Abstract
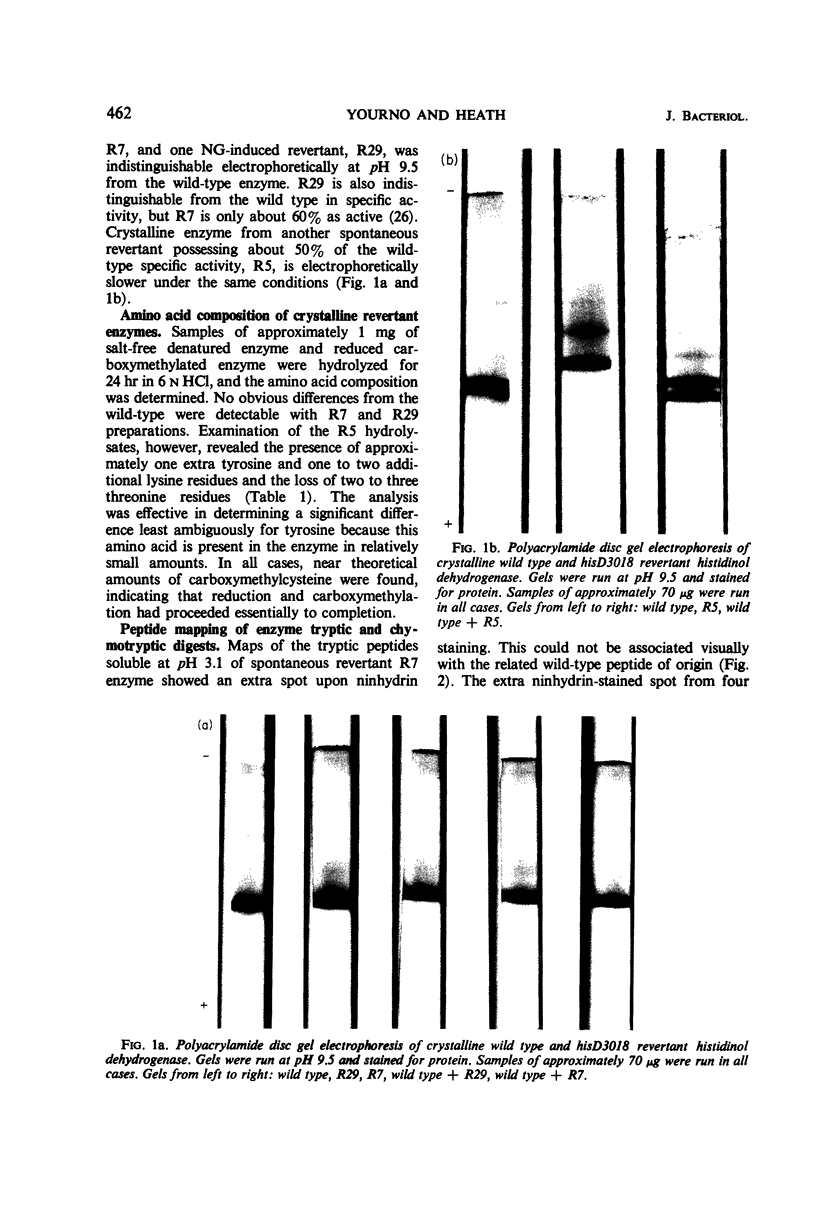
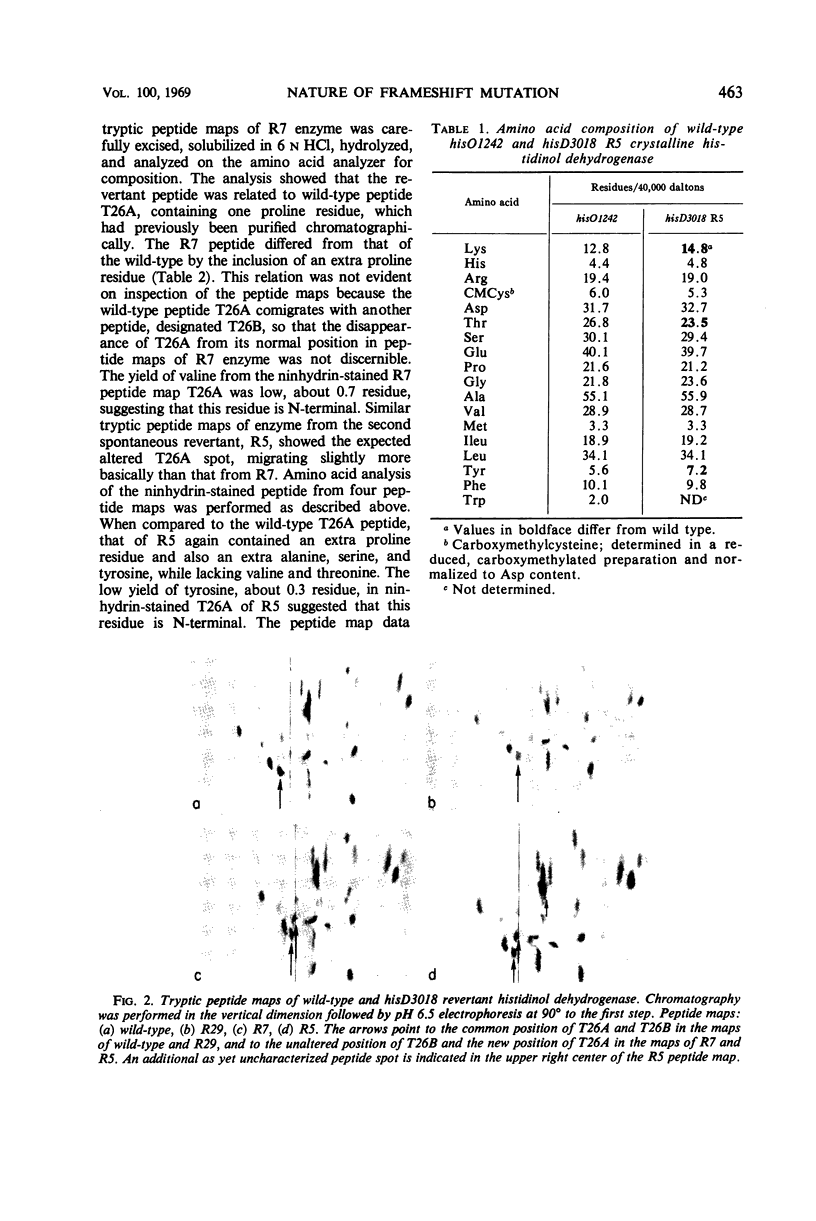
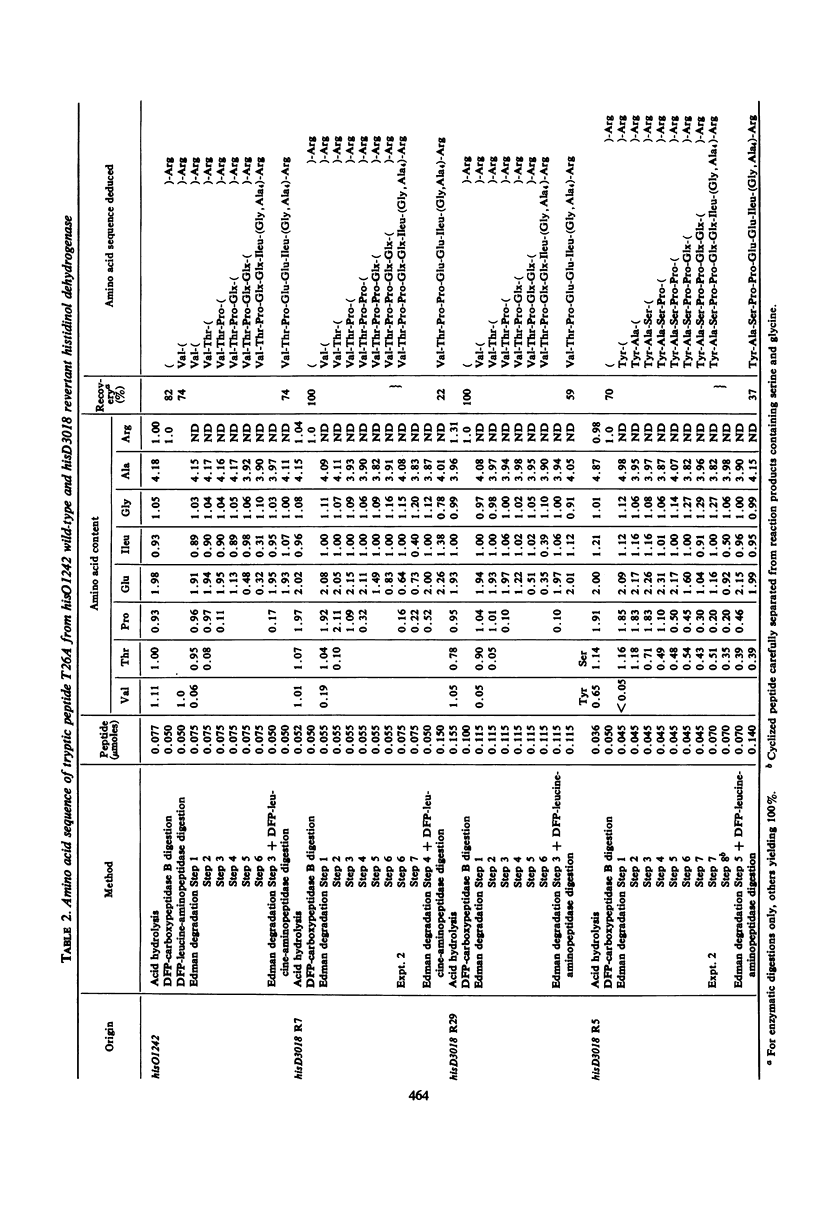
Histidinol dehydrogenase from three differing revertants of ICR-191A-induced frameshift hisD3018 has been purified and examined for amino acid replacements. The enzyme from one spontaneously arising revertant, R7, contains an extra proline residue, whereas that from another, R5, contains an extensive frameshifted sequence, four amino acid residues of which have been identified to date. The amino acid replacement data are in agreement with the in vitro code word assignments and allow the characterization of the hisD3018 frameshift as an addition of one nucleotide pair, most likely guanine plus cytosine. Enzymatic data for those ICR-191A-induced revertants of hisD3018 arising within the hisD gene indicate that the enzyme is wild type and, therefore, that ICR-191A can cause deletions as well as additions of single base pairs. The wild-type amino acid sequence is restored in enzyme from an N-methyl-N′-nitro-N-nitrosoguanidine (NG)-induced revertant, R29, suggesting that NG is a base-deleting as well as a base-substituting mutagen. The unusual response of hisD3018 to external suppressors is considered in terms of reinitiation of protein synthesis out of phase, coupled with suppression of a nonpermissive missense codon so generated, and of an alternative hypothesis invoking a true frameshift suppressor transfer ribonucleic acid with an extended or deleted anticodon.