Abstract
Objectives. We examined the effects of a brief counseling intervention designed to reduce HIV risk behaviors and sexually transmitted infections (STIs) among patients receiving STI services in Cape Town, South Africa.
Methods. After randomization to either a 60-minute risk reduction counseling session or a 20-minute HIV–STI educational session, patients completed computerized sexual behavior assessments. More than 85% of the participants were retained at the 12-month follow-up.
Results. There were 24% fewer incident STIs and significant reductions in unprotected vaginal and anal intercourse among participants who received risk reduction counseling relative to members of the control condition. Moderator analyses showed shorter lived outcomes for heavy alcohol drinkers than for lighter drinkers. The results were not moderated by gender.
Conclusions. Brief single-session HIV prevention counseling delivered to STI clinic patients has the potential to reduce HIV infections. Counseling should be enhanced for heavier drinkers, and sustained outcomes will require relapse prevention techniques. Disseminating effective, brief, and feasible behavioral interventions to those at highest risk for HIV infection should remain a public health priority.
Although South Africa has less than 1% of the world's population, it accounts for nearly 10% of the global burden of AIDS. It is estimated that currently 5.5 million South Africans (12.3% of the country's total population of 44.8 million) are infected with HIV.1 A number of different factors probably account for the high incidence of HIV in South Africa, including sexual mixing patterns, social migration, high rates of alcohol abuse, sexual coercion in relationships characterized by gender power imbalances, and delayed rollout of HIV prevention programs.2–5
Perhaps most critical in driving HIV infections are other co-occurring sexually transmitted infections (STIs), which increase susceptibility to HIV by degrading naturally protective mucosal immunological mechanisms, migrating vulnerable cells to the genital tract, and affording HIV a portal of entry into the bloodstream. STIs also facilitate transmission of the virus from HIV-infected partners by increasing their HIV infectiousness.6 As a result of these factors, in combination with high HIV prevalence rates, South Africans who contract STIs are among the highest-risk populations for HIV infection in the world.2
Although behavioral interventions have been shown to be effective in reducing sexual risks among STI clinic patients,7 several of these interventions have relied on multiple group sessions that have proven difficult to implement.8,9 In response to the urgent need for effective, feasible, and affordable interventions designed to prevent HIV among STI clinic patients, researchers have developed brief single-session HIV risk reduction counseling interventions intended for use in both resource-rich10–13 and resource-poor STI clinics.14 When performed in conjunction with HIV testing, brief prevention counseling has shown promise in reducing sexual risk behaviors and decreasing STIs.15,16
Brief risk reduction counseling has also demonstrated promising outcomes when delivered outside of HIV testing. For example, Crosby et al.17 examined a single-session personalized counseling intervention for men receiving STI clinic services in the United States. The intervention led to increases in condom use, reductions in unprotected sex, reductions in sexual partners, and 38% fewer new STI diagnoses relative to a standard of care control group. Overall, single-session sexual risk reduction counseling can be as effective as interventions that require multiple sessions and consume far greater resources.7,18
The brief risk reduction counseling intervention reported here is grounded in cognitive–behavioral theories of health behavior change and is designed for use with all STI patients, including those who refuse HIV testing. We previously tested this intervention in a small trial conducted in Cape Town, South Africa. We observed a 63% reduction in unprotected vaginal and anal intercourse over a 6-month follow-up period, compared with the 30% reduction observed in an HIV education control condition.19 In addition, condom use among participants increased from 65% to 88%. The overall findings were promising and suggested that a brief single-session counseling intervention may be effective in reducing the risk of HIV and other STIs in South Africa.
We report the outcomes of a randomized clinical trial designed to test the effects of a brief single-session risk reduction counseling session intended for use in resource-poor STI clinics. We hypothesized that brief theory-based risk reduction counseling sessions would reduce unprotected vaginal and anal intercourse and prevent STIs during 12 months of observation. We also examined potential moderators of the intervention effects. We included participant gender as a factor in the analyses because there are differences in STI risks between men and women, especially given the gender dynamics in sexual relationships and that men ultimately control the use of condoms. We also tested alcohol use and use of other drugs as moderators of risk reduction outcomes because they are known cofactors for HIV transmission risk behaviors in South Africa.20,21
METHODS
Participants were 414 men and 203 women receiving services at an urban STI clinic in Cape Town, South Africa. The participating clinic is one of the largest public STI clinics in Cape Town. Patients historically have visited this clinic from areas throughout Cape Town because they are assured greater confidentiality than they are at neighborhood clinics. The patient population is approximately 25% female, and 90% of patients are indigenous (Black) Africans. Approximately half of all patients have previously received STI services. The estimated HIV prevalence among clinic patients is 25%, based on reactive tests among the approximately 50% of patients who accept HIV testing.
Participant Recruitment and Enrollment
The study activities commenced in August 2005, and enrollment occurred between February 2006 and June 2007. Potential participants were STI patients referred by a nurse clinician to participate in a prevention study that involved receiving a single counseling session and completing follow-up assessments over 12 months. To be referred for the study, patients were required to be 18 years old or older and to have been seen at the clinic for STI diagnostic or treatment services. Patients who elected to enroll in the study were scheduled for and completed a computerized baseline assessment and a single counseling session. Active recruitment procedures were used, and sampling occurred throughout all hours of clinic operation.
Outcomes from previous HIV risk reduction counseling studies involving a model similar to that used in the current study suggested a 25% reduction in recurring STIs.9,15 At an alpha level of 0.05, a sample size of 610 was determined sufficient to allow for the detection of intervention effects on incident STIs with a power of 0.80.
Study Design and Procedures
STI clinic patients were initially screened with a single-page survey that collected basic demographic information. Patients who met the entry criteria were offered the opportunity to enroll in the trial. Participants completed baseline assessments administered via audio computer-assisted self-interview (ACASI) techniques.
