Introduction
Pathological and imaging studies indicate brain volume is reduced in schizophrenia; however, the timing and nature of the processes involved remains uncertain (Roberts 1991). If smaller brain volumes were present from birth, as suggested by neurodevelopmental hypotheses, head size should also be smaller in schizophrenia (Stevens 1994). Despite debate and commentary in the literature, the number of direct studies of head size in schizophrenia is relatively few. Measurements of cerebral volume in imaging studies are variable; these may include just brain parenchyma, all intracranial contents, and occasionally the skull. Recent studies indicate that sex, height, and ethnicity are important factors to consider when head size is assessed (Bale et al 1991; Bushby et al 1992). The current study is a preliminary investigation of 100 schizophrenic subjects, directly assessing head size using standard methods and controlling for effects of sex, height, and ethnicity. The hypothesis was that schizophrenic subjects would have smaller than normal head sizes, and that microcephaly (< 3rd centile) would be more common than macrocephaly (> 97th centile).
Materials and Methods
The patient group consisted of inpatients at three chronic psychiatric hospitals (67 male, 33 female; 17–86 years old), who met DSM-III-R criteria for chronic schizophrenia or schizoaffective disorder and provided informed consent. Published control values of adult head circumference for height were used (Bushby et al 1992). A local volunteer control group (37 men, 37 women; 22–71 years old), was also used. Subjects and controls were Caucasian; those with medical disorders associated with abnormalities in head size, e.g., spina bifida, mental handicap, and non-Caucasians were excluded. Maximum occipitofrontal head circumference (OFC) was measured to the nearest 0.1 cm using a nonstretch tape measure. Height was measured with the subject standing straight up without shoes, or from height recorded on admission (n = 29).
Chi-square tests were used to determine significant differences from expected frequencies from centile charts (Bushby et al 1992). Two-tailed Student’s t tests were used to compare differences between patients and volunteer controls on mean OFC, height, and age. Analyses of covariance were used to examine the effect of diagnosis on OFC, controlling for height. Correlations of OFC with age and years of education were assessed using Pearson’s r.
Computerized tomography (CT) scans were available for a subsample of 18 subjects (13 male, 5 female). These were studied using a standardized rating scale developed to assess ventricular and cortical cerebrospinal fluid spaces in schizophrenia (Flynn et al 1996). An analysis of covariance was performed to determine the effect of head circumference on lateral and third ventricle, and cortical sulcal size after controlling for age and height. Only scans from male subjects were considered, since there were too few female subjects for analyses.
Results
Comparison to Normal Adult Centile Charts
Head circumference for height values for male (Figure 1) and female (Figure 2) subjects are displayed on Bushby’s centile charts (Bushby et al 1992). Significantly more (n = 10, 14.9%) male patients than expected had OFC for height greater than the 97th centile (chi-square = 32.74, df = 1, p< .0001); large head sizes occurred across all heights. Findings were similar for OFC for height greater than the 50th centile (n = 48, 71.6%; chi-square = 12.55, df = 1, p = .0004). In contrast, only 2 female patients (6.1%) had an OFC for height greater than the 97th centile (chi-square = 1.06, df = 1, p = .30), and 22 (66.7%) were at or above the 50th centile (chi-square = 3.67, df = 1, p = .055). Results for microcephaly were not significantly different from expected for either male (chi-square = 0.52, df = 1, p = .47) or female (chi-square = 1.02, df = 1, p = .31) subjects.
Figure 1.

Head circumference for height in 67 male subjects with schizophrenia (●) plotted on normative centile bands for Caucasian male subjects (Bushby et al 1992).
Figure 2.
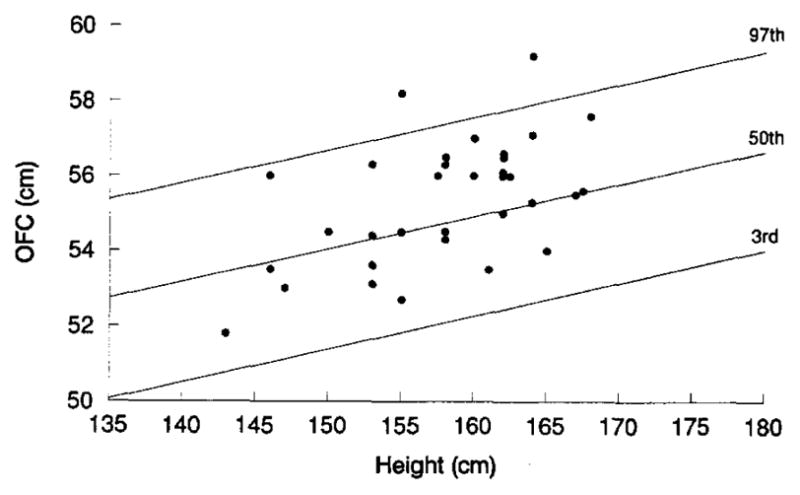
Head circumference for height in 33 female subjects with schizophrenia (●) plotted on normative centile bands for Caucasian female subjects (Bushby et al 1992).
