SUMMARY
Background
Resistance to loop diuretics is common in patients with ascites. Diminished glomerular filtration rate (GFR) is thought to mediate resistance to loop diuretics. Midodrine, a commonly used alpha-1 agonist, has been shown to improve GFR in non-azotemic patients with cirrhosis.
Aim
To conduct a randomized, double-blind, placebo-controlled, cross-over study to test the hypothesis that midodrine significantly increases natriuretic response of IV furosemide in non-azotemic cirrhotics with ascites.
Methods
All subjects participated in both phases, which were (i) furosemide IV infusion + oral midodrine 15 mg administered 30 min before furosemide (ii) furosemide IV infusion + oral placebo administered 30 min before furosemide. Primary outcomes were 6-h urine sodium excretion and 6-h total urine volume.
Results
A total of 15 patients (men: 8; age: 52.7 ± 7.6 years; serum creatinine: 1.06 ± 0.2 mg/dL) were studied. Total 6-h urine sodium excretion was 109 ± 42 mmol in the furosemide + midodrine treatment phase and was not significantly different from that in the furosemide + placebo treatment phase (126 ± 69 mmol, P = 0.6). Similarly, mean 6-h total urine volume was not significantly different between two groups (1770 ± 262 mL vs. 1962 ± 170 mL, P = 0.25).
Conclusions
Oral midodrine does not increase the natriuretic response to furosemide in non-azotemic cirrhotic patients with ascites. Orally administered midodrine does not increase natriuretic response to furosemide in non-azotemic cirrhotic patients with ascites.
INTRODUCTION
Approximately 5 million Americans are estimated to have chronic liver disease and cirrhosis. Cirrhosis was the 12th leading cause of death in 2006 with approximately 27 000 deaths according to CDC vital statistics data.1 Ascites is a major complication of cirrhosis and is an important prognosticator with 40% mortality in 2–5 years after its onset.2 Patients with advanced cirrhosis and ascites are hospitalized frequently for optimizing their diuretic regimen, correcting electrolyte abnormalities, large volume paracenteses and transjugular intrahepatic portasystemic shunt (TIPS) placement. Direct health care cost for liver diseases was in excess of 1.4 billion dollars per year estimated in 2000.3 The medical treatment for cirrhotic ascites is sodium-restricted diet and diuretics.4, 5 The usual diuretic regimen consists of a combination of spironolactone and furosemide. This therapy is effective in majority of the patients; however, some individuals become resistant to diuretic therapy and pose a major therapeutic challenge in clinical practice.4–6 Several mechanisms for this loop diuretic resistance have been investigated and they include hypoalbuminaemia, diminished glomerular filtration rate (GFR), altered pharmacokinetics and pharmacodynamics of loop diuretics, and simultaneous usage of NSAIDs.7–11 Of these, diminished GFR is perhaps one of the important mechanisms for loop diuretic resistance in patients with cirrhotic ascites. In a previous study, Villeneuve et al.8 investigated the furosemide pharmacokinetics and pharmacodynamics in eight normal volunteers and 14 patients with cirrhosis, eight of whom were resistant to diuretic therapy. This study showed that cirrhosis was associated with a reduction in the pharmacodynamic response to furosemide and this reduction was not due to altered tubular sensitivity to furosemide, but rather was directly related to diminished GFR, which is common in patients with decompensated cirrhosis.8
Midodrine, an alpha-1 agonist acting directly on the peripheral alpha-receptors, has been widely used in the therapy of orthostatic hypotension and various secondary hypotensive disorders. It has recently been shown that single-dose administration of midodrine leads to a significant improvement in systemic and renal haemodynamics in non-azotemic cirrhotic patients with ascites.12, 13 Angeli et al. reported that 15 mg of oral midodrine increased renal plasma flow by 40%, GFR by 21% and urinary sodium excretion by 28%. When combined, the studies by Villeneuve et al. and Angeli et al. suggest that diuretic resistance in cirrhotic patients with ascites is possibly due to diminished GFR, and orally administered midodrine could significantly improve GFR in non-azotemic cirrhotics with ascites.
