Nrf2 mRNA expression was quantified using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as internal standard (GeneBank accession number NM_002046). Nrf2 and GAPDH transcripts were measured by quantitative reverse transcription PCR (qRT-PCR) using ccNrf2Fw1 + ccNrf2Rv1 and GAPDHFw + GAPDHRv primers (Table 1). Two PCR reactions per sample corresponding to each available individual (N=5 per group) were run for qRT-PCR on a 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). Final PCR reactions consisted of 10 μl SYBR Green PCR Master Mix (Applied Biosystems), 6 μl H2O, 0.5 μl of each primer (20 μmol l–1) and 3 μl cDNA (150 ng of total RNA). After an initial denaturing step at 95°C for 5 min, amplifications were performed for 40 cycles at 95°C for 30 s, 60°C for 35 s and a final step of 55 s at 72°C, with a single fluorescence measurement and a final melting curve program decreasing 0.3°C every 20 s from 95 to 60°C. Positive and negative controls were included. Standard curves of Nrf2 and GAPDH were run to determine amplification efficiency, which was 99.2% for Nrf2 and 99.5% for GAPDH, using dilutions from 5×10–4 to 5×10–9 ng μl–1 of PCR fragments.
Statistics
Differences among age groups were detected by one-way ANOVA with Bonferroni post hoc tests. Groups were considered statistically different at P<0.05. Statistical analyses were performed using the SYSTAT© 12.0 software (Cranes Software International Ltd, Richmond, CA, USA). Results are presented as means ± s.e.m.
RESULTS
Protein detection using antibodies
For hooded seal GCLm, a single band was observed at ∼28 kDa. For PrxVI, a single band of ∼25 kDa was detected (supplementary material Fig. S1). Using the Nrf2 (H-300) antibody, which maps near the N-terminus of human Nrf2, a single band was detected at ∼68 kDa. In the nuclear extracts, additional weaker bands of approximately 37 and >100 kDa were observed (supplementary material Fig. S1). A single band at ∼15 kDa was also detected with the Glrx1 (FL-106) antibody, whereas multiple bands were observed at ∼75 kDa and above 25 and 15 kDa using the Trx1 (N-20) antibody. The use of an immunization peptide revealed that the band at ∼12 kDa is Trx1 (supplementary material Fig. S2). All the observed bands indicate that the approximate molecular weight of each of the studied hooded seal proteins is very similar to that of human, bovine, rodent and porcine proteins (Swiss-Prot Protein knowledgebase).
Maturation increases GSH and peroxide-removing enzymes in the muscle of hooded seals
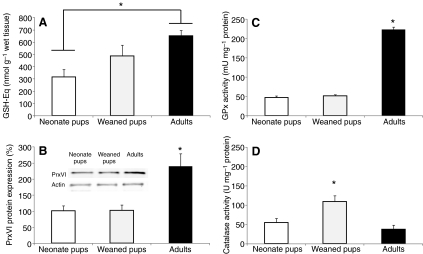
GSH-Eq, PrxVI expression, GPx and catalase activities were measured in the skeletal muscle of newborn, weaned and adult hooded seals to determine whether maturation increases GSH and peroxide-removing enzymes in these mammals during their transition from land to aquatic lifestyles. GSH content was higher (P<0.05) in adults (647±47 nmol g–1 wet tissue) than in newborns (313±61 nmol g–1 wet tissue) (Fig. 1A). PrxVI protein expression was twofold higher (P<0.01) in adults (238±38%) than in neonates (100±15%) or weaned pups (101±16%) (Fig. 1B) whereas GPx activity was more than fourfold higher (P<0.001) in adults (223±7 mU mg–1 protein) than in neonates (47±3 mU mg–1 protein) or weaned pups (51±3 mU mg–1 protein) (Fig. 1C). Catalase activity was higher (P<0.05) in weaned pups (108±15 U mg–1 protein) than in neonates (54±11 U mg–1 protein) or adults (36±10 U mg–1 protein) (Fig. 1D).
Fig. 1.