Comparison to Volunteer Control Sample
Mean OFC for male patients was significantly greater than for male controls: 58.64 cm (SD 1.64) vs. 57.61 cm (SD 1.66) (t = −3.05, df = 102, p = .0029). Mean height was not different between male patients and controls: 176.32 cm (SD 8.08) and 174.03 cm (SD 8.56) (t = −1.36,df = 102,p = .18). In contrast, female patients had shorter mean height than controls (157.92 cm, SD 6.54, vs. 162.84 cm, SD 6.18; t = 3.23, df = 68, p = .0019), but did not differ on mean OFC: 55.34 cm (SD 1.66) vs. 55.78 cm (SD 1.43) (t = 1.21, df = 68, p = .23). Analyses of covariance after adjusting for height revealed diagnostic status to be a significant factor for head circumference in male (F = 7.25, df = 1,p = .008) but not female (F = .01, df = 1, p = .91) subjects. Patients and controls did not differ in mean age: 41.6 years (SD 14.5) and 39.9 years (SD 10.7) (t = −.90, df = 172, p = .37). There was no significant correlation of head circumference with age (r = .12, p = .31) or years of education (r = .072, p = .55) in controls.
CT Male Patient Subsample: Relationship of Head Circumference to Brain Structure
The subsample included 3 subjects with OFC above the 97th centile. OFC was directly related to size of the lateral ventricle (F = 13.86, p = .005) and cortical sulci (F = 6.63, p = .03) after controlling for age and height. There was no significant relationship between OFC and third ventricle size.
Discussion
Our hypothesis that patients with schizophrenia would have smaller head sizes was not supported. Significantly more male patients had larger than expected OFC for height; 1 in every 6 had relative macrocephaly. Sex differences were prominent. Diagnosis was not a significant factor in female head size. Results were similar using two different control groups, and distributions appeared unimodal. These results are consistent with previous reports suggesting cranial size is increased (Weinberger et al 1987) or at least is not decreased in schizophrenia (Andreasen et al 1990; Breier et al 1992; DeMyer et al 1988; Jernigan et al 1991; Mathew et al 1985; Pearlson et al 1991), particularly in male subjects (Andreasen et al 1990; Jernigan et al 1991; Mathew et al 1985; Reveley and Reveley 1987).
The findings are unlikely to have resulted from systematic errors in measurement, since OFC was measured using standard methods and raters at three different sites. Also, the largest previous study using direct head circumference measurements (Weinberger et al 1987) reported mean values very close to those for male subjects and controls in the current study. Even if height was systematically underestimated by 2.5 cm, results for male subjects would continue to be significant; however, methodological differences between this and other studies (Andreasen et al 1986; Grove et al 1991; Ingraham et al 1991; Jones and Lewis 1991; Pearlson et al 1989; Zipursky et al 1991) could account for variable findings, since a range of brain imaging and morphometric assessments were used.
What could cause a finding of no decreased head size, despite evidence for reduced brain tissue volume in schizophrenia? Since in schizophrenia head circumference at birth is no different from controls in male but is smaller in female subjects (McNeil et al 1993) (the same direction as sex differences in the current study), changes in head and/or brain size likely occur during maturation. Early brain injuries may cause skull thickening and increased ventricular and sulcal fluid in some cases (Dyke et al 1933). Of interest in this regard was the increased lateral ventricular and cortical sulcal size associated with increased OFC in a subsample of the present study; however, because of the small sample size this finding must be regarded as very preliminary, and only a contribution to the development of a testable hypothesis. As well, brain tissue reductions on a regional or global basis could also occur later during maturation, after head size is established (Stevens 1994).
Alternatively, observed head size increases may be due to overgrowth, secondary to pleiotropic expression of a developmental gene, as in neurofibromatosis type 1 or fragile X syndrome. In general, macrocephaly is not necessarily apparent at birth, becomes established and progresses during childhood, and is more often seen in male subjects (Lorber and Priestly 1981; Riccardi 1992). Another possibility is that acquired factors could enlarge head size after onset of schizophrenia by some unknown mechanism.
This study confirms the importance of controlling for sex and height when studying adult head size. Further studies are needed to investigate the following issues: brain imaging in subjects with increased head size, replication in other ethnic groups, association of increased head size with other abnormalities of craniofacial development, macrocephaly as a subtype, and head size in families. Learning more about the trajectory of changes in both head and brain size during development may help contribute to solving some of the mysteries regarding the origins and pathogenesis of schizophrenia.
Acknowledgments
The study was supported by the Scottish Rite Research Program and Canadian Psychiatric Research Foundation. Dr. Honer was supported by the B.C. Health Research Foundation.
The authors thank Jacqueline McAlduff, RN and Rochelle Roy, RN for help with data collection, and the staff of the Queen Street Mental Health Centre and Refractory Psychosis Program of Riverview Hospital.