Immediately after the baseline assessment, participants were randomly assigned to receive either the experimental 60-minute behavioral skill-building HIV risk reduction counseling session or a 20-minute HIV educational control intervention. Participants were scheduled for follow-up assessments 1, 3, 6, 9, and 12 months after counseling. Participants received 100 South African rand (approximately $10) as compensation for returning to the clinic and completing the baseline assessments. Payments escalated incrementally to 200 rand at the 12-month follow-up.
Randomization and Blinding
The study recruitment and scheduling staff used a pregenerated list of appointment times to assign participants to the experimental or the control condition. Participants were enrolled in the study and assigned to the next time slot available for a baseline assessment. Participants who returned to the clinic for their baseline assessment were then assigned to either the experimental or control condition via a pregenerated assignment scheme. Assignment was not breached throughout the trial. Recruitment, screening, and assessment staff remained blinded to condition throughout the study, and counselors never conducted assessments.
Intervention Conditions
Experimental condition: Brief theory-based HIV risk reduction skills counseling.
The experimental intervention was grounded in the information–motivation–behavioral skills model of behavior change.22 As a means of protecting against counselor drift, the intervention was completely manualized, and a tabletop flipchart guided the counselor and the participant through the session content. As described elsewhere,19 the information component of the counseling (20 minutes in duration) reviewed facts about HIV transmission and risk behaviors, discussed the local prevalence of HIV, clarified misconceptions, dispelled myths about AIDS, and described HIV antibody testing. After participants had reviewed how people contract HIV, attention turned to their own personal risks for HIV infection.
The motivation component (20 minutes in duration) integrated motivational counseling techniques that included motivation for change and strengthening commitment to change. Addressing alcohol use as a risk factor was embedded within the motivational counseling component. The intervention included the World Health Organization's brief alcohol counseling model as the basis for alcohol risk reduction.23,24 Participants were given their baseline Alcohol Use Disorders Identification Test (AUDIT) score as feedback and shown how the score represents potential drinking hazards. Alcohol risk reduction was tailored to the level of drinking indicated by the AUDIT score. Decisional balance techniques, including confidence and perceived importance of reducing alcohol-related risks, were used to elicit self-motivating statements for alcohol reduction.
The final component of the risk reduction counseling was behavioral self-management and sexual communication skill building (20 minutes in duration). Counselors engaged participants in a functional analysis of their risk by having them discuss personal risk situations and identify cues related to their sexual risks. Counselors taught participants how to recognize environmental and cognitive–affective cues that serve as triggers for high-risk situations, including mood states, substance use, and sexual partner characteristics. Participants were asked to think of ways to manage triggers that might contribute to their personal risk and were taught strategies to reduce their risk by redirecting sexual activities toward safer sex alternatives, carrying condoms, and avoiding sex after drinking.
Behavioral rehearsal role-plays were used to enhance risk reduction skills. Correct male and female condom use was also demonstrated and modeled, allowing participants to practice condom application on wooden anatomical models with corrective feedback from the counselor. The session ended with participants creating personalized goals and a risk reduction plan that they took with them.
Control condition: HIV information and education.
The active control condition was an HIV–STI education counseling session that consisted of the same 20 minutes of HIV–STI information included in the first part of the experimental intervention. This session represented a didactic educational experience similar to that used in past research.15,16 This condition was also manualized and used a tabletop flipchart to guide the session. The session ended by soliciting questions from participants and providing them with a written information summary that they took with them.
Counselor Training and Intervention Quality Assurance
The counselors were one African man and one African woman with minimal counseling experience outside the study protocol. The same pair of counselors delivered both the experimental and control interventions to avoid confounding counselors with treatment conditions. Both counselors were bilingual (English and Xhosa), and both delivered the interventions to men and women in keeping with standard clinic services. Each counselor attended weekly 2-hour supervision and debriefing meetings with the project manager and a professionally registered counseling psychologist.
Measures
All measures were administered at the baseline and 1-, 3-, 6-, 9-, and 12-month follow-up assessments in English and Xhosa, the 2 languages spoken by nearly all clinic patients. Participants viewed the instruments on a 15-inch color monitor, used headphones to listen to items read by a machine voice, and responded by clicking a mouse. Research has shown that ACASIs yield reliable responses to sexual behavior interviews.25 Participants were briefly instructed on how to use the mouse prior to the baseline assessment. The measures consisted of 254 items that gathered descriptive data (demographics, HIV risk history, alcohol and drug use), data on primary outcomes (STI diagnoses abstracted from medical records and behavioral outcomes, including sexual risk behaviors, preventive behaviors, and alcohol-related risk behaviors), and data on secondary outcomes (theoretical constructs such as HIV knowledge, alcohol outcome expectancies, and self-efficacy for risk reduction).
Descriptive information.
Participants reported their age, gender, education, ethnicity, marital status, and other basic demographic information. In addition, we asked whether participants had been tested for HIV and, if so, the result of their most recent test. Participants also completed the AUDIT, a 10-item self-report instrument that gathers information on quantity and frequency of alcohol use; the test was designed to identify individuals for whom the use of alcohol places them at risk for developing alcohol problems.26–28 AUDIT scores range from 0 to 40, and scores of 8 or above identify individuals who may be at risk for alcohol problems.27 The AUDIT has been used in South Africa and is reliable and valid.29 The instrument's first 2 items assess frequency and quantity of alcohol use. We calculated an index of current drinking frequency and quantity by taking the product of these 2 items. The alcohol index therefore weighted the quantity of alcohol typically consumed by frequency of use.
Sexually transmitted infections.
