Abstract
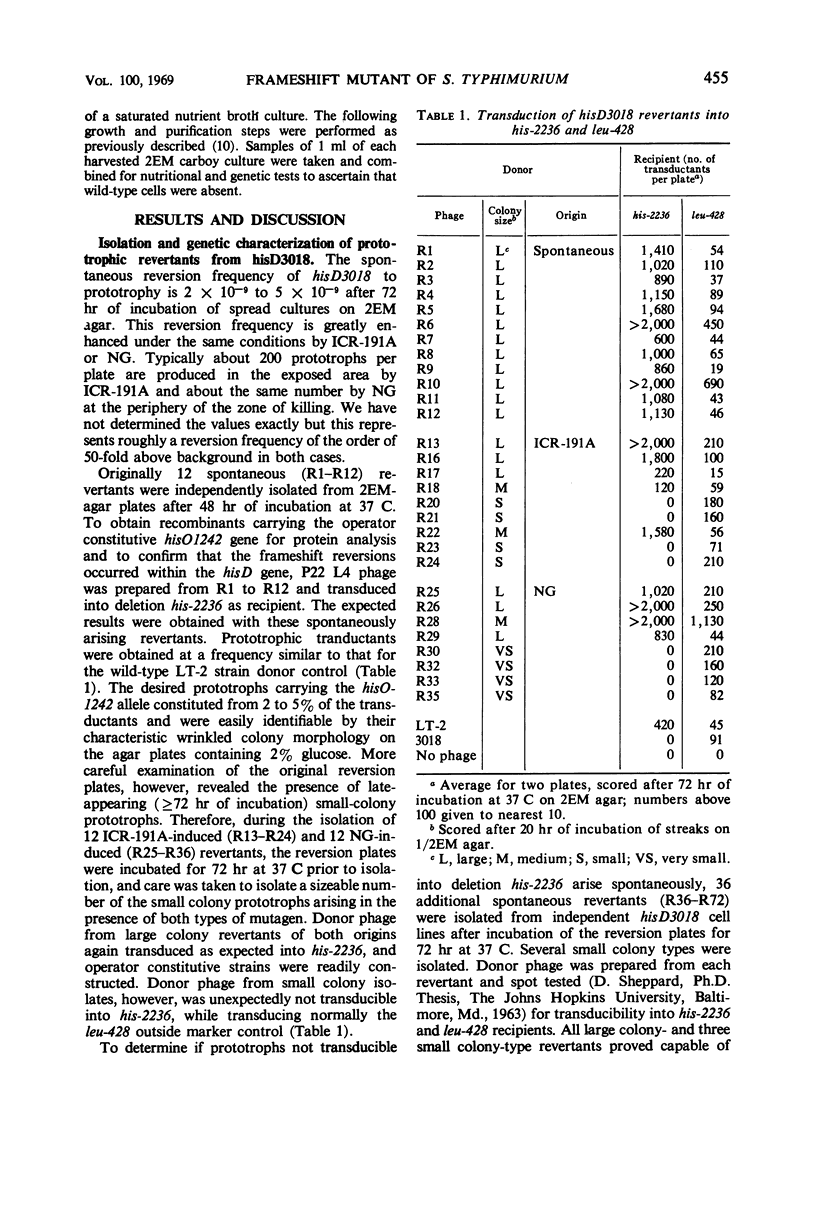
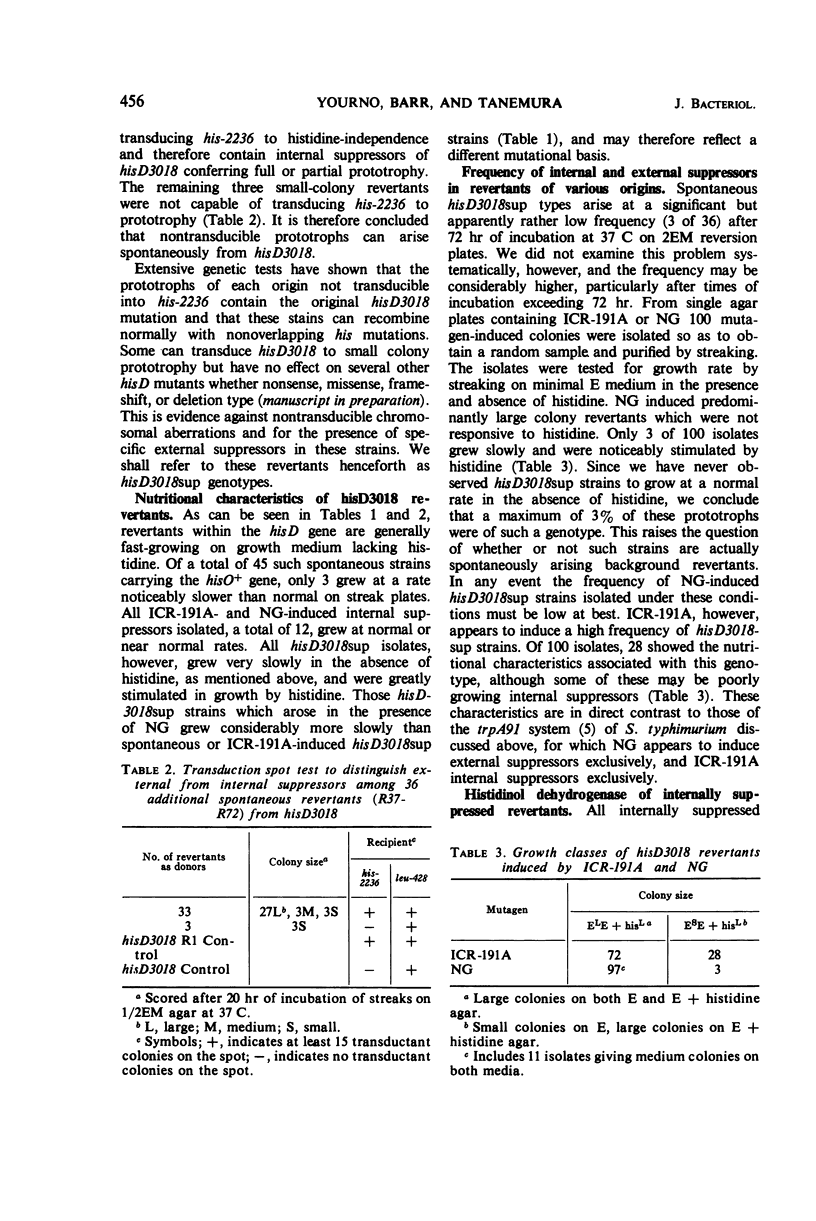
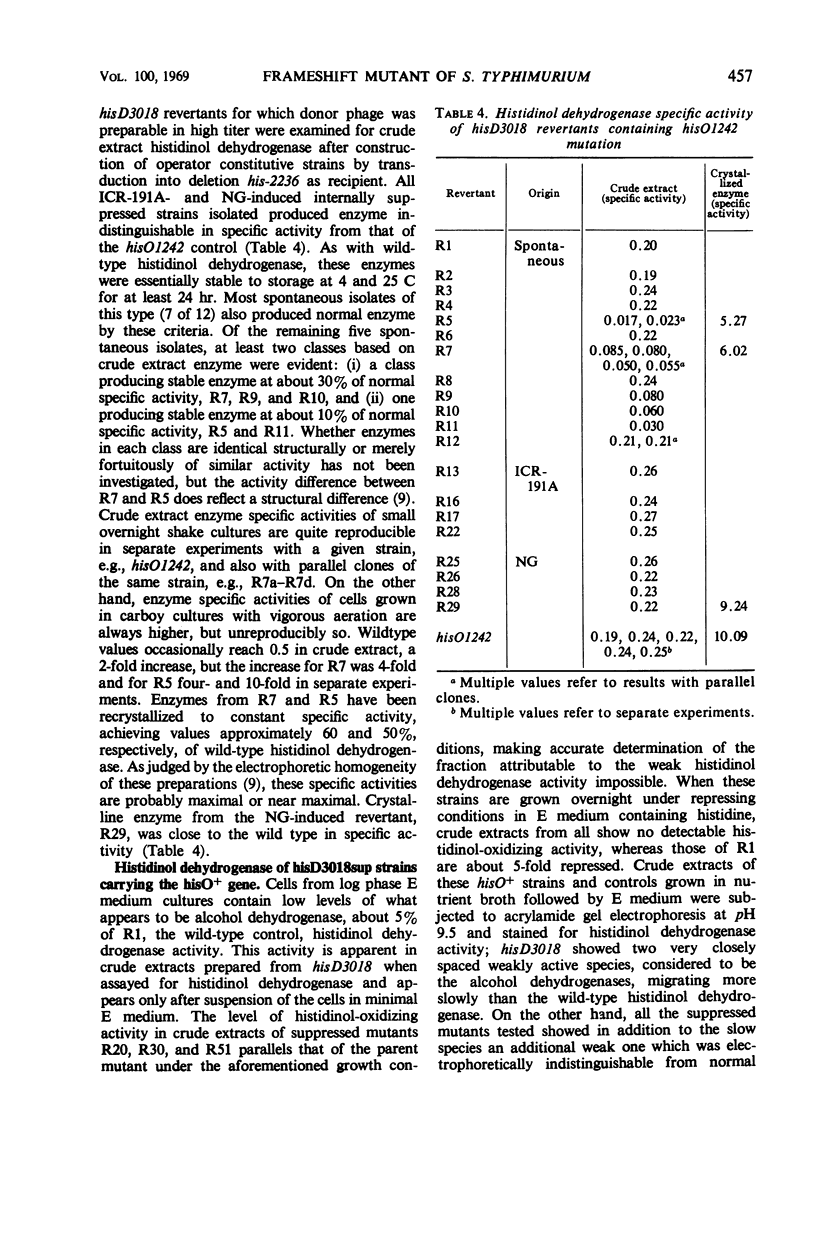
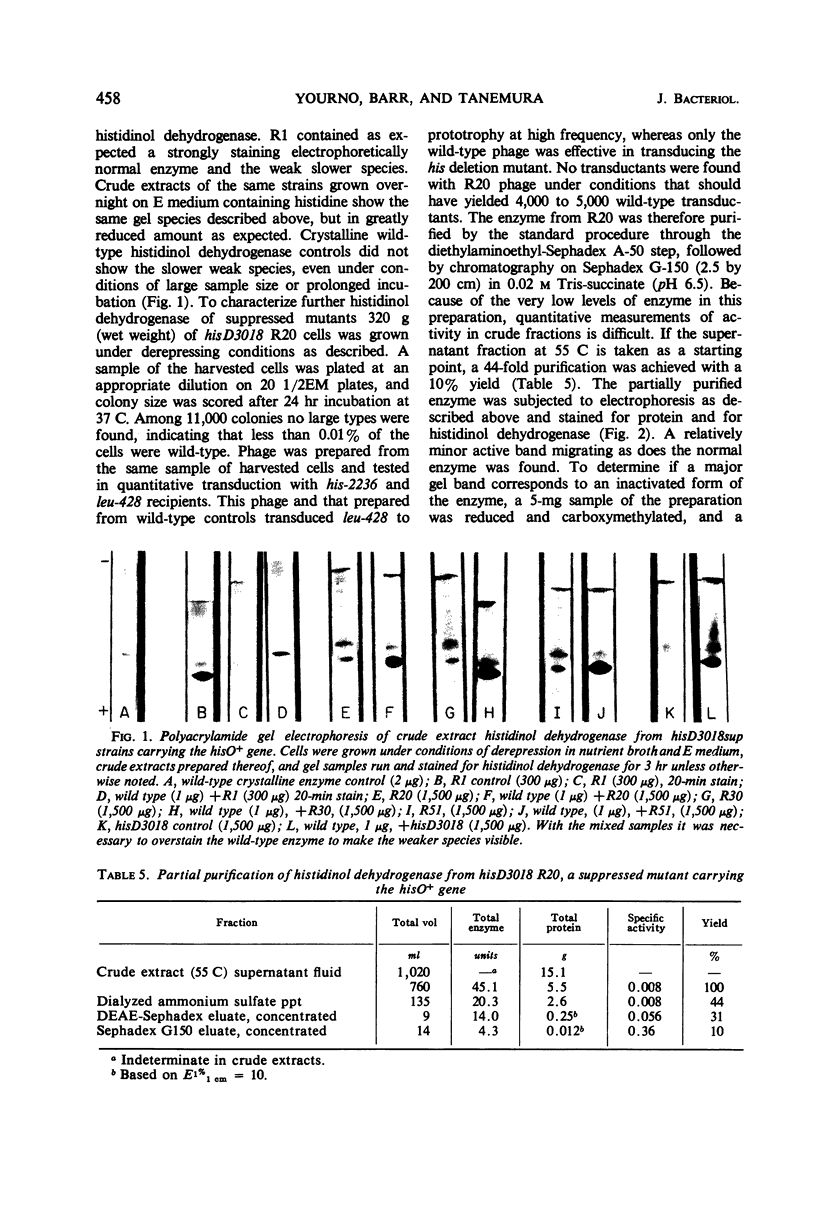
Prototrophic revertants of ICR-191A-induced frameshift mutant hisD3018 have been induced spontaneously by ICR-191A and N-methyl-N′-nitro-N-nitrosoguanidine (NG) treatment. In each case two genetically distinct prototroph classes were differentiated by transducibility into his deletion recipients: (i) transducible, generally fast-growing revertants within the hisD gene producing from 10 to 100% of normal amounts of histidinol dehydrogenase and (ii) nontransducible slow-growing