On the basis of this background, we hypothesized that midodrine co-administration could enhance the efficacy of furosemide in patients with cirrhotic ascites through its effects on renal haemodynamics and/or the pharmacokinetics and pharmacodynamics of furosemide. We conducted a randomized, double-blind, placebo-controlled, cross-over study of non-azotemic cirrhotics with ascites to assess whether midodrine co-administration enhances the natriuretic response to furosemide.
MATERIALS AND METHODS
Patients
After Indiana University School of Medicine institutional review board approval on 17 April 2002, 16 adult cirrhotic patients with clinically detectable ascites (age ≥18, Child–Pugh score ≥7) were screened prior to enrolment. During the screening procedure, medical history was obtained, a physical examination performed, an electrocardiogram (EKG) obtained and blood and urine sampling for chemistry 17, complete blood count (CBC) with differential and platelets, and urinalysis. Patients having co-morbid conditions that would affect response to a diuretic were excluded including congestive heart failure, creatinine clearance <60 mL/min or untreated endocrinopathies, actively consuming alcohol, or who have a TIPS. Patients after signing informed consent were admitted to the General Clinical Research Center (GCRC) at which time any diuretics except spironolactone were discontinued. This is because spirinolactone effect is persistent for a longer duration and hence discontinuation would not necessarily mean loss of effect and may interfere with steady state. They were started on a metabolic diet containing 30 mmol of sodium per day and 60–80 mmol of potassium per day. Our previous study has shown that this level of sodium restriction allows discontinuation of other diuretics in such patients without affecting blood pressure control, body weight or rapid fluid accumulation14 Daily weight was taken and electrolytes and creatinine were measured in serum and in 24-h urine collection.
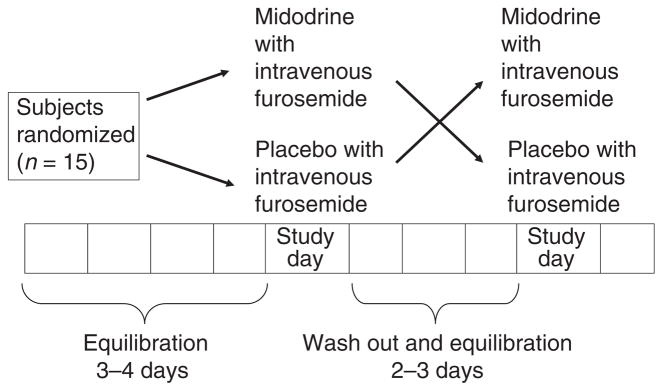
Patients equilibrated on the metabolic diet until sodium balance was attained as noted by two consecutive daily 24-h urinary sodium excretion values that varied ≤20% and/or change in two consecutive daily weights of ≤0.5 kg. Patients were then randomized to one of the phases of the protocol (Figure 1). The two phases of the study were:
Figure 1.
Graphical representation of the study protocol. A state of equilibration was achieved by acquisition of sodium balance defined as change in body weight ≤0.5 kg on two consecutive days while on metabolic diet (30 mmol Na, 80 mmol K/day).
Midodrine (15 mg orally 30 min before) and furosemide 40 mg intravenously,
Placebo (orally given 30 min before) and furosemide 40 mg intravenously.
