Abstract
Objectives. We investigated how persons from key populations at higher risk of HIV exposure interpreted the process and outcomes of the Step Study HIV-1 vaccine trial, which was terminated early, and implications for willingness to participate in and community support for HIV vaccine research.
Methods. We used qualitative methods and a community-based approach in 9 focus groups (n = 72) among ethnically and sexually diverse populations and 6 semistructured key informant interviews in Ontario, Canada, in 2007 to 2008.
Results. Participants construed social meaning from complex clinical and biomedical phenomena. Social representations and mental models emerged in fears of vaccine-induced infection, conceptualizations of unfair recruitment practices and increased risk behaviors among trial participants, and questioning of informed consent. Narratives of altruism and the common good demonstrated support for future trials.
Conclusions. Public discourse on HIV vaccine trials is a productive means of interpreting complex clinical trial processes and outcomes in the context of existing beliefs and experiences regarding HIV vaccines, medical research, and historical disenfranchisement. Strategic engagement with social representations and mental models may promote meaningful community involvement in biomedical HIV prevention research.
Scientific discourse, including studies and commentaries circulated in peer-reviewed medical and public health journals, reveals sometimes-contentious disagreement among biomedical researchers, public health officials, and clinicians, particularly at the vanguard of discovery. In the context of HIV vaccine research, opposing views have characterized the soundness of scientific and economic rationales for launching large-scale clinical trials1–4 and interpretation of trial results.5–7 It is nevertheless a basic tenet of scientific discourse that clinical trials, the majority of which do not result in an efficacious product, are mechanisms to inform evolving discovery.
Contested clinical trials and biomedical outcomes also may constitute controversial social phenomena. Yet throughout a history of international HIV chemoprophylaxis trial shutdowns and sometimes-acerbic debate,4,8,9 considerably less attention has focused on the social processes and outcomes of clinical trials; these too might be used to advance an evolving social science of biomedical HIV prevention research, including evidence to support critical processes of knowledge translation and community engagement.9,10
THE STEP STUDY
From 2004 to 2009, the HIV Vaccine Trials Network, the National Institute of Allergy and Infectious Diseases, and Merck Research Laboratories conducted an international phase IIb test-of-concept clinical trial (the Step Study; HVTN 502) to assess the efficacy of an experimental HIV-1 vaccine in decreasing HIV acquisition rates or lowering the viral load set point among those who contracted HIV.11 The overall trial involved 3000 high-risk, HIV-negative participants in North America, the Caribbean, South America, and Australia aged 18 to 45 years.11 One of us (P. A. N.), in collaboration with the Canadian Immunodeficiency Research Collaborative at Maple Leaf Medical Clinic, which operated the Toronto trial site (n = 55), received independent funding in 2005 to conduct sociobehavioral research as an adjunct to the local trial.
In the fall of 2007, the sponsors halted the trial because interim results indicated the test vaccine would not reach its projected goals. Furthermore, and unexpectedly, the rate of HIV acquisition was significantly higher among a subset of volunteers who received the test vaccine rather than the placebo.11,12 The trial termination and its aftermath resulted in confusion and debate in biomedical discourse, ultimately contributing to a shift in direction for HIV vaccine research, including calls for greater selectivity in advancing candidate vaccines to human trials.13,14
APPROACHING THE STEP STUDY THROUGH PUBLIC DISCOURSE
Case studies of controversial events provide an entrée to explore social meaning.15,16 Social meaning is revealed in social representations and mental models that are used to make sense of complex events. Mental models—subjective representations of structures of an event, including settings, participants, and actions15—derive from the web of preexisting knowledge, attitudes, and beliefs that individuals have about a particular subject.17
In the field of public health, Fischhoff et al.17 and Morgan et al.18 have explored mental models that underlie social constructions of risk. These mental models, in turn, may influence health and risk behaviors (e.g., undue fears that present barriers to vaccine uptake).18,19 Slovic has identified mental models of health risks that affect priorities in public health funding allocations, sometimes influenced more by public perceptions than by scientific evidence.19 Public discourse has material consequences.
Public health and social science approaches to HIV vaccine trials have contributed to an important evidence base on individual attitudes and knowledge associated with willingness to participate in clinical trials.20–22 Considerably less attention has focused on social representations and mental models that may underlie public perceptions of HIV vaccine trials and decisions about participation. After the early termination of the Step Study HIV vaccine trial, we investigated how members of key populations at high risk for HIV exposure, including potential trial participants, interpreted the process and outcomes of the trial and the implications for willingness to participate in and community support for HIV vaccine research.
METHODS
We used a modified grounded theory methodology23 and a community-based approach.24 Representatives from community agencies serving key populations at high risk for HIV assisted in articulating research questions and in data collection and interpretation.