Maturation increases glutathione content and peroxide-removing enzymes in skeletal muscle of hooded seals. (A) Total glutathione content (GSH-Eq), (B) 1-cys peroxiredoxin (PrxVI) protein expression, (C) glutathione peroxidase (GPx) and (D) catalase activities in neonates, weaned pups and adult hooded seals. One unit of GPx is defined as the amount of enzyme that oxidizes 1 μmol NADPH min–1 at 25°C. One unit of catalase is defined as the amount of enzyme needed to reduce 1 μmol H2O2 min–1 at 25°C. Data are means ± s.e.m. *, P<0.01.
Trx, TrxR and Glrx increase with maturation in hooded seals
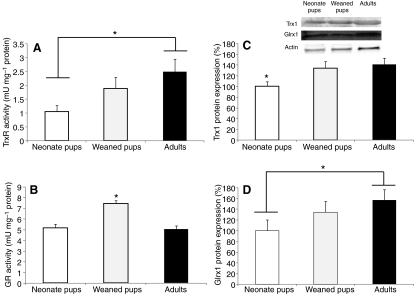
GR and TrxR activities, as well as Trx1 and Glrx1 protein expression, were measured in newborn, weaned and adult hooded seals to determine whether maturation increases these redox regulators. TrxR activity was higher (P=0.034) in adults (2.5±0.4 mU mg–1 protein) than in neonates (1.0±0.2 mU mg–1 protein) (Fig. 2A) whereas GR activity was higher (P<0.001) in weaned pups (7.4±0.3 mU mg–1 protein) than in neonates (5.1±0.4 mU mg–1 protein) or adults (4.9±0.3 mU mg–1 protein) (Fig. 2B). Trx1 protein expression was 30–40% higher (P<0.05) in weaned pups and adults than in neonates (Fig. 2C) whereas Glrx1 protein content was 50% higher (P<0.05) in adults than in neonates (Fig. 2D). Taken together, these results suggest that the antioxidant capacity to remove H2O2 develops with maturation in hooded seals.
Fig. 2.
Thioredoxin, thioredoxin reductase and glutaredoxin increase with maturation in hooded seals. (A) Thioredoxin reductase (TrxR) activity, (B) glutathione disulphide reductase (GR) activity, (C) thioredoxin (Trx) protein expression and (D) glutaredoxin 1 (Glrx1) protein expression in neonates, weaned pups and adult hooded seals. One unit of TrxR activity is defined as the NADPH-dependent production of 2 μmol 2-nitro-5-thiobenzoate min–1 at 22°C. One unit of GR is defined as the amount of enzyme needed to oxidize 1 nmol of NADPH to NADP+ per minute at 25°C. Data are means ± s.e.m. *, P<0.05.
Nrf2 coding sequence of the hooded seal
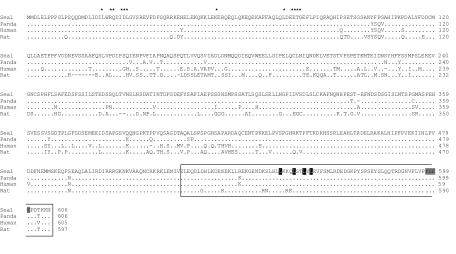
The complete Nrf2 coding sequence from C. cristata (ccNrf2; GenBank accession no. JF429697) was obtained, cloned and sequenced from muscle cDNA. The ccNrf2 sequence is 1822 bp long and encodes a polypeptide of 606 amino acids with a predicted molecular weight of 67.6 kDa, which is similar to other mammalian homologs (Fig. 3). The ccNrf2 predicted amino acid sequence shows high identity to Nrf2 of the giant panda (Ailuropoda melanoleuca, 97%), the domestic dog (Canis lupus familiaris, 95%), the wild boar (Sus scrofa, 90%), humans (Homo sapiens, 90%) and the rat (Rattus norvegicus, 80%). The conserved leucine zipper domain is located in the N terminus (residues 520 to 606) and includes the key residues for nuclear export signal (Leu554, -558, -560 and -562) and nuclear location signal (Lys597, -599 and –600; Ser598). Also, ccNrf2 has the conserved N-terminal domain (Leu23 and -30; Gln26; Asp27, -77 and -29; Gly30 and -81; Glu57, -79 and -82; Thr80) essential for the Keap1-mediated degradation of Nrf2 by the proteosomal system (Fig. 3).
Fig. 3.