Protocol
Patients fasted (except for distilled water) from midnight before study until at least 4 h after the administration of study medications. The daily 24-h urine collection was completed and a baseline serum sample was obtained. A 10 mL/kg oral distilled water load was given along with midodrine hydrochloride (15 mg) or oral placebo 30 min prior to 40 mg of intravenous furosemide administration. We chose this 30-min interval on the basis of pharmacokinetics of midodrine. The time of intravenous furosemide administration was counted as zero time. Urine and serum were collected at 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6 and 8 h. We determined the renal haemodynamics by measuring the GFR using iothalamate clearance. On the evening prior to furosemide administration, a bolus of iothalamate (Conray 60) was given intravenously followed by an overnight intravenous infusion (0.5 mL/min) until 8 h after dosing with study medications. Iothalamate was given to all, but one patient who was allergic to iodine. The bolus dose was determined based on a predicted plasma level using an estimated GFR from Cockcroft formula. The volume of distribution was assumed to be 0.35 L/kg and the infusion rate was held constant at 250 μL/h and GFR was calculated using a previously described formula.15 Before the furosemide was administered, three samples of urine and serum were collected 30 min apart to confirm that iothalamate had reached a steady-state and to measure the baseline GFR (−1, −0.5 and 0 h). Additional serum samples were collected at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6 and 8 h after the diuretic was administered to measure the relative change in GFR caused by the furosemide plus midodrine or furosemide plus placebo. Urine losses were replaced for 8 h with equal volumes of one-half normal saline intravenously to prevent the volume depletion and the development of acute diuretic tolerance. (Note that the concentration of urinary sodium elicited by loop diuretics is reasonably approximated by 0.45% normal saline; thus, this methodology maintains volume status in the patient.) Twenty-four hours after the beginning of the acute study, patients were assessed to determine whether or not they are in negative sodium balance; if so, sodium losses were replaced with normal saline. Patients were continued on the diet until they attained a body weight and 24-h urinary sodium excretion within 0.5 kg and/or 20% respectively of that before the first arm of the study. This served as washout phase and allowed an individual patient to be in a comparable state of sodium balance before each phase of study and hence they served as their own control.
Assays
Serum and urine samples were measured for electrolytes, creatinine, iothalamate and study drugs. From these values, urinary excretion rates of solute, iothalamate and creatinine clearances were determined. The midodrine, furosemide and iothalamate levels were measured by HPLC using methods published previously.12–14, 16–19
Data analysis and statistical methods
Diuretic response was assessed by measuring urine volume, sodium excretion and by relating urinary furosemide excretion rate to sodium excretion rate. The primary end point was 6-h urinary sodium excretion.
The change in GFR was analysed as the mean maximal change between the two phases of the study. We measured the effect of midodrine co-administration on the response to furosemide (urinary volume and sodium excretion), the pharmacokinetics (elimination half-life, Vd, serum clearance) and pharmacodynamics (by relating the excretion rate of furosemide to the excretion rate of sodium) of the furosemide, and the renal haemodynamics (GFR by iothalamate clearance). We also examined the effects of midodrine co-administration on the pharmacokinetics of furosemide, natriuretic response to furosemide and the renal haemodynamics in patients with ascites. Descriptive statistics were used to describe the cohort and standard statistical tests (t-tests for continuous variables and chi-square for categorical variables) were used to test for statistical significance which was defined as P < 0.05.
Cross over studies tend to have the weakness of carryover effects. To obviate this, a washout period between two phases was incorporated as mentioned above. T1/2 of desglymidodrine, the active metabolite, is around 3–4 h and that for midodrine is around 25 min. Given wash out interval of 24–48 h in our study, we did not anticipate any carry-over effect theoretically. In our previous study,14 the mean difference between urinary sodium excretion with the premixed combination of furosemide and albumin premixed and furosemide alone was 9.24 ± 56.6 (s.d.). This difference was not statistically significant (P = 0.57). For the current study, we assumed that an absolute difference (increase) of 45 mmol for midodrine combined with furosemide was the smallest clinically significant difference worth detecting. A sample size of 15 subjects will provide an 80% power by a one-sided test at the 5% level. The power calculation assumed testing using a paired t-test. For this preliminary study, we assumed that the response to midodrine co-administration can be only in one direction (can only increase the urinary sodium) and thus one-sided t-test was used.