Data Collection
We conducted the study from September 2007 to September 2008 in Toronto and Ottawa, Canada, the cities with the highest HIV prevalence in the province of Ontario. Ontario (population = 12.6 million) has the highest proportion of HIV-positive persons in Canada (population = ∼34.3 million)25; of the estimated 65 000 persons living with HIV nationally, 44.4% live in Ontario.26 In 2008, Toronto (population = 2.5 million), Ontario's largest city, accounted for 65.3% and Ottawa (population = 870 000), the second largest city, for 11.3% of new HIV infections in the province.27
Similar to the United States, in Canada men who have sex with men are estimated to account for the greatest proportion (44%) of new infections; 17% are diagnosed in injection drug users.26 Ethnic/racial disparities in HIV prevalence are found among Black Canadians from African and Caribbean countries (2.2% of Canada's population and 16.0% of new infections) and among Aboriginal peoples (3.8% of Canada's population and 12.5% of new HIV infections) in 2008.26
Toronto and Ottawa each have 1 central AIDS service organization; both agreed to collaborate in our study. We partnered with 5 additional community organizations in Toronto, selected because they served different key populations at high risk of HIV exposure and to ensure inclusion of men and women. Ottawa does not have a similar diversity of organizations providing HIV-related services; thus we relied on the central AIDS service organization, which is networked with many communities.
Staff and peer research assistants at the 7 community organizations conducted recruitment by notifying clients of the study and posting flyers on-site. To protect client confidentiality, we conducted all contact with potential participants through community organizations. Inclusion criteria were being 18 years old or older and identifying as 1 of the designated study populations. All participants provided written consent and received a $30 honorarium and transportation reimbursement.
We conducted 9 focus groups lasting 90 minutes: Aboriginal men (1) and women (1), African and Caribbean Black women (2), gay men and men who have sex with men (2), male (1) and female (1) injection drug users, and female sex workers (1). In 6 groups, participants' HIV status was negative or unknown; the other 3 comprised HIV-positive participants (Aboriginal men, 1 group of African and Caribbean women, and men who have sex with men). Because Toronto was the Ontario site of the Step Study, had a larger population, and had higher HIV prevalence than Ottawa did, we conducted 6 groups in Toronto and 3 in Ottawa.
Subsequent to the focus groups, we conducted 6 key informant interviews with community advocates and health care providers. Criteria for inclusion of key informants were referral by a community organization as someone with expertise on at least 1 of the study populations and 5 or more years of community or health care experience.
We conducted focus groups and key informant interviews in a private room on-site at community agencies. Three key informants were interviewed off-site at a mutually agreed-upon location. Two experienced facilitators, a doctoral candidate (C. L.) and a service provider–peer researcher from the selected population, co-led each group. Facilitators explained ground rules at the beginning of each group, including respect for confidentiality and diversity of opinion. We used a semistructured interview guide (see box on the next page) for focus groups and analogous questions for key informants, whose responses reflected their perspectives on community reactions. At the end of each focus group, facilitators distributed a brief, anonymous, self-administered sociodemographic questionnaire. We conducted focus groups as part of an iterative process in which we assessed emerging themes in subsequent focus groups, a form of member checking.28 Key informants also reflected on participant data, which contributed to data analysis and interpretation. We achieved theoretical saturation: no new themes emerged in the final focus groups and interviews.28,29
Semistructured Interview Guide for Study of Reactions to the Step Study HIV Vaccine Trial: Ontario, Canada, 2007–2008
| 1. To start with, we're going to discuss what vaccines are. You may have received a vaccine at some time in your life. We want to know what you know or what other members of your community may have heard about vaccines. |
| 2. What, if anything, have you or people from your community heard about vaccines to protect against HIV/AIDS or HIV vaccine trials, that is, medical studies to test possible HIV vaccines? |
| 3. What kinds of concerns might people in your community have about participating in an HIV vaccine trial? What might we tell people at risk for HIV in your community to help them decide about whether to participate or not in an HIV vaccine trial? |
| Now, I would like to give you a little information about an HIV vaccine trial that recently took place, with one of the trial locations in downtown Toronto at the Maple Leaf Medical Clinic: A test vaccine that was being developed to prevent HIV/AIDS failed in an experimental study. The study volunteers were all free of HIV at the start of the HIV vaccine trial; but they were people who were at high risk for getting HIV due to risk behaviors: most were gay, bisexual men or MSM or female sex workers. They were all repeatedly counseled in the vaccine trial about how to reduce their risk of getting HIV, including use of condoms, during the trial. But the test vaccine didn't work to protect against HIV infection and the trial was stopped early. Some of the volunteers became infected due to risk behaviors. |
| 4. Please tell me your immediate reactions upon hearing about this. Or, if you heard about this trial shutdown before, please describe your thoughts and reactions. |
| Finally, a very surprising outcome was that some of the volunteers who got the test vaccine (vs the placebo or inactive substance) seemed to be placed at higher risk for HIV. Everyone in the trial was then told whether they had gotten the test vaccine or placebo; and it was explained that IF they came into contact with HIV through unprotected sex or sharing needles or drug injecting equipment, they might be more likely to get HIV than someone who didn't get the test vaccine. |
| 5. Any further reactions upon hearing about this trial? |
| 6. To what extent do you think people in your community would understand that the negative results were accidental and unexpected? |
| 7. To what extent would people in your community understand that the vaccine itself didn't give anyone HIV, but it made them more susceptible (or likely to become infected) IF they came into contact with HIV? [explain here if necessary to clarify] |
| 8. How might we explain this trial and the results to people in your community? To what extent might it affect your or others in your community's willingness to participate in an HIV vaccine trial in the future? |
| 9. Finally, is there anything else that you would like to add that we might not have asked you about? Is there anything that you think is particularly important in your culture or community when talking about HIV vaccines and HIV vaccine trials? |
Note. MSM = men who have sex with men.