Alignment of the predicted hooded seal Nrf2 amino acid sequence (ccNrf2) compared with its mammalian homologs. Seal: hooded seal Cystophora cristata (JF429697). Panda: giant panda Ailuropoda melanoleuca, 97%. Human: Homo sapiens (NP_006155). Rat: Rattus norvegicus (NP_113977). Leucine zipper domain (outlined), nuclear export signal (black shading), nuclear location signal (gray shading) and residues for the Keap1-mediated proteosomal degradation (*) are shown.
Nrf2 and its downstream genes during post-natal development in hooded seals
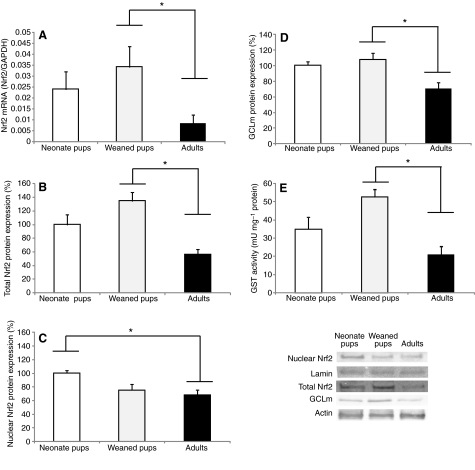
Nrf2 mRNA expression levels and protein content was measured in crude extracts and nuclear fractions to determine whether maturation increases this transcriptional regulator. Nrf2 transcript levels decreased fourfold (P<0.01) in adult seals compared with weaned pups (Fig. 4A). Nrf2 protein content in crude extracts was also higher (P<0.01) in weaned pups (134±12%) than in adults (56±7%) (Fig. 4B), whereas nuclear Nrf2 was higher (P<0.05) in neonates (100±4%) than in adults (67±7%) (Fig. 4C). GCLm protein expression and GST activity, both of which are known to be regulated by Nrf2, were also higher (P<0.01) in weaned pups (107±8%; 52±4 mU mg–1 protein) than in adults (70±7%; 21±4 mU mg–1 protein) (Fig. 4D,E), suggesting that the development of the antioxidant system in hooded seals is not related to increased Nrf2 expression.
Fig. 4.
Nrf2 and two of its downstream targets during the postnatal development of the hooded seal. (A) Nrf2 mRNA expression, (B) total Nrf2 (crude extract), (C) Nrf2 nuclear content, (D) glutamate-cysteine ligase modulatory subunit (GCLm) protein expression and (E) glutathione S-transferase (GST) activity in neonates, weaned pups and adult hooded seals. Nrf2 relative mRNA levels were calculated by quantitative real-time RT-PCR and compared with GAPDH levels. One unit of GST is defined as the amount of enzyme that synthesizes 1 μmol product min–1 at 25°C. Data are means ± s.e.m. *, P<0.05.
DISCUSSION
Maturation in hooded seals is characterized by the development of their diving capacity (Burns et al., 2007; Burns et al., 2010; Folkow et al., 2010; Lestyk et al., 2009) and is associated with increases in 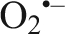 production but not with increases in oxidative damage (Vázquez-Medina et al., 2011a). In the present study we found that the antioxidant system of the hooded seal develops with maturation, likely contributing to the prevention of the accumulation of oxidative damage.
production but not with increases in oxidative damage (Vázquez-Medina et al., 2011a). In the present study we found that the antioxidant system of the hooded seal develops with maturation, likely contributing to the prevention of the accumulation of oxidative damage.