RESULTS
Patient characteristics
Sixteen patients were consented for the study, but one subject chose not to participate further during the initial equilibration phase. Fifteen patients (eight men, mean ± s.d. age: 52.7 ± 7.6 years) completed the study and their demographic details are given in Table 1. All patients had cirrhosis due to parenchymal liver disease with hepatitis C and/or previous alcohol consumption as the predominant aetiology. Their mean ± s.d. of Child–Pugh and MELD scores were 8.4 ± 1.4 and 12.1 ± 2.5 respectively. One patient did not receive iothalamate due to history of allergic reactions to iodinated contrast. Their mean ± s.d. duration of stay in GCRC was 8 ± 0.7 days and their mean ± s.d. duration for acquiring equilibration prior to midodrine and placebo phases was 3.4 ± 1.2 days and 3.4 ± 1.4 days respectively.
Table 1.
Selected demographic and clinical characteristics of study participants*
| n = 15 | |
|---|---|
| Age (years) | 52.7 ± 7.6 |
| Men | 8 |
| Weight at screening (kg) | 80.7 ± 14 |
| Systolic blood pressure (mmHg) | 114 ± 15.4 |
| Serum bilirubin (mg/dL) | 1.82 ± 0.9 |
| Serum albumin (gm/dL) | 3 ± 0.5 |
| Serum creatinine (mg/dL) | 1.06 ± 0.2 |
| BUN (mg/dL) | 15.6 ± 8 |
| Serum sodium (mmol/L) | 134.1 ± 5.34 |
| Child–Pugh score | 8.4 ± 1.4 |
| MELD score | 12.14 ± 2.50 |
Values represent mean ± s.d.
Effect of midodrine on response to furosemide
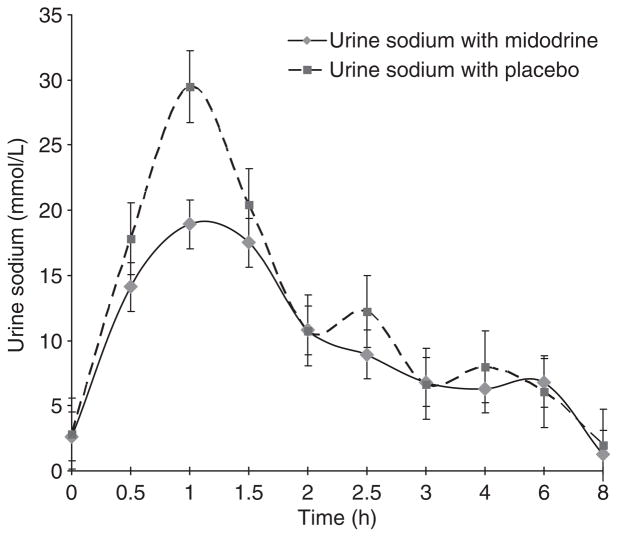
The effects of midodrine on the response to furosemide were assessed by measuring total urine volume and sodium excretion as well as urine sodium excretion rate over a 6-h period following furosemide administration with either midodrine or placebo pretreatment. Six-hour urine volume in midodrine-furosemide phase was 1770 ± 262 mL and was not significantly different from placebo-midodrine phase (1962 ± 170 mL, P = 0.25). Similarly, 6-h urine sodium excretion was 109 ± 42 mmol in midodrine-furosemide phase and was not significantly different from placebo-midodrine phase (126 ± 69 mmol, P = 0.7) (Table 2). Second, we examined the effect of midodrine on urinary sodium excretion rate following furosemide administration over a 6-h period. As shown in Figure 2, urinary sodium excretion rates were identical between two treatment phases (P-value by two-tailed t-test 0.9).
Table 2.
Effect of midodrine on responses to furosemide (from 0 to 6 h)
| Furosemide with placebo | Furosemide with midodrine | P value | |
|---|---|---|---|
| Total urine volume (mL) | 1962 ± 170 | 1770 ± 262 | 0.25 |
| Total urinary sodium (mMol) | 126 ± 69 | 109 ± 42 | 0.6 |
Figure 2.
Urinary sodium excretion rate produced by furosemide (given at time 0), with midodrine and placebo (administered 30 min before IV furosemide) in cirrhotic patients with ascites. There was no difference between two groups (P = 0.9).