Occurrences of newly diagnosed STIs were coded from patients’ clinic charts as the primary biological endpoint. We contracted a nurse with more than 20 years of experience working in Cape Town STI clinics, including the clinic that served as the site in this study, to code the chart-abstracted STI data. The nurse coder was blind to conditions and did not record any identifying participant information. Data were retrieved from patient files on the clinic premises with the permission of patients. Because STIs are treated presumptively in South Africa, confirmed diagnoses underestimate the actual number of STIs. We therefore included in our analyses any occurrence of urethral or vaginal discharge that resulted in STI treatment as well as diagnoses of incident STIs. Because participants could have had multiple STIs over the year of observation, we treated STI diagnoses as a continuous count variable. Only STIs detected within 12 months after baseline were coded for outcomes.
Sexual risk and protective behaviors.
Participants responded to items assessing their number of male and female sexual partners and frequency of sexual behaviors in the preceding month (specifically vaginal and anal intercourse with and without condoms). A 30-day retrospective period was selected because previous research has shown reports of numbers of partners and sexual events over this interval to be reliable.30 Participants were instructed to think back over the past month and estimate the number of sexual partners and number of sexual occasions in which they practiced each behavior.
In addition, we calculated the percentage of intercourse occasions in which condoms were used via the following ratio: condom-protected vaginal intercourse + condom-protected anal intercourse/total vaginal intercourse + total anal intercourse. Participants also indicated the number of times they had consumed alcohol (defined as beer, wine, or other alcoholic beverages) or used other drugs before sex in the preceding month. An open response format was used so that continuous frequencies of occurrences could be recorded.
HIV prevention knowledge.
A 12-item test was used to assess HIV risk- and prevention-related knowledge. Items were adapted from a measure reported by Carey and Schroder31 and reflected information about HIV transmission, condom use, and AIDS-related knowledge; response options were yes, no, and don't know. Example items included “Is AIDS spread by kissing?” and “Can a person get AIDS by sharing kitchens and bathrooms with someone who has AIDS?” AIDS knowledge test scores were expressed as percentage of correct responses (Kuder–Richardson 20 coefficient = 0.71); don't know responses were scored as incorrect.
Alcohol outcome expectancies.
We adapted an alcohol outcome expectancy measure from items used in previous research.32–34 The scale included 9 items (e.g., “I am a better sex partner after I have been drinking” and “When I'm drinking, I do things I wouldn't usually do”) reflecting expected sexual enhancement and expected loss of control after drinking. Responses were made on 4-point scales ranging from strongly disagree (1) to strongly agree (4) (α = 0.90).
Risk reduction self-efficacy.
Defined as the personal sense of confidence that one can perform specific behaviors under specified conditions, self-efficacy is commonly used as a proxy for behavioral skills.35,36 The self-efficacy scale we used consisted of 6 items, including “I am confident about suggesting using condoms with a new sex partner” and “I am certain that I can use a condom when having sex.” Items were responded to on a 4-point scale (1 = disagree, 4 = agree), scored for mean responses; higher scores indicated stronger self-efficacy (α = 0.69).
Data Analyses
We initially conducted analyses to examine the integrity of the randomization procedures and study design. An intent-to-treat approach was used in all primary outcome analyses. Outcome analyses tested models that included baseline scores as covariates and main effects for intervention condition, participant gender, and time of assessment as well as the interactions between factors. Planned contrasts tested for simple effects of interactions.
The primary outcome analyses tested our study hypotheses regarding intervention effects on sexual risk behaviors and chart-abstracted STIs; generalized estimating equations (GEEs) were used in conducting these analyses. We selected GEEs for all main outcome analyses because this methodology is based on a quasi-likelihood theory allowing for overdispersion in outcome variables.37 GEEs corrected for the within-subject correlation characteristic of our repeated measures design.38 We used an auto-regressive correlation structure to account for the within-subject correlation resulting from successive observations. Poisson distributions were used for continuous count data (e.g., sexual partners, sexual behaviors, STI rates), and linear distributions were used for scaled data (e.g., theoretical constructs, condom use percentages). Participant gender was included in the main outcome analyses.
To examine alcohol use as a moderating variable, we repeated all of the analyses with alcohol consumption level (lighter drinkers [AUDIT score < 8] vs heavier drinkers [AUDIT score ≥ 8]) included as a factor. Thus, main effects of intervention, gender, alcohol use, and assessment time as well as interactions were included in these models. For STI outcomes, moderator models were also tested with number of partners, unprotected sex, and substance use before sex during the follow-up periods. SPSS version 18.0 (SPSS Inc, Chicago, IL) was used in conducting all of the analyses; the statistical significance level was set at P < .05.
RESULTS
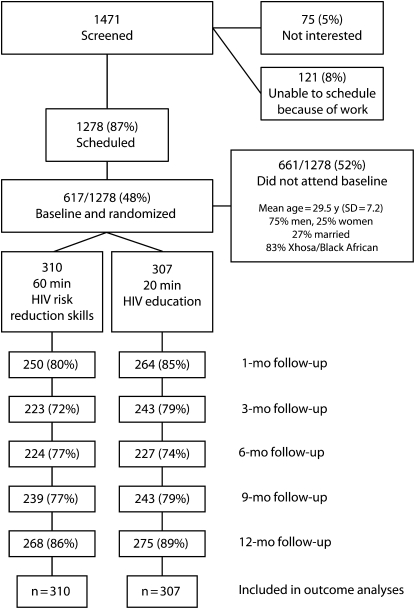
Figure 1 shows the flow of participants through the trial. Overall, we retained 88% of participants at the 12-month follow-up; this rate was higher than the 73% retention at the 6-month follow-up. Results of the preliminary analyses to determine the integrity of the study design showed that there were no differences between participants in the experimental and control conditions with respect to any demographic characteristics, substance use, theoretical constructs, or sexual behaviors. Nor did we observe any differences between participants who completed the baseline assessment and those who did not (Table 1). Analyses also showed that attrition across conditions was balanced.
FIGURE 1.