Data Analysis
We digitally recorded all focus groups and interviews; these were transcribed verbatim. We then used narrative thematic techniques from grounded theory and a constant comparative method to analyze the data.23,28 All transcriptions were uploaded into NVivo version 7 software (QSR International, Victoria, Australia).
We used multiple forms of coding to examine latent patterns in participant narratives: open coding to identify, name, describe, and categorize phenomena in the text; axial coding to connect codes to one another; and selective coding to identify themes.23,28,29 We resolved differences in coding by consensus. We then ranked themes according to the number of groups in which they arose. Finally, we used theoretical coding28,29 to organize themes into a conceptual model. We used triangulation of data sources (men and women from different racial/ethnic and sexual minority groups, and key informants and focus group participants) and methods (focus groups and interviews) to enhance the validity of the findings.30
RESULTS
We report sociodemographic characteristics of focus group participants (n = 72) in Table 1. Overall, participants' mean age was 39.5 years; 60% were women and more than two thirds were people of color. Half self-identified as gay, lesbian, bisexual, or queer. Approximately half (46%) had a high school degree or less education, and approximately one quarter (28%) were employed.
TABLE 1.
Sociodemographic Characteristics of Focus Group Participants in Study of Reactions to the Step Study HIV Vaccine Trial: Ontario, Canada, 2007–2008
| Characteristic | Range (Mean) or No. (%) |
| Age, y | 21–66 (39.5) |
| Monthly income, $ | 0–4000 (1272) |
| Gender | |
| Men | 29 (40) |
| Women | 43 (60) |
| Ethnicity (n = 59) | |
| Aboriginal | 12 (20) |
| African/Caribbean | 21 (35) |
| Asian/South Asian/mixed | 3 (6) |
| Latino | 4 (7) |
| White | 19 (32) |
| Born in Canada | |
| Yes | 46 (65) |
| No | 25 (35) |
| Sexual orientation | |
| Gay | 22 (31) |
| Lesbian | 3 (4) |
| Bisexual | 4 (6) |
| Heterosexual | 36 (50) |
| Queer/other | 7 (10) |
| Relationship status | |
| Single | 46 (64) |
| Married | 8 (11) |
| Common law | 7 (10) |
| Separated/divorced | 6 (8) |
| Other | 5 (7) |
| Education (n = 69) | |
| < high school diploma | 16 (23) |
| High school diploma | 16 (23) |
| Some college | 22 (32) |
| Bachelor's degree | 11 (16) |
| Graduate degree | 4 (6) |
| Employment status | |
| Full time | 12 (17) |
| Part time | 8 (11) |
| Unemployed | 17 (24) |
| Social assistance | 11 (16) |
| Disability | 22 (31) |
| Retired | 1 (1) |
| HIV serostatus | |
| Negative/unknown | 50 (69) |
| Positive | 22 (31) |
Note. Sample size = 72.
Key informants were 5 women and 1 man, 3 Whites, 1 African, 1 Caribbean, and 1 Aboriginal. Two were coordinators of community-based HIV education and prevention projects, 2 were community service providers engaged in HIV education and advocacy, 1 was a program coordinator for female sex workers, and 1 was a physician who treated HIV-positive patients and conducted clinical trials.
We identified 9 themes in qualitative analysis, presented in 3 conceptual categories according to the stage of the trial process: pretrial, trial implementation, and posttrial. Table 2 shows themes in rank order according to the number of groups in which each theme arose, focus groups (and groups with > 50% of participants endorsing the theme) and key informants who endorsed each theme, and illustrative quotations from focus group participants and key informants.
TABLE 2.