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger H., Brammar W. J., Yanofsky C. Analysis of amino acid replacements resulting from frameshift and missense mutations in the tryptophan synthetase A gene of Escherichia coli. J Mol Biol. 1968 Jul 14;34(2):219–238. doi: 10.1016/0022-2836(68)90248-9. [DOI] [PubMed] [Google Scholar]
- Berger H., Brammar W. J., Yanofsky C. Spontaneous and ICR191-A-induced frameshift mutations in the A gene of Escherichia coli tryptophan synthetase. J Bacteriol. 1968 Nov;96(5):1672–1679. doi: 10.1128/jb.96.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brammar W. J., Berger H., Yanofsky C. Altered amino acid sequences produced by reversion of frameshift mutants of tryptophan synthetase A gene of E. coli. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1499–1506. doi: 10.1073/pnas.58.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRICK F. H., BARNETT L., BRENNER S., WATTS-TOBIN R. J. General nature of the genetic code for proteins. Nature. 1961 Dec 30;192:1227–1232. doi: 10.1038/1921227a0. [DOI] [PubMed] [Google Scholar]
- Carbon J., Berg P., Yanofsky C. Studies of missense suppression of the tryptophan synthetase A-protein mutant A36. Proc Natl Acad Sci U S A. 1966 Aug;56(2):764–771. doi: 10.1073/pnas.56.2.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstark A., Eisenstark R., Van Sickle R. Mutation of Salmonella typhimurium by nitrosoguanidine. Mutat Res. 1965 Feb;2(1):1–10. doi: 10.1016/0027-5107(65)90002-3. [DOI] [PubMed] [Google Scholar]