Participants’ progress through the randomized trial phases: Cape Town, South Africa, 2006–2008.
TABLE 1.
Characteristics of Participants, by Condition: Cape Town, South Africa, 2006–2008
| Characteristic | HIV Risk Reduction (n = 310), No. (%) or Mean ±SD | Control (n = 307), No. (%) or Mean ±SD | χ2 or ta |
| Gender | 0.2 | ||
| Men | 205 (66) | 209 (68) | |
| Women | 105 (34) | 98 (32) | |
| Race/ethnicity | 0.1 | ||
| Black | 286 (92) | 289 (94) | |
| White/mixed | 24 (8) | 18 (6) | |
| Preferred language | 0.1 | ||
| English | 84 (27) | 85 (27) | |
| Xhosa | 226 (73) | 222 (73) | |
| Employed | 205 (66) | 186 (61) | 2.0 |
| Marital status | 0.8 | ||
| Single | 208 (67) | 216 (70) | |
| Cohabitating | 38 (12) | 32 (10) | |
| Married | 64 (21) | 59 (20) | |
| Alcohol use in past mo | 152 (49) | 150 (48) | 0.0 |
| Current alcohol use | 1.5 | ||
| Never | 109 (35) | 102 (33) | |
| Monthly | 57 (18) | 56 (18) | |
| 2–4 times/mo | 93 (30) | 103 (33) | |
| 2–3 times/wk | 36 (12) | 32 (10) | |
| ≥ 4 times/wk | 15 (5) | 11 (4) | |
| AUDIT score ≥ 8 | 140 (45) | 126 (41) | 0.8 |
| Tested for HIV | 98 (31) | 81 (26) | 2.0 |
| HIV positive | 22 (7) | 25 (8) | 0.1 |
| Age, y | 29.2 ±7.1 | 29.2 ±7.1 | 0.1 |
| Education, y | 11.0 ±2.3 | 11.2 ±2.2 | 0.9 |
| AUDIT score | 7.52 ±7.79 | 7.49 ±7.89 | 0.1 |
Note. AUDIT = Alcohol Use Disorders Identification Test.
χ2 for numbers of participants, t for means.
Primary Outcomes
Analyses of the sexual behavior outcomes demonstrated significant between-condition differences in unprotected vaginal, unprotected anal, and combined unprotected vaginal and anal intercourse over the preceding month, after controlling for baseline (Table 2). Also, for combined unprotected intercourse there was a significant interaction between intervention condition and assessment time (Wald χ24 = 9.82, P < .05). Analyses showed significant between-condition differences with respect to combined unprotected intercourse at the 1-, 3-, and 6-month follow-ups. However, the differences were not significant at the 9- and 12-month follow-ups.
TABLE 2.
Sexual Risk, Risk Reduction, and Alcohol-Related Outcomes, by Condition: Cape Town, South Africa, 2006–2008
| HIV Risk Reduction, Mean (SD) | Control, Mean (SD) | Wald χ2 | |
| No. of sexual partners | 0.01 | ||
| Baseline | 1.67 (2.45) | 1.55 (3.12) | |
| 1-mo follow-up | 1.22 (1.02) | 1.40 (1.79) | |
| 3-mo follow-up | 1.13 (0.85) | 1.33 (3.21) | |
| 6-mo follow-up | 1.18 (1.15) | 1.22 (1.41) | |
| 9-mo follow-up | 1.31 (1.93) | 1.14 (0.87) | |
| 12-mo follow-up | 1.14 (0.92) | 1.11 (0.81) | |
| No. of occasions of unprotected vaginal intercourse | 7.47** | ||
| Baseline | 1.85 (3.21) | 2.43 (5.13) | |
| 1-mo follow-up | 0.48 (1.26) | 1.25 (4.34) | |
| 3-mo follow-up | 0.32 (1.16) | 0.87 (2.95) | |
| 6-mo follow-up | 0.43 (1.69) | 0.86 (2.79) | |
| 9-mo follow-up | 0.47 (1.39) | 0.65 (2.41) | |
| 12-mo follow-up | 0.54 (1.55) | 0.69 (2.36) | |
| No. of occasions of unprotected anal intercourse | 5.30* | ||
| Baseline | 0.31 (1.20) | 0.32 (1.80) | |
| 1-mo follow-up | 0.04 (0.30) | 0.20 (1.07) | |
| 3-mo follow-up | 0.03 (0.24) | 0.16 (1.20) | |
| 6-mo follow-up | 0.07 (0.61) | 0.20 (1.13) | |
| 9-mo follow-up | 0.11 (0.81) | 0.19 (1.34) | |
| 12-mo follow-up | 0.08 (0.64) | 0.08 (0.56) | |
| Total no. of occasions of unprotected intercourse | 8.67** | ||
| Baseline | 2.16 (3.61) | 2.75 (5.84) | |
| 1-mo follow-up | 0.52 (1.30) | 1.45 (4.88) | |
| 3-mo follow-up | 0.36 (1.22) | 1.03 (3.55) | |
| 6-mo follow-up | 0.50 (1.80) | 1.06 (3.20) | |
| 9-mo follow-up | 0.58 (1.71) | 0.84 (3.10) | |
| 12-mo follow-up | 0.62 (1.70) | 0.78 (2.46) | |
| Condom use, % | 2.69 | ||
| Baseline | 70 (36) | 69 (37) | |
| 1-mo follow-up | 90 (24) | 89 (25) | |
| 3-mo follow-up | 94 (18) | 89 (26) | |
| 6-mo follow-up | 93 (20) | 89 (26) | |
| 9-mo follow-up | 92 (21) | 91 (22) | |
| 12-mo follow-up | 90 (24) | 91 (25) | |
| No. of occasions of substance use in sexual contexts | 3.82* | ||
| Baseline | 2.05 (7.30) | 1.53 (4.04) | |
| 1-mo follow-up | 0.64 (2.17) | 1.21 (5.79) | |
| 3-mo follow-up | 0.32 (1.26) | 0.92 (4.32) | |
| 6-mo follow-up | 0.45 (1.78) | 0.78 (2.86) | |
| 9-mo follow-up | 0.57 (2.11) | 2.11 (2.81) | |
| 12-mo follow-up | 0.55 (2.17) | 2.17 (2.13) | |
| Alcohol use quantity/frequency index | 6.36** | ||
| Baseline | 2.85 (3.81) | 2.84 (3.69) | |
| 1-mo follow-up | 1.56 (2.30) | 2.02 (2.58) | |
| 3-mo follow-up | 1.36 (2.03) | 2.02 (2.74) | |
| 6-mo follow-up | 1.39 (2.14) | 1.76 (2.59) | |
| 9-mo follow-up | 1.46 (2.44) | 1.79 (2.44) | |
| 12-mo follow-up | 1.41 (2.25) | 1.71 (2.49) |
Note. Sexual behaviors refer to the previous month. All statistical tests adjusted for baseline rates.