References
- Andreasen NA, Nasrallah HA, Dunn V, et al. Structural abnormalities in the frontal system in schizophrenia. Arch Gen Psychiatry. 1986;43:136–144. doi: 10.1001/archpsyc.1986.01800020042006. [DOI] [PubMed] [Google Scholar]
- Andreasen NA, Ehrhardt JC, Swayze VW, et al. Magnetic resonance imaging of the brain in schizophrenia. Arch Gen Psychiatry. 1990;47:35–44. doi: 10.1001/archpsyc.1990.01810130037006. [DOI] [PubMed] [Google Scholar]
- Bale SJ, Amos CI, Parry DM, et al. Relationship between head circumference and height in normal adults and in the nevoid basal cell carcinoma syndrome and neurofibromatosis type I. Am J Med Genet. 1991;40:206–210. doi: 10.1002/ajmg.1320400217. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan R, Elkashef A, et al. Brain morphology and schizophrenia—A magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch Gen Psychiatry. 1992;49:921–926. doi: 10.1001/archpsyc.1992.01820120009003. [DOI] [PubMed] [Google Scholar]
- Bushby KMD, Cole T, Matthews JNS, et al. Centiles for adult head circumference. Arch Dis Child. 1992;67:1286–1287. doi: 10.1136/adc.67.10.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMyer MK, Gilmor RL, Hendrie HC, et al. Magnetic resonance brain images in schizophrenic and normal subjects: Influence of diagnosis and education. Schizophr Bull. 1988;14:21–37. doi: 10.1093/schbul/14.1.21. [DOI] [PubMed] [Google Scholar]
- Dyke CG, Davidoff LM, Masson CB. Cerebral hemiatrophy with homolateral hypertrophy of the skull and sinuses. Surg Gynecol Obstet. 1933;57:588–600. [Google Scholar]
- Flynn SW, Falkai P, Altman S, et al. The Computerized Tomography Rating Scale for Schizophrenia (CTRSS): Validity and reliability [abstract] Schizophr Res. 1996;18:192. [Google Scholar]
- Grove WM, Lebow BS, Medus C. Head size in relationship to schizophrenia and schizotypy. Schizophr Bull. 1991;17:157–161. doi: 10.1093/schbul/17.1.157. [DOI] [PubMed] [Google Scholar]
- Ingraham LJ, Bridge TP, Parker E, et al. Cerebral size affects localized density measured by computed tomography. Arch Gen Psychiatry. 1991;48:178–179. doi: 10.1001/archpsyc.1991.01810260086013. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Zisook S, Heaton RK, et al. Magnetic resonance imaging abnormalities in lenticular nuclei and cerebral cortex in schizophrenia. Arch Gen Psychiatry. 1991;48:881–890. doi: 10.1001/archpsyc.1991.01810340013002. [DOI] [PubMed] [Google Scholar]
- Jones GH, Lewis JE. Head circumference in elderly long-stay patients with schizophrenia. Br J Psychiatry. 1991;159:435–438. doi: 10.1192/bjp.159.3.435. [DOI] [PubMed] [Google Scholar]
- Lorber J, Priestly BL. Children with large heads: A practical approach to diagnosis in 557 children, with special reference to 109 children with megalencephaly. Dev Med Child Neurol. 1981;23:494–504. doi: 10.1111/j.1469-8749.1981.tb02023.x. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Partain CL, Prakash R, et al. A study of the septum pellucidum and corpus callosum in schizophrenia with MR imaging. Acta Psychiatr Scand. 1985;72:414–421. doi: 10.1111/j.1600-0447.1985.tb02634.x. [DOI] [PubMed] [Google Scholar]
- McNeil TF, Cantor-Graae E, Nordstrom LG, et al. Head circumference in ‘preschizophrenic’ and control neonates. Br J Psychiatry. 1993;162:517–523. doi: 10.1192/bjp.162.4.517. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Kim WS, Kubos KL, et al. Ventricle-brain ratio, computed tomographic density, and brain area in 50 schizophrenics. Arch Gen Psychiatry. 1989;46:690–697. doi: 10.1001/archpsyc.1989.01810080020003. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Barta PE, Schraml FV, et al. Reply to “Brain size in schizophrenia” by R.B. Zipursky et al. Arch Gen Psychiatry. 1991;48:180–181. [Google Scholar]
- Reveley MA, Reveley AM. Hat size in schizophrenia [letter] Arch Gen Psychiatry. 1987;44:674. [PubMed] [Google Scholar]
- Riccardi VM. Neurofibromatosis: Phenotype, Natural History, and Pathogenesis. Baltimore: Johns Hopkins University Press; 1992. [Google Scholar]
- Roberts GW. Schizophrenia: A neuropathological perspective. Br J Psychiatry. 1991;158:8–17. doi: 10.1192/bjp.158.1.8. [DOI] [PubMed] [Google Scholar]
- Stevens JR. Brain atrophy or dystrophy in schizophrenia: When did it happen? Arch Gen Psychiatry. 1994;51:927. doi: 10.1001/archpsyc.1994.03950110087013. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Iadarola M, et al. Hat size in schizophrenia [letter] Arch Gen Psychiatry. 1987;44:672. [PubMed] [Google Scholar]
- Zipursky RB, Lim KO, Pfefferbaum A. Brain size in schizophrenia [letter] Arch Gen Psychiatry. 1991;48:179–180. [Google Scholar]