- Engelhardt D. L., Webster R. E., Wilhelm R. C., Zinder N. In vitro studies on the mechanism of suppression of a nonsense mutation. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1791–1797. doi: 10.1073/pnas.54.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H. M., Abelson J., Landy A., Brenner S., Smith J. D. Amber suppression: a nucleotide change in the anticodon of a tyrosine transfer RNA. Nature. 1968 Mar 16;217(5133):1019–1024. doi: 10.1038/2171019a0. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Zipser D. A mutation which creates a new site for the re-initiation of polypeptide synthesis in the z gene of the lac operon of Escherichia coli. J Mol Biol. 1968 Dec;38(3):305–314. doi: 10.1016/0022-2836(68)90388-4. [DOI] [PubMed] [Google Scholar]
- Gupta N. K., Khorana H. G. Missense suppression of the tryptophan synthetase A-protein mutant A78. Proc Natl Acad Sci U S A. 1966 Aug;56(2):772–779. doi: 10.1073/pnas.56.2.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana H. G., Büchi H., Ghosh H., Gupta N., Jacob T. M., Kössel H., Morgan R., Narang S. A., Ohtsuka E., Wells R. D. Polynucleotide synthesis and the genetic code. Cold Spring Harb Symp Quant Biol. 1966;31:39–49. doi: 10.1101/sqb.1966.031.01.010. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- Nirenberg M., Caskey T., Marshall R., Brimacombe R., Kellogg D., Doctor B., Hatfield D., Levin J., Rottman F., Pestka S. The RNA code and protein synthesis. Cold Spring Harb Symp Quant Biol. 1966;31:11–24. doi: 10.1101/sqb.1966.031.01.008. [DOI] [PubMed] [Google Scholar]
- RICHARDS F. M., VITHAYATHIL P. J. The preparation of subtilisn-modified ribonuclease and the separation of the peptide and protein components. J Biol Chem. 1959 Jun;234(6):1459–1465. [PubMed] [Google Scholar]