*P ≤ .05; **P < .01.
There was a trend toward an intervention effect on the use of substances before sex, with the intervention group reporting fewer occurrences of alcohol and other drug use before sex during the follow-up period. The between-condition difference on the alcohol use frequency and quantity index was significant, with members of the risk reduction counseling group reporting less alcohol use at the follow-ups than those in the control condition. No intervention effects were observed for reductions in number of sexual partners, although there was an overall assessment time effect (Wald χ21 = 9.78, P < .05); significant reductions in numbers of partners occurred across groups from baseline throughout the follow-ups. There were no intervention effects or assessment time effects on condom use percentage.
Moderator analyses focusing on sexual behavior outcomes revealed significant main effects by gender, including number of sexual partners (Wald χ21 = 14.26, P < .01), substance use before sex (Wald χ21 = 47.77, P < .01), and frequency and quantity of alcohol use (Wald χ21 = 50.08, P < .01). In each case, risk levels were higher among men than they were among women. However, there were no interactions between gender and intervention condition, failing to show any moderator effects of gender on the intervention outcomes.
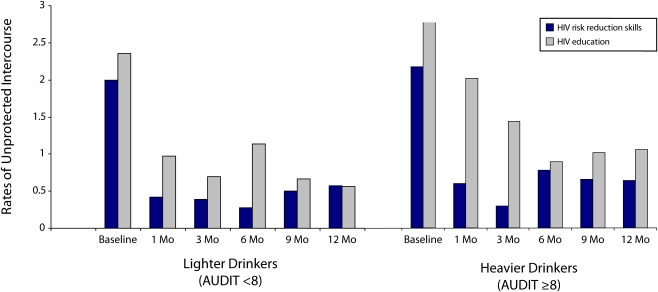
By contrast, alcohol use significantly interacted with intervention condition on several main outcomes. When alcohol use was included in the model, the main effect of intervention condition on combined unprotected sexual behaviors remained significant (Wald χ21 = 6.56, P < .01). However, the 3-way interaction between intervention condition, assessment time, and alcohol use was also significant (Wald χ21 = 20.10, P < .01). As shown in Figure 2, the intervention effect for lighter drinkers was similar to that for the overall sample, with significant reductions in unprotected intercourse between conditions that had dissipated by the 12-month follow-up. However, heavier drinkers in the control condition demonstrated the highest and most persistent high-risk behavior.
FIGURE 2.
Frequencies of sexual intercourse among lighter and heavier drinkers, by condition: Cape Town, South Africa, 2006–2008.
Note. AUDIT = Alcohol Use Disorders Identification Test.
Sexually Transmitted Infections
Results of analyses on incident STIs over the 12 months after counseling indicated that participants in the risk reduction counseling group were less likely to return to the clinic with an STI than were participants in the control condition (Wald χ21 = 3.35, P = .06). Overall, 12.9% of the members of the risk reduction counseling group returned to the clinic with another STI over the year, compared with 16.9% of control participants, representing 24% fewer infections in the experimental group.
In addition to participant gender, we tested 3 potential moderators of STI outcomes: number of sexual partners reported at the follow-ups, unprotected sex, and use of substances before sex. Results showed that when moderator variables were taken into account, participants in the risk reduction counseling group had contracted significantly fewer STIs over the follow-up period (Table 3). The only significant interaction between the intervention and a moderator variable was that involving number of sexual partners; participants in the risk reduction counseling intervention who had only 1 or no sex partners at the follow-up assessments had significantly fewer STIs than did their counterparts with multiple partners and the participants in the control condition.
TABLE 3.
Sexually Transmitted Infections Over the 12-Month Postintervention Period, by Condition: Cape Town, South Africa, 2006–2008
| HIV Risk Reduction |
Control |
Wald χ2 |
|||||
| Moderator Variablea | % | Mean (SD) | % | Mean (SD) | Condition | Moderator | Condition × Moderator |
| No. of sexual partners in preceding mobc | 4.05* | 1.19 | 6.12** | ||||
| 0 or 1 | 6.1 | 0.10 (0.46) | 20.8 | 0.29 (0.64) | |||
| ≥2 | 23.0 | 0.30 (0.61) | 21.1 | 0.24 (0.49) | |||
| No. of unprotected vaginal/anal sex actscd | 6.64* | 1.51 | 3.15 | ||||
| 0 | 8.9 | 0.13 (0.49) | 20.8 | 0.28 (0.63) | |||
| ≥1 | 17.5 | 0.24 (0.59) | 21.2 | 0.26 (0.54) | |||
| No. of substance use episodes before sexcd | 4.65* | 0.06 | 1.09 | ||||
| 0 | 11.9 | 0.13 (0.39) | 19.7 | 0.27 (0.62) | |||
| ≥1 | 12.2 | 0.25 (0.78) | 23.0 | 0.27 (0.53) | |||
Variables include participants who attended all 5 follow-ups.