Subsequently, we examined the effect of midodrine on GFR as measured by iothalamate clearance. As shown in Figure 3, GFR were identical between midodrine and placebo phases, suggesting that 15 mg of midodrine had no effect on GFR in this group of cirrhotic patients (P-value by two-tailed t-test 0.4).
Figure 3.
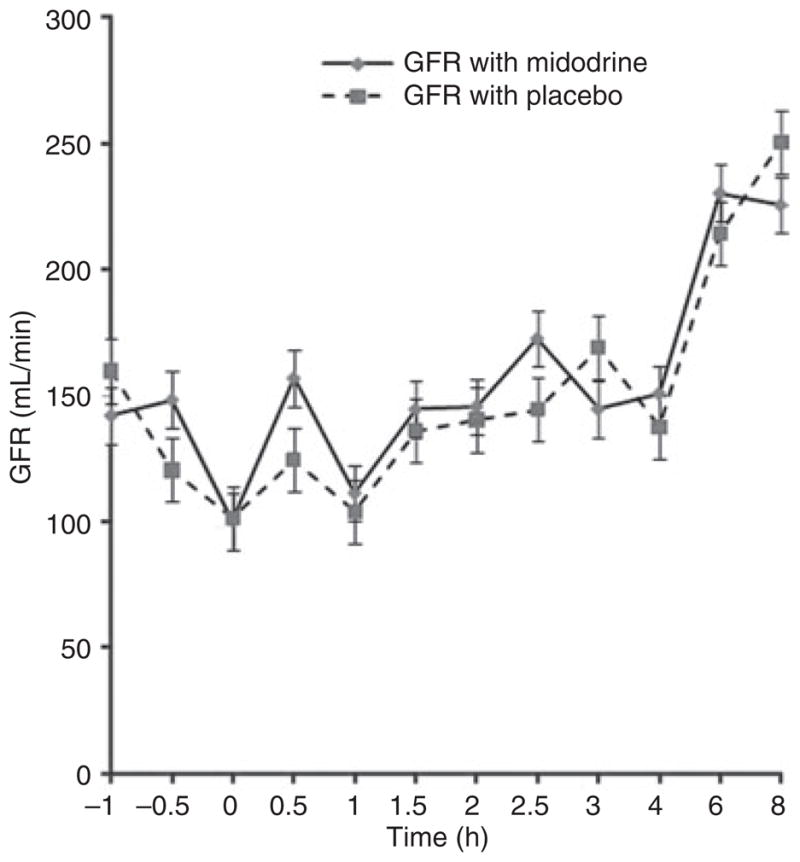
Glomerular filtration rate during the study period with midodrine and placebo. There was no difference between two groups (P = 0.4).
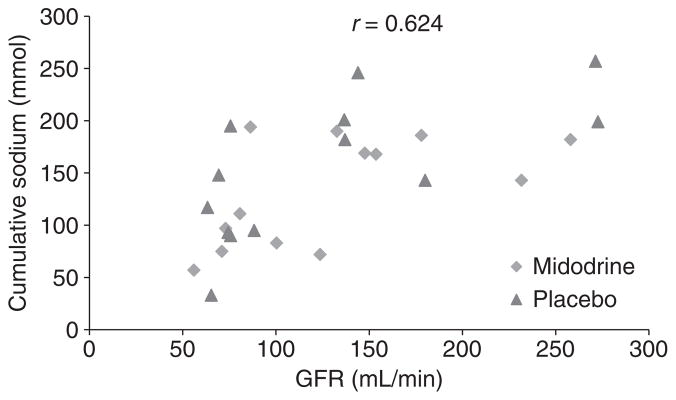
Our study reconfirmed the previously observed significant relationship between GFR and natriuretic response to furosemide. When baseline GFR was plotted against urinary sodium excretion, there was a statistically significant positive relationship between baseline GFR and natriuresis following furosemide administration (r = 0.62, P = 0.003) (Figure 4).
Figure 4.