prototrophs with very low levels of enzyme activity of which at least some arose by external suppression. These nontransducible revertants, whether arising spontaneously or in the presence of ICR-191A or NG, contain histidinol dehydrogenase which is electrophoretically similar to the wild-type enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Whitfield H. J., Jr Frameshift mutagenesis in Salmonella. Cold Spring Harb Symp Quant Biol. 1966;31:221–225. doi: 10.1101/sqb.1966.031.01.030. [DOI] [PubMed] [Google Scholar]
- LOPER J. C., ADAMS E. PURIFICATION AND PROPERTIES OF HISTIDINOL DEHYDROGENASE FROM SALMONELLA TYPHIMURIUM. J Biol Chem. 1965 Feb;240:788–795. [PubMed] [Google Scholar]
- Martin R. G., Silbert D. F., Smith W. E., Whitfield H. J., Jr Polarity in the histidine operon. J Mol Biol. 1966 Nov 14;21(2):357–369. doi: 10.1016/0022-2836(66)90104-5. [DOI] [PubMed] [Google Scholar]
- Riyasaty S., Atkins J. F. External suppression of a frameshift mutant in salmonella. J Mol Biol. 1968 Jun 28;34(3):541–557. doi: 10.1016/0022-2836(68)90179-4. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Antón D. N., Hartman P. E. Histidine regulatory mutants in Salmonella typhimurium. I. Isolation and general properties. J Mol Biol. 1966 Dec 28;22(2):305–323. doi: 10.1016/0022-2836(66)90134-3. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Whitfield H. J., Jr, Martin R. G., Ames B. N. Classification of aminotransferase (C gene) mutants in the histidine operon. J Mol Biol. 1966 Nov 14;21(2):335–355. doi: 10.1016/0022-2836(66)90103-3. [DOI] [PubMed] [Google Scholar]
- Yourno J., Heath S. Nature of the hisD3018 frameshift mutation in Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):460–468. doi: 10.1128/jb.100.1.460-468.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourno J., Ino I. Purification and crystallization of histidinol dehydrogenase from Salmonella typhimurium LT-2. J Biol Chem. 1968 Jun 25;243(12):3273–3276. [PubMed] [Google Scholar]