Diving seals undergo a number of cardiovascular adjustments that allow them to maximize the use of their oxygen stores but at the same time result in ischemia and hypoxemia (Elsner, 1999; Kooyman and Ponganis, 1998; Meir et al., 2009). After a dive, perfusion of ischemic tissues presents seals with the potential for oxidant production and oxidative stress due to the accumulation of the ATP degradation product HX and the activation of XO during ischemia (Elsner et al., 1998; Kuppusamy and Zweier, 1989; McCord, 1985; Thompson-Gorman and Zweier, 1990). Comparative studies have shown that antioxidant protection is higher in seals than in non-diving mammals, which likely allows seals to cope with recurrent ischemia/reperfusion cycles associated with their diving lifestyle (Elsner et al., 1998; Murphy and Hochachka, 1981; Vázquez-Medina et al., 2006; Vázquez-Medina et al., 2007; Wilhelm Filho et al., 2002; Zenteno-Savín et al., 2002). The present study shows that GSH content gradually increases with maturation in hooded seals, and that PrxVI expression and GPx activity are higher in adult hooded seals than in neonates or weaned pups. Both PrxVI and GPx use GSH as a cofactor to remove H2O2. H2O2 is the main oxidant produced after ischemia/reperfusion due to the HX/XO pathway, which is activated after experimental ischemia in seal organs (Brown et al., 1988; Elsner et al., 1998). Our results suggest that the capacity to remove H2O2 increases as hooded seals transition from terrestrial to aquatic lifestyles. This is consistent with the previously reported higher 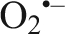 production, MnSOD protein content and SOD activity in adult seals than in pups because SOD dismutates
production, MnSOD protein content and SOD activity in adult seals than in pups because SOD dismutates 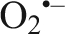 into H2O2 (Vázquez-Medina et al., 2011a). This also suggests that GPx and Prx, and not catalase, are the main enzymes responsible for H2O2 removal in seal muscle under basal conditions. Although GPx activity has been shown to be higher in muscle of ringed seals than in pigs under basal conditions, catalase activity is higher in pigs and no difference exists between terrestrial and marine mammal red blood cells (Vázquez-Medina et al., 2006; Wilhelm Filho et al., 2002).
into H2O2 (Vázquez-Medina et al., 2011a). This also suggests that GPx and Prx, and not catalase, are the main enzymes responsible for H2O2 removal in seal muscle under basal conditions. Although GPx activity has been shown to be higher in muscle of ringed seals than in pigs under basal conditions, catalase activity is higher in pigs and no difference exists between terrestrial and marine mammal red blood cells (Vázquez-Medina et al., 2006; Wilhelm Filho et al., 2002).
The results also show that Trx1 and Glrx1 expression as well as TrxR activity increase with maturation in hooded seals, suggesting that these enzymes contribute to counteract the potential increases in peroxide production. Thioredoxins are the major cellular protein disulfide reductases; therefore, they serve as electron donors for enzymes such as Prx and GPx (Arnér and Holmgren, 2000; Björnstedt et al., 1994). Higher Trx1 and Glrx1 expression, as well as higher TrxR activity, are consistent with higher PrxVI protein content and GPx activity and suggest that the Trx and Glrx systems are key modulators of the intracelular redox balance in adult hooded seals.
Although Nrf2 mRNA and protein were detected in crude extracts and nuclear fractions from the three age groups, neither transcript nor Nrf2 protein content increased with maturation, suggesting that Nrf2 activation is not stimulated by maturation-related increases in oxidant production in hooded seals (Immenschuh and Baumgart-Vogt, 2005; Jaiswal, 2004). The latter is consistent with the decreases observed in three downstream enzymes controlled by Nrf2 (GST, GCLm and catalse) in adult seals compared with weaned pups (Chan and Kan, 1999; Cho et al., 2002; Itoh et al., 1997; Yang et al., 2005). These results can be explained by the observed increases in PrxVI expression and GPx activity, as increased intracellular levels of H2O2 are among the main mechanisms responsible for the activation of Nrf2, and both GPx and PrxVI scavenge H2O2. Lower Nrf2 and catalase in adults than in weaned pups, as well as higher GPx and PrxVI in adults than in pups, also suggests that intracellular H2O2 production is kept at physiological levels during development in hooded seals because GPx and Prx are responsible for the removal of physiological concentrations of H2O2 whereas catalase activity and Nrf2 translocation increase in response to increased H2O2 production (Halliwell and Gutteridge, 2007; Prestera and Talalay, 1995). Although PrxVI has been shown to be controlled by Nrf2 (Chowdhury et al., 2009), its transcription is also responsive to hormonal regulation and PrxVI levels increase with postnatal development in terrestrial mammals (Fisher, 2010; Kim et al., 2002).