Strong relationship between baseline glomerular filtration rate (GFR) and 6-h urinary sodium excretion following furosemide administration (r = 0.62, P = 0.003).
Furosemide pharmacokinetics
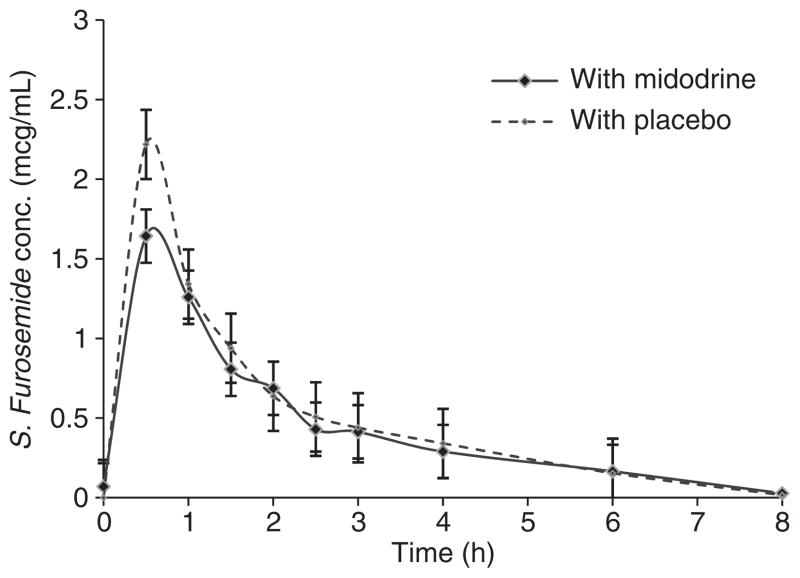
As expected, midodrine had no effect on furosemide pharmacokinetics. From Figure 5, it can be observed that serum furosemide concentrations were identical between midodrine and placebo pretreated phases.
Figure 5.
Furosemide pharmacokinetics with midodrine and placebo were not different (P = 0.7).
DISCUSSION
In our rigorously conducted randomized controlled study, single dose midodrine co-administration did not enhance the diuretic efficacy of furosemide. In addition, our study failed to show any significant effect on renal haemodynamics. Our study represents the first investigation that rigorously examined if midodrine enhances diuretic effects of furosemide.
Midodrine, a selective alpha-1 agonist approved by the FDA to treat symptomatic orthostatic hypotension, is increasingly being utilized in hepatology practice for a multitude of uses including in hepatorenal syndrome and cirrhosis-related haemodynamic complications.20 Although it is used extensively in clinical practice, studies that systematically examined its effect on renal haemodynamics or natriuresis are limited. In the classic study by Angeli et al.,12 an oral dose of 15 mg midodrine significantly improved renal haemodynamics including renal plasma flow and GFR and increased urinary sodium excretion in non-azotemic cirrhotics with ascites. In another study, Kalambokis et al.21 have examined the effect of a 7-day treatment with midodrine (10 mg t.i.d.) in non-azotemic cirrhotics with (n = 12) and without (n = 11) ascites. While midodrine improved systemic haemodynamics and natriuresis in both patients with and without ascites, suppression of renin-aldosterone system was noted only in those with ascites. On the basis of this observation, the authors speculated that improved natriuresis induced by midodrine is probably mediated through increased effective arterial blood volume. While both of these studies showed that midodrine by itself may promote natriuresis, they did not examine if it ameliorates the resistance to loop diuretics. There are additional studies which examined the effect of midodrine on renal haemodynamics, but in combination with octreotide and/or albumin.21, 22 While these are interesting studies, they do not specifically address if midodrine has any role in the management of ascites or in improving pharmacodynamic effect of furosemide. Our study confirmed the previously noted positive relationship between GFR and natriuresis induced by furosemide. We believe that midodrine lacked effect on furosemide because it did not improve GFR in our cohort of patients.