Average number of sexual partners reported at each follow-up.
Model includes named moderator, intervention condition, gender, Alcohol Use Disorders Identification Test score, and interaction terms.
Participants reporting no unprotected acts (or no substance use before sex) at each follow-up were coded as no; participants reporting unprotected acts (substance use before sex) at one or more follow-ups were coded as yes.
*P < .05; **P < .01.
Secondary Outcomes
Results showed a significant intervention effect on AIDS-related knowledge; members of the control condition demonstrated more accurate AIDS knowledge than did members of the risk reduction counseling condition (Table 4). In addition, we observed significant between-condition differences on the alcohol outcome expectancy measure; at the follow-ups, participants who received risk reduction counseling were significantly less likely than were those in the control condition to believe that alcohol enhances sexual experiences. No significant differences were observed for the self-efficacy scale. There were also no interactions between intervention condition and assessment time on the theoretical constructs.
TABLE 4.
Intervention-Related Theoretical Construct Scores, by Condition: Cape Town, South Africa, 2006–2008
| HIV Risk Reduction, Mean (SD) | Control, Mean (SD) | Wald χ2 | |
| HIV prevention knowledge score (% correct) | 7.03** | ||
| Baseline | 75 (22) | 79 (17) | |
| 1-mo follow-up | 83 (18) | 89 (13) | |
| 3-mo follow-up | 86 (17) | 89 (14) | |
| 6-mo follow-up | 85 (19) | 88 (14) | |
| 9-mo follow-up | 84 (20) | 87 (15) | |
| 12-mo follow-up | 83 (20) | 87 (16) | |
| Alcohol outcome expectancies score | 5.11* | ||
| Baseline | 1.80 (0.87) | 1.83 (0.83) | |
| 1-mo follow-up | 1.58 (0.77) | 1.73 (0.90) | |
| 3-mo follow-up | 1.46 (0.68) | 1.64 (0.85) | |
| 6-mo follow-up | 1.44 (0.73) | 1.66 (0.90) | |
| 9-mo follow-up | 1.48 (0.76) | 1.71 (0.91) | |
| 12-mo follow-up | 1.58 (0.83) | 1.66 (0.90) | |
| Risk reduction self-efficacy score | 0.62 | ||
| Baseline | 3.84 (2.13) | 3.82 (2.15) | |
| 1-mo follow-up | 4.44 (2.05) | 4.41 (2.07) | |
| 3-mo follow-up | 4.72 (1.95) | 4.69 (1.97) | |
| 6-mo follow-up | 4.87 (1.88) | 4.81 (1.86) | |
| 9-mo follow-up | 4.98 (1.74) | 4.75 (1.89) | |
| 12-mo follow-up | 5.06 (1.70) | 4.86 (1.87) |
Note. All statistical tests adjusted for baseline scores.
*P < .05; **P < .01.
DISCUSSION
The brief risk reduction skills counseling intervention tested here demonstrated significant reductions in incident STIs relative to an information control condition. We observed 24% fewer STIs over the 1-year follow-up among participants who received risk reduction counseling than among those in the information condition. In addition, there were significant reductions in unprotected vaginal and anal intercourse as well as risk-related substance use, including expectancies that alcohol enhances sexual experiences.
Our findings are consistent with previous prevention intervention trials involving STI patients7 and extend our initial trial conducted with a smaller sample that followed participants for only 6 months.19 The current findings show that unprotected intercourse outcomes were no longer significant by 9 months and that the intervention had no effects on number of partners or condom use. In addition, we observed significantly greater HIV prevention knowledge in the control condition, illustrating the notion that increased knowledge does not lead to meaningful behavior change.39,40
These findings should, however, be considered in the context of the between-condition reductions in number of partners, reductions in unprotected sex, and increases in condom use observed after the baseline assessment. Consistent with past interventions for STI clinic patients, the diagnosis and treatment experience, along with standard of care interventions, had an impact on risk behaviors.10,15,41 Thus, an effective counseling intervention designed to further reduce STI and HIV risks must contribute to behavior change over and above the standard of care.
Unlike previous brief interventions with STI clinic patients,41 we did not find differences in intervention effects between men and women. However, the observed outcomes were significantly moderated by alcohol use. The moderator analyses showed that the intervention effects were less robust and durable for heavier drinkers than for lighter drinkers. In addition, the intervention effects on STIs were moderated by number of sexual partners at the follow-ups, with the greatest protection among participants receiving the risk reduction counseling and reporting fewer partners.
These findings pinpoint areas in which the risk reduction counseling tested in this trial requires strengthening. Specifically, it is apparent that the substance use component requires greater potency given the substantial moderating role of heavy drinking. The intervention also requires a booster session between 3 and 6 months after initial counseling. Previous research has established the added value of booster sessions in sustaining behavior change.42 A booster session that consists of brief counseling to reinforce successful risk reduction, review skills practiced in the initial intervention, and address challenges that contribute to relapse is likely to bolster intervention effects over time.43,44
Limitations
The results of this trial should be interpreted in light of its methodological limitations. The trial was conducted in a single STI clinic in Cape Town, a better resourced city than most any in southern Africa, rendering the generalizability of the findings unknown. The external validity of the results is further reduced by the fact that half of the individuals scheduled for counseling failed to attend the baseline session. This rate of loss is similar to previous trials involving STI clinic patients,8,10,15 and we did not detect differences in the information from our screening instrument between participants who did and did not attend the baseline session.