An alternative explanation for the observed lower Nrf2 levels in adults than in weaned pups is that Nrf2 nuclear translocation in hooded seals responds to particular events, such as a prolonged ischemic episode induced by a particularly extended dive. Although adult hooded seals are able to dive to depths in excess of 1000 m for nearly 1 h, and can remain submerged for approximately 80% of their time at sea, they perform routine dives of >20 min (Folkow and Blix, 1999). In addition, the higher Nrf2, catalase, GCLm and GST levels observed in weaned pups than in adults can be explained by the physiological condition of the weaned seals. We have shown that fasting increases catalase, GCLm and GST in postweaning elephant seals (Vázquez-Medina et al., 2010; Vázquez-Medina et al., 2011b) but apparently not in fasting adult female seals (Vázquez-Medina et al., 2011b).
In summary, our results show that the antioxidant system of hooded seals develops with maturation, likely preparing them to cope with the potential diving-induced oxidant production. Results also show that peroxide-removing enzymes such as GPx, PrxVI, Trx and Glrx1 increase with maturation, suggesting that they contribute to the regulation of intracellular redox state in hooded seal muscle. Lastly, Nrf2 of the hooded seal has functional and regulatory domains similar to those of most terrestrial mammals, although its nuclear translocation does not seem to be controlled by potential increases in maturation-related oxidant production.
Supplementary Material
ACKNOWLEDGEMENTS
This work would not have taken place without the generous support of Dr M. O. Hammill (DFO). We thank the Canadian Coast Guard helicopter pilots, H. McRae and B. Kendall, who provided logistic support in sample collection. The Château Madelinot and R. Simon provided laboratory space. L. Measures, S. Pillet and S. Turgeon assisted with sample collection. J.P.V.-M. is supported by fellowships from UC MEXUS, CONACYT, Mexico's Secretaría de Educación Pública and The University of California.
LIST OF ABBREVIATIONS
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GCLm
glutamate-cysteine ligase modulatory subunit
- Glrx1
glutaredoxin 1
- GPx
glutathione peroxidases
- GR
glutathione disulphide reductase
- GSH
glutathione
- GSH-Eq
total glutathione content
- GSSG
glutathione disulphide
- GST
glutathione-S-transferases
- HX
hypoxanthine
- Nrf2
NF-E2-related factor 2
- PrxVI
peroxyredoxin VI
- SOD
superoxide dismutase
- Trx1
thioredoxin 1
- TrxR
thioredoxin reductase
- XO
xanthine oxidase
FOOTNOTES
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/214/17/2903/DC1
FOOTNOTES
J.G.S.-O. is supported by a UC MEXUS-CONACTY postdoctoral fellowship. Research was funded by grants from SEMARNAT-CONACYT (T.Z.-S.), UC Mexus-CONACYT (R.M.O., T.Z.-S.) and NHLBI (R.M.O.). Deposited in PMC for release after 12 months.
REFERENCES
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403-410 [DOI] [PubMed] [Google Scholar]
- Arnér E. S. J., Holmgren A. (2000). Physiological functions of thioredoxin and thioredoxin reductase. FEBS J. 267, 6102-6109 [DOI] [PubMed] [Google Scholar]
- Björnstedt M., Xue J., Huang W., Akesson B., Holmgren A. (1994). The thioredoxin and glutaredoxin systems are efficient electron donors to human plasma glutathione peroxidase. J. Biol. Chem. 269, 29382-29384 [PubMed] [Google Scholar]
- Bloom D. A., Jaiswal A. K. (2003). Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H: quinone oxidoreductase-1 gene expression. J. Biol. Chem. 278, 44675-44682 [DOI] [PubMed] [Google Scholar]
- Bowen W. D., Boness D. J., Oftedal O. T. (1987). Mass transfer from mother to pup and subsequent mass loss by the weaned pup in the hooded seal, Cystophora cristata. Can. J. Zool. 65, 1-8 [Google Scholar]
- Brown J. M., Terada L. S., Grosso M. A., Whitmann G. J., Velasco S. E., Patt A., Harken A. H., Repine J. E. (1988). Xanthine oxidase produces hydrogen peroxide which contributes to reperfusion injury of ischemic, isolated, perfused rat hearts. J. Clin. Invest. 81, 1297-1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J., Lestyk K., Folkow L., Hammill M., Blix A. (2007). Size and distribution of oxygen stores in harp and hooded seals from birth to maturity. J. Comp. Physiol. B 177, 687-700 [DOI] [PubMed] [Google Scholar]