Strengths of our study include its robust study design and rigorously controlled setting in the GCRC where the study was performed. All patients had equilibration and washout phases, and because of the crossover study design, they served as their own controls minimizing any confounding caused by inter-individual variation. However, it suffers from some shortcomings that require further discussion. Although our study definitively excludes any significant effect that a single dose of midodrine would have on the diuretic efficacy of furosemide, it does not address if midodrine administered at a higher dosage or over a long-term would have a different effect on furosemide. In addition to GFR, ideally one would like to measure the effective renal plasma flow (ERPF) for precise estimation of renal haemodynamics. Para-aminohippurate (PAH), a substance commonly used to measure the ERPF, is not available for human usage in the United States. Given the fact that GFR correlates closely with ERPF, it is unlikely that inability to measure ERPF will be a significant limitation. Another limitation is the use of intravenous furosemide (as opposed to oral) in this experiment. To provide clinically relevant benefit, midodrine would have to enhance the diuretic effects of orally administered furosemide. However, the inter-individual variability in the absorption of oral furosemide is high and the number of patients needed to overcome such variability is prohibitively high for a proof-of-concept study such as this.
In summary, our study shows that a single dose of oral midodrine neither significantly increases the diuretic effects of intravenously administered furosemide nor improves GFR in cirrhotics with near normal GFR. Alternative strategies are needed to alleviate the resistance to loop diuretics. As diuretic effects of furosemide positively correlate with GFR, improving GFR would be an important avenue to explore.
Footnotes
Declaration of personal interests: V. Misra has recruited and followed up patients, abstracted data, wrote and edited the manuscript. R. Vuppalanchi and C. Kahi recruited and followed up patients and edited the manuscript. D. Jones has performed and analysed furosemide and iothalamate assays, and edited the manuscript. M. Hamman has performed assays and edited the manuscript. P. Kwo has edited the manuscript. N. Chalasani has developed concept, recruited and followed up subjects and edited the manuscript. Drs. Chalasani, Vuppalanchi and Kwo have multiple consulting agreements with pharmaceutical companies, but none represent a conflict to the current paper. Drs. Misra, Jones, Kahi and Hamman have no conflicts of interest.
Declaration of funding interests: This study was supported by American College of Gastroenterology Clinical Research Award (N.C.), National Institutes of Health Grant K24DK069290 (to NC) and M01RR000750 (to the General Clinical Research Center). The study was presented at as a President’s Plenary abstract at the 2008 American College of Gastroenterology annual meeting (Orlando) and VM is the recipient of the AstraZeneca Senior Research Fellow Award.
References
- 1.Hoyert DL, Heron MP, Murphy SL, et al. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57:1–135. [PubMed] [Google Scholar]
- 2.Sherlock S, Dooley J. Ascites. In: Sherlock S, editor. Diseases of the Liver and Biliary System. 11. London: Blackwell; 2002. p. 139. [Google Scholar]
- 3.Ruhl CE, Sayer B, Byrd-Holt DD, et al. Costs of digestive diseases. In: Everhart J, editor. The Burden of Digestive Diseases in the United States. Washington, DC: US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. US Government Printing Office NIH Publication No. 09-6443; 2008. pp. 137–46. [Google Scholar]
- 4.Runyon BA. Historical aspects of treatment of patients with cirrhosis and ascites. Semin Liver Dis. 1997;17:163–73. doi: 10.1055/s-2007-1007195. [DOI] [PubMed] [Google Scholar]