It is not possible from our data to determine which intervention components, including the alcohol components, were necessary for producing risk behavior change. The differences in the time required by the 2 conditions (60 minutes vs 20 minutes) may have contributed to the observed outcomes. As is the case with nearly all behavioral interventions, we were not able to blind our intervention counselors to the experimental conditions. In addition, participants completed 6 assessments across 12 months, which may have influenced their behavior over time.10 Another limitation was our use of a self-efficacy scale as a proxy for behavioral skills rather than a direct assessment of these skills. With these constraints in mind, we believe that brief HIV risk reduction counseling for STI patients has the potential to prevent HIV infections.
Conclusions
The value of brief interventions designed to reduce HIV transmission risks increases as prevention resources become scarce. Although effective, multiple-session, and small group interventions have proven difficult to implement,8,45,46 interventions that target those most at risk and in places of high HIV prevalence are urgently needed in developed47 and developing countries.48
The current study demonstrates the efficacy of a single-session risk reduction model for people who have contracted STIs other than HIV in a city with an HIV prevalence rate of nearly 20%. There are numerous opportunities for implementing such an intervention, including routine STI clinical services and counseling conducted after an HIV test. Brief risk reduction counseling in the midst of a teachable moment, such as an STI diagnosis, has the potential to significantly affect HIV transmission at a time when prevention options are few and prevention resources are shrinking. Thus, implementing simple and potent interventions in areas with high HIV prevalence rates should be a public health priority.
Acknowledgments
This research was supported by the National Institute of Mental Health (grant R01-MH074371).
Human Participant Protection
This study was approved by the University of Connecticut institutional review board and the Research Ethics Committee of the Human Sciences Research Council of South Africa. Participants provided written informed consent.
References
- 1.2006 Report on the Global AIDS Epidemic. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2006 [Google Scholar]
- 2.Udjo EO. A re-look at recent statistics on mortality in the context of HIV/AIDS with particular reference to South Africa. Curr HIV Res. 2008;6(2):143–151 [DOI] [PubMed] [Google Scholar]
- 3.Nattrass N. Poverty, sex and HIV. AIDS Behav. 2009;13(5):833–840 [DOI] [PubMed] [Google Scholar]
- 4.Chigwedere P, Essex M. AIDS denialism and public health practice. AIDS Behav. 2010;14(2):237–247 [DOI] [PubMed] [Google Scholar]
- 5.Wouters E, van Rensburg HC, Meulemans H. The national strategic plan of South Africa: what are the prospects of success after the repeated failure of previous AIDS policy? Health Policy Plan. 2010;25(3):171–185 [DOI] [PubMed] [Google Scholar]
- 6.Ward H, Ronn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5(4):305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott-Sheldon LA, Fielder RL, Carey MP. Sexual risk reduction interventions for patients attending sexually transmitted disease clinics in the United States: a meta-analytic review, 1986 to early 2009. Ann Behav Med. 2010;40(2):191–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute of Mental Health Multisite HIV Prevention Trial Group The NIMH Multisite HIV Prevention Trial: reducing HIV sexual risk behavior. Science. 1998;280:1889–1894 [DOI] [PubMed] [Google Scholar]
- 9.Shain RN, Piper JM, Holden AE, et al. Prevention of gonorrhea and chlamydia through behavioral intervention: results of a two-year controlled randomized trial in minority women. Sex Transm Dis. 2004;31(7):401–408 [DOI] [PubMed] [Google Scholar]
- 10.Carey MP, Senn TE, Vanable PA, Coury-Doniger P, Urban MA. Brief and intensive behavioral interventions to promote sexual risk reduction among STD clinic patients: results from a randomized controlled trial. AIDS Behav. 2010;14(3):504–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belcher L, Kalichman S, Topping M, et al. A randomized trial of a brief HIV risk reduction counseling intervention for women. J Consult Clin Psychol. 1998;66(5):856–861 [DOI] [PubMed] [Google Scholar]
- 12.Kalichman SC, Rompa D, Coley B. Experimental component analysis of a behavioral HIV-AIDS prevention intervention for inner-city women. J Consult Clin Psychol. 1996;64(4):687–693 [DOI] [PubMed] [Google Scholar]
- 13.Picciano JF, Roffman RA, Kalichman SC, Walker DD. Lowering obstacles to HIV prevention services: effects of a brief, telephone-based intervention using motivational enhancement therapy. Ann Behav Med. 2007;34(2):177–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen S, Tice J, Van de Perre P, et al. Effect of serotesting with counselling on condom use and seroconversion among HIV discordant couples in Africa. BMJ. 1992;304(6842):1605–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamb M, Fishbein M, Douglas J, et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases. JAMA. 1998;280(13):1161–1167 [DOI] [PubMed] [Google Scholar]
- 16.Metcalf CA, Douglas JM, Jr, Malotte CK, et al. Relative efficacy of prevention counseling with rapid and standard HIV testing: a randomized, controlled trial (RESPECT-2). Sex Transm Dis. 2005;32(2):130–138 [DOI] [PubMed] [Google Scholar]