- Burns J., Skomp N., Bishop N., Lestyk K., Hammill M. (2010). Development of aerobic and anaerobic metabolism in cardiac and skeletal muscles from harp and hooded seals. J. Exp. Biol. 213, 740-748 [DOI] [PubMed] [Google Scholar]
- Chan K., Kan Y. W. (1999). Nrf2 is essential for protection against acute pulmonary injury in mice. Proc. Natl. Acad. Sci. USA 96, 12731-12736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. Y., Jedlicka A. E., Reddy S. P. M., Kensler T. W., Yamamoto M., Zhang L. Y., Kleeberger S. R. (2002). Role of NRF2 in protection against hyperoxic lung injury in mice. Am. J. Respir. Cell Mol. Biol. 26, 175-182 [DOI] [PubMed] [Google Scholar]
- Chowdhury I., Mo Y., Gao L., Kazi A., Fisher A. B., Feinstein S. I. (2009). Oxidant stress stimulates expression of the human peroxiredoxin 6 gene by a transcriptional mechanism involving an antioxidant response element. Free Radic. Biol. Med. 46, 146-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner R. (1999). Living in water: solutions to physiological problems. In Biology of Marine Mammals (ed. Reynolds J. E. I., Rommel S. A.), pp. 73-116 Washington, DC: Smithsonian Institution Press; [Google Scholar]
- Elsner R., Øyasæter S., Almaas R., Saugstad O. D. (1998). Diving seals, ischemia-reperfusion and oxygen radicals. Comp. Biochem. Physiol. 119A, 975-980 [DOI] [PubMed] [Google Scholar]
- Fisher A. B. (2011). Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid. Redox Signal. 15, 831-844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkow L. P., Blix A. S. (1999). Diving behaviour of hooded seals (Cystophora cristata) in the Greenland and Norwegian Seas. Polar Biol. 22, 61-74 [Google Scholar]
- Folkow L., Nordøy E., Blix A. (2010). Remarkable development of diving performance and migrations of hooded seals (Cystophora cristata) during their first year of life. Polar Biol. 33, 433-441 [Google Scholar]
- Furtado-Filho O. V., Polcheira C., Machado D. P., Mourão G., Hermes-Lima M. (2007). Selected oxidative stress markers in a South American crocodilian species. Comp. Biochem. Physiol. 146C, 241-254 [DOI] [PubMed] [Google Scholar]
- Griffith O. W. (1980). Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 106, 207-212 [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. (2007). Free Radicals in Biology and Medicine. New York: Oxford University Press; [Google Scholar]
- Immenschuh S., Baumgart-Vogt E. (2005). Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid. Redox Signal. 7, 768-777 [DOI] [PubMed] [Google Scholar]
- Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I. (1997). An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 236, 313-322 [DOI] [PubMed] [Google Scholar]
- Jaiswal A. K. (2004). Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 36, 1199-1207 [DOI] [PubMed] [Google Scholar]
- Kim H. S., Pak J. H., Gonzales L. W., Feinstein S. I., Fisher A. B. (2002). Regulation of 1-cys peroxiredoxin expression in lung epithelial cells. Am. J. Respir. Cell Mol. Biol. 27, 227-233 [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Kang M. I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. (2004). Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 24, 7130-7139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A., Kang M. I., Watai Y., Tong K. I., Shibata T., Uchida K., Yamamoto M. (2006). Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell. Biol. 26, 221-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooyman G., Ponganis P. (1998). The physiological basis of diving to depth: birds and mammals. Annu. Rev. Physiol. 60, 19-32 [DOI] [PubMed] [Google Scholar]
- Kuppusamy P., Zweier J. (1989). Characterization of free radical generation by xanthine oxidase. Evidence for hydroxyl radical generation. J. Biol. Chem. 264, 9880-9884 [PubMed] [Google Scholar]