- 5.Runyon BA. Management of adult patients with ascites caused by cirrhosis. Hepatology. 1998;27:264–72. doi: 10.1002/hep.510270139. [DOI] [PubMed] [Google Scholar]
- 6.Arroyo V, Gines P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164–76. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 7.Brater DC. Diuretic therapy. N Engl J Med. 1998;339:387–95. doi: 10.1056/NEJM199808063390607. [DOI] [PubMed] [Google Scholar]
- 8.Villeneuve JP, Verbeeck RK, Wilkinson GR, et al. Furosemide kinetics and dynamics in patients with cirrhosis. Clin Pharmacol Ther. 1986;40:14–20. doi: 10.1038/clpt.1986.132. [DOI] [PubMed] [Google Scholar]
- 9.Sawhney VK, Gregory PB, Swezey SE, et al. Furosemide disposition in cirrhotic patients. Gastroenterology. 1981;81:1012–6. [PubMed] [Google Scholar]
- 10.Mirouze D, Zipser RD, Reynolds TB. Effect of inhibitors of prostaglandin synthesis on induced diuresis in cirrhosis. Hepatology. 1983;3:50–5. doi: 10.1002/hep.1840030108. [DOI] [PubMed] [Google Scholar]
- 11.Planas R, Arroyo V, Rimola A, et al. Acetylsalicylic acid suppresses the renal hemodynamic effect and reduces the diuretic action of furosemide in cirrhosis with ascites. Gastroenterology. 1983;84:247–52. [PubMed] [Google Scholar]
- 12.Angeli P, Volpin R, Piovan D, et al. Acute effects of the oral administration of midodrine, an alpha-adrenergic agonist, on renal hemodynamics and renal function in cirrhotic patients with ascites. Hepatology. 1998;28:937–43. doi: 10.1002/hep.510280407. [DOI] [PubMed] [Google Scholar]
- 13.Voelker JR, Cartwright-Brown D, Anderson S, et al. Comparison of loop diuretics in patients with chronic renal insufficiency. Kidney Int. 1987;32:572–8. doi: 10.1038/ki.1987.246. [DOI] [PubMed] [Google Scholar]
- 14.Chalasani N, Gorski JC, Horlander JC, Sr, et al. Effects of albumin/furosemide mixtures on responses to furosemide in hypoalbuminemic patients. J Am Soc Nephrol. 2001;12:1010–6. doi: 10.1681/ASN.V1251010. [DOI] [PubMed] [Google Scholar]
- 15.Al-Uzri A, Holliday MA, Gambertoglio JG, et al. An accurate practical method for estimating GFR in clinical studies using a constant subcutaneous infusion. Kidney Int. 1992;41:1701–6. doi: 10.1038/ki.1992.243. [DOI] [PubMed] [Google Scholar]
- 16.Chennavasin P, Seiwell R, Brater DC. Pharmacokinetic-dynamic analysis of the indomethacin-furosemide interaction in man. J Pharmacol Exp Ther. 1980;215:77–81. [PubMed] [Google Scholar]
- 17.Chennavasin P, Seiwell R, Brater DC, et al. Pharmacodynamic analysis of the furosemide-probenecid interaction in man. Kidney Int. 1979;16:187–95. doi: 10.1038/ki.1979.120. [DOI] [PubMed] [Google Scholar]
- 18.Kaojarern S, Day B, Brater DC. The time course of delivery of furosemide into urine: an independent determinant of overall response. Kidney Int. 1982;22:69–74. doi: 10.1038/ki.1982.134. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal R. Chromatographic estimation of iothalamate and p-aminohippuric acid to measure glomerular filtration rate and effective renal plasma flow in humans. J Chromatogr B Biomed Sci Appl. 1998;705:3–9. doi: 10.1016/s0378-4347(97)00497-0. [DOI] [PubMed] [Google Scholar]
- 20.Karwa R, Woodis CB. Midodrine and octreotide in treatment of cirrhosis-related hemodynamic complications. Ann Pharmacother. 2009;43:692–9. doi: 10.1345/aph.1L373. [DOI] [PubMed] [Google Scholar]
- 21.Kalambokis G, Fotopoulos A, Economou M, et al. Effects of a 7-day treatment with midodrine in non-azotemic cirrhotic patients with and without ascites. J Hepatol. 2007;46:213–21. doi: 10.1016/j.jhep.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Tandon P, Ross TT, Lesley M, et al. The effect of 1 month of therapy with midodrine, octreotide-LAR and albumin in refractory ascites: a pilot study. Liver Int. 2009;29:169–74. doi: 10.1111/j.1478-3231.2008.01778.x. [DOI] [PubMed] [Google Scholar]