- Riyasaty S., Atkins J. F. External suppression of a frameshift mutant in salmonella. J Mol Biol. 1968 Jun 28;34(3):541–557. doi: 10.1016/0022-2836(68)90179-4. [DOI] [PubMed] [Google Scholar]
- Singer B., Fraenkel-Conrat H. Chemical modification of viral RNA. VI. The action of N-methyl-N'-nitro-N-nitrosoguanidine. Proc Natl Acad Sci U S A. 1967 Jul;58(1):234–239. doi: 10.1073/pnas.58.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Jacob F., Monod J. On the subunit structure of wild-type versus complemented beta-galactosidase of Escherichia coli. J Mol Biol. 1968 Feb 28;32(1):1–13. doi: 10.1016/0022-2836(68)90140-x. [DOI] [PubMed] [Google Scholar]
- Whitfield H. J., Jr, Martin R. G., Ames B. N. Classification of aminotransferase (C gene) mutants in the histidine operon. J Mol Biol. 1966 Nov 14;21(2):335–355. doi: 10.1016/0022-2836(66)90103-3. [DOI] [PubMed] [Google Scholar]
- Yourno J., Barr D., Tanemura S. Externally suppressible frameshift mutant of Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):453–459. doi: 10.1128/jb.100.1.453-459.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourno J. Composition and subunit structure of histidinol dehydrogenase from Salmonella typhimurium. J Biol Chem. 1968 Jun 25;243(12):3277–3288. [PubMed] [Google Scholar]
- Yourno J., Ino I. Purification and crystallization of histidinol dehydrogenase from Salmonella typhimurium LT-2. J Biol Chem. 1968 Jun 25;243(12):3273–3276. [PubMed] [Google Scholar]