- Lestyk K., Folkow L., Blix A., Hammill M., Burns J. (2009). Development of myoglobin concentration and acid buffering capacity in harp (Pagophilus groenlandicus) and hooded (Cystophora cristata) seals from birth to maturity. J. Comp. Physiol. B 179, 985-996 [DOI] [PubMed] [Google Scholar]
- McCord J. (1985). Oxygen-derived free radicals in postischemic tissue injury. N. Engl. J. Med. 312, 159-163 [DOI] [PubMed] [Google Scholar]
- Meir J. U., Champagne C. D., Costa D. P., Williams C. L., Ponganis P. J. (2009). Extreme hypoxemic tolerance and blood oxygen depletion in diving elephant seals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R927-R929 [DOI] [PubMed] [Google Scholar]
- Murphy B. J., Hochachka P. W. (1981). Free amino acid profiles in blood during diving and recovery in the Antarctic Weddell seal. Can. J. Zool. 59, 455-459 [Google Scholar]
- Prestera T., Talalay P. (1995). Electrophile and antioxidant regulation of enzymes that detoxify carcinogens. Proc. Natl. Acad. Sci. USA 92, 8965-8969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Russell D. W. (2001). Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673-4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Gorman S. L., Zweier J. L. (1990). Evaluation of the role of xanthine oxidase in myocardial reperfusion injury. J. Biol. Chem. 265, 6656-6663 [PubMed] [Google Scholar]
- Valdivia P. A., Zenteno-Savín T., Gardner S. C., Alonso Aguirre A. (2007). Basic oxidative stress metabolites in eastern Pacific green turtles (Chelonia mydas agassizii). Comp. Biochem. Physiol. 146C, 111-117 [DOI] [PubMed] [Google Scholar]
- Vázquez-Medina J. P., Zenteno-Savín T., Elsner R. (2006). Antioxidant enzymes in ringed seal tissues: potential protection against dive-associated ischemia/reperfusion. Comp. Biochem. Physiol. 142C, 198-204 [DOI] [PubMed] [Google Scholar]
- Vázquez-Medina J. P., Zenteno-Savín T., Elsner R. (2007). Glutathione protection against dive-associated ischemia/reperfusion in ringed seal tissues. J. Exp. Mar. Biol. Ecol. 345, 110-118 [Google Scholar]
- Vázquez-Medina J. P., Crocker D. E., Forman H. J., Ortiz R. M. (2010). Prolonged fasting does not increase oxidative damage or inflammation in postweaned northern elephant seal pups. J. Exp. Biol. 213, 2524-2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Medina J. P., Olguín-Monroy N. O., Maldonado P. D., Santamaría A., Königsberg M., Elsner R., Hammilll M. O., Burns J. M., Zenteno-Savín T. (2011a). Maturarion increases superoxide radical production without increasing oxidative damage in the skeletal muscle of hooded seals (Cystophora cristata). Can. J. Zool. 89, 206-212 [Google Scholar]
- Vázquez-Medina J. P., Zenteno-Savín T., Crocker D. E., Forman H. J., Ortiz R. M. (2011b). Prolonged fasting increases glutathione biosynthesis in postweaned northern elephant seals. J. Exp. Biol. 14, 1294-1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm Filho D., Sell F., Ribeiro L., Ghislandi M., Carrasquedo F., Fraga C. G., Wallauer J. P., Simões-Lopes P. C., Uhart M. M. (2002). Comparison between the antioxidant status of terrestrial and diving mammals. Comp. Biochem. Physiol. 133A, 885-892 [DOI] [PubMed] [Google Scholar]
- Yang H., Magilnick N., Lee C., Kalmaz D., Ou X., Chan J. Y., Lu S. C. (2005). Nrf1 and Nrf2 regulate rat glutamate-cysteine ligase catalytic subunit transcription indirectly via NF-κB and AP-1. Mol. Cell. Biol. 25, 5933-5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenteno-Savín T., Clayton-Hernandez E., Elsner R. (2002). Diving seals: are they a model for coping with oxidative stress? Comp. Biochem. Physiol. 133C, 527-536 [DOI] [PubMed] [Google Scholar]
- Zenteno-Savín T., St Leger J., Ponganis P. J. (2010). Hypoxemic and ischemic tolerance in emperor penguins. Comp. Biochem. Physiol. 152C, 18-23 [DOI] [PubMed] [Google Scholar]
- Zhang D. D., Hannink M. (2003). Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 23, 8137-8151 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.