- 17.Crosby R, DiClemente RJ, Charnigo R, Snow G, Troutman A. A brief, clinic-based, safer sex intervention for heterosexual African American men newly diagnosed with an STD: a randomized controlled trial. Am J Public Health. 2009;99(suppl 1):S96–S103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson BT, Scott-Sheldon LA, Smoak ND, Lacroix JM, Anderson JR, Carey MP. Behavioral interventions for African Americans to reduce sexual risk of HIV: a meta-analysis of randomized controlled trials. J Acquir Immune Defic Syndr. 2009;51(4):492–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalichman SC, Simbayi LC, Vermaak R, Cain D, Jooste S, Peltzer K. HIV/AIDS risk reduction counseling for alcohol using sexually transmitted infections clinic patients in Cape Town, South Africa. J Acquir Immune Defic Syndr. 2007;44(5):594–600 [DOI] [PubMed] [Google Scholar]
- 20.Kalichman SC, Simbayi LC, Kaufman M, Cain D, Jooste S. Alcohol use and sexual risks for HIV/AIDS in sub-Saharan Africa: systematic review of empirical findings. Prev Sci. 2007;8(2):141–151 [DOI] [PubMed] [Google Scholar]
- 21.Parry CD, Myers B, Morojele NK, et al. Trends in adolescent alcohol and other drug use: findings from three sentinel sites in South Africa (1997–2001). J Adolesc. 2004;27(4):429–440 [DOI] [PubMed] [Google Scholar]
- 22.Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychol Bull. 1992;111(3):455–474 [DOI] [PubMed] [Google Scholar]
- 23.Babor TF, Grant M, Acuda W, et al. A randomized clinical trial of brief interventions in primary care: summary of a WHO project. Addiction. 1994;89(6):657–660 [DOI] [PubMed] [Google Scholar]
- 24.Hall W, Saunders JB, Babor TF, et al. The structure and correlates of alcohol dependence: WHO collaborative project on the early detection of persons with harmful alcohol consumption—III. Addiction. 1993;88(12):1627–1636 [DOI] [PubMed] [Google Scholar]
- 25.Morrison-Beedy D, Carey MP, Tu X. Accuracy of audio computer-assisted self-interviewing (ACASI) and self-administered questionnaires for the assessment of sexual behavior. AIDS Behav. 2006;10(5):541–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Silva P, Jayawardana P, Pathmeswaran A. Concurrent validity of the Alcohol Use Disorders Identification Test (AUDIT). Alcohol. 2008;43(1):49–50 [DOI] [PubMed] [Google Scholar]
- 27.Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Addiction. 1995;90(10):1349–1356 [DOI] [PubMed] [Google Scholar]
- 28.Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56(4):423–432 [DOI] [PubMed] [Google Scholar]
- 29.Kalichman SC, Simbayi LC, Jooste S, Cain D. Frequency, quantity, and contextual use of alcohol among sexually transmitted infection clinic patients in Cape Town, South Africa. Am J Drug Alcohol Abuse. 2007;33(5):687–698 [DOI] [PubMed] [Google Scholar]
- 30.Napper LE, Fisher DG, Reynolds GL, Johnson ME. HIV risk behavior self-report reliability at different recall periods. AIDS Behav. 2010;14(1):152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carey MP, Schroder KE. Development and psychometric evaluation of the brief HIV Knowledge Questionnaire. AIDS Educ Prev. 2002;14(2):172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown SA, Christiansen BA, Goldman MS. The Alcohol Expectancy Questionnaire: an instrument for the assessment of adolescent and adult alcohol expectancies. J Stud Alcohol. 1987;48(5):483–491 [DOI] [PubMed] [Google Scholar]
- 33.Brown SA, Goldman MS, Inn A, Anderson LR. Expectations of reinforcement from alcohol: their domain and relation to drinking patterns. J Consult Clin Psychol. 1980;48(4):419–426 [DOI] [PubMed] [Google Scholar]
- 34.Aarons GA, Goldman MS, Greenbaum PE, Coovert MD. Alcohol expectancies: integrating cognitive science and psychometric approaches. Addict Behav. 2003;28(5):947–961 [DOI] [PubMed] [Google Scholar]
- 35.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215 [DOI] [PubMed] [Google Scholar]
- 36.Bandura A. Self-Efficacy: The Exercise of Control. New York, NY: WH Freeman; 1997 [Google Scholar]
- 37.Pan W. Akaike's information criterion in generalized estimating equations. Biometrics. 2001;57(1):120–125 [DOI] [PubMed] [Google Scholar]
- 38.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060 [PubMed] [Google Scholar]
- 39.Ferris FD, von Gunten CF, Emanuel LL. Knowledge: insufficient for change. J Palliat Med. 2001;4(2):145–147 [DOI] [PubMed] [Google Scholar]
- 40.Clark AM, Freydberg CN, McAlister FA, Tsuyuki RT, Armstrong PW, Strain LA. Patient and informal caregivers’ knowledge of heart failure: necessary but insufficient for effective self-care. Eur J Heart Fail. 2009;11(6):617–621 [DOI] [PubMed] [Google Scholar]
- 41.Kalichman SC, Weinhardt LS, Benotsch E, et al. Experimental components analysis of brief theory-based HIV-AIDS risk reduction counseling for sexually transmitted infection patients. Health Psychol. 2005;24(2):198–208 [DOI] [PubMed] [Google Scholar]
- 42.Metcalf CA, Malotte CK, Douglas JM, Jr, et al. Efficacy of a booster counseling session 6 months after HIV testing and counseling: a randomized, controlled trial (RESPECT-2). Sex Transm Dis. 2005;32(2):123–129 [DOI] [PubMed] [Google Scholar]
- 43.Swanson J, Cooper A. The role of alcohol and drug relapse prevention in the treatment and prevention of HIV disease. J Int Assoc Physicians AIDS Care. 1998;4(4):14–19 [PubMed] [Google Scholar]
- 44.Roffman RA, Stephen RS, Curtin L, et al. Relapse prevention as an interventive model for HIV risk reduction in gay and bisexual men. AIDS Educ Prev. 1998;10(1):1–18 [PubMed] [Google Scholar]
- 45.Holtgrave DR. The role of quantitative policy analysis in HIV prevention technology transfer. Public Health Rep. 2004;119(1):19–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veniegas RC, Kao UH, Rosales R, Arellanes M. HIV prevention technology transfer: challenges and strategies in the real world. Am J Public Health. 2009;99(suppl 1):S124–S130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Sadr WM, Mayer KH, Hodder SL. AIDS in America—forgotten but not gone. N Engl J Med. 2010;362(11):967–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merson MH. The HIV-AIDS pandemic at 25—the global response. N Engl J Med. 2006;354(23):2414–2417 [DOI] [PubMed] [Google Scholar]