Themes and Quotations From Focus Group Participants From Key Populations at High Risk of HIV Exposure and Key Informants on Reactions to the Step Study HIV Vaccine Trial: Ontario, Canada, 2007–2008
| Themes | Endorsement | Quotations |
| Preventive misconception | Focus groups (6 Toronto, 2 Ottawa): HIV-negative Aboriginal women,a HIV-negative African/Caribbean women,a HIV-positive African/Caribbean women, female sex workers,a HIV-negative MSM,a HIV-positive MSM,a female IDUs, male IDUs | “They had the Superman syndrome; they acted like they conquered the disease; because they had the vaccine because they didn't realize it was still a trial.” (Aboriginal woman) |
| “The understanding of vaccine to the general public means I am immune: you have given me the invisible cloak; you've given me the Superman suit. I'm all good.” (African/Caribbean key informant) | ||
| Key informants: African/Caribbean women, clinical researcher, female sex workers, IDUs | “They're going to practice even more high risk. I can guarantee it.” (HIV-negative MSM) | |
| “Counseling about risk reduction was a big part of the study. I don't think it really makes that much of a difference in that really high-risk population. There's probably a small proportion of the people that we could talk to until we're blue in the face and they're going to do what they do.” (clinical researcher key informant) | ||
| HIV vaccine as a common good | Focus groups (5 Toronto, 2 Ottawa): HIV-negative African/Caribbean women,a HIV-positive African/Caribbean women,a female sex workers,a HIV-negative MSM,a female IDUs, male IDUs | “We have to continue to be part of those trials in one way or another; it's the only way to find a cure.” (HIV-negative MSM) |
| “At the end of the day having the vaccine would help anybody who's experienced rape or sexual assault. As women, especially sex workers, they are at a higher risk of being vulnerable to assaults and violence; having a vaccine would benefit us all.” (female sex worker key informant) | ||
| Key informant: female sex workers | “They need to take it back to the lab and rework it.” (HIV-negative African/Caribbean woman) | |
| Targeted recruitment of vulnerable participants | Focus groups (6 Toronto, 1 Ottawa): HIV-negative African/Caribbean women,a female sex workers,a female IDUs,a male IDUs, HIV-negative Aboriginal women, HIV-negative MSM, HIV-positive MSM | “Why didn't they use college girls? Why did they use sex trade workers?” (HIV-negative African/Caribbean woman) |
| “You want the whole community; everyone should be involved. There should be the upper-class, White, working, professional male, and the down-to-the-street addicted individual. There should be every race, every class. That way it's being tested so that all populations can say this is something that is correct data.” (female sex worker key informant) | ||
| Key informants: Aboriginal peoples, African/Caribbean women, clinical researcher, female sex workers, IDUs, MSM | “Definitely there are some people who are too vulnerable, injection drug users, for one; they're too busy focusing on their fix. I don't think they can focus on anything else.” (Aboriginal key informant) | |
| Dissemination of previous HIV vaccine trial results | Focus groups (3 Toronto, 3 Ottawa): HIV-positive Aboriginal men,a HIV-negative African/Caribbean women,a HIV-positive African/Caribbean women,a female sex workers,a HIV-negative MSM,a HIV-positive MSMa | “‘This is why we had to stop the trial’; be very clear and very honest because while many people are suspicious, I wouldn't say our community is completely unwilling to engage in advances of medicine; but we'd like to do it feeling that we've come in with the best knowledge possible.” (African/Caribbean key informant) |
| Key informants Aboriginal peoples, African/Caribbean women, clinical researcher, female sex workers, IDUs, MSM | “What were the trials like in labs? Did they do it on mice? Did they do it on pigs? Did they do it on monkeys? What was their rationale to now bring it out and say, ‘Okay, I think we're ready’?” (HIV-negative African/Caribbean woman) | |
| “Basically I would like to know everything from A to Z and go from there.” (HIV-positive MSM) | ||
| “It's going to be hard to find people to go to trials; I think it's going to be really hard.” (HIV-negative MSM) | ||
| Mistrust and conspiracy | Focus groups (4 Toronto, 2 Ottawa): HIV-positive Aboriginal men,a HIV-negative Aboriginal women,a HIV-negative African/Caribbean women,a HIV-positive African/Caribbean women, female IDUs, male IDUs | “The damage has already been done, the conspiracy theory comes back: ‘The government is trying to wipe us all out. They don't really care about us as people… . Now that I've had the vaccine, I'm at higher risk.’ I don't know if someone from my population would be able to grasp that as not the vaccine's fault.” (female sex worker key informant) |
| Key informants: Aboriginal peoples, African/Caribbean owmen, female sex workers | “Trickery, what a piece of trickery. How are you going to tell me I have a vaccine and then tell me I'm going to get HIV at the same time? I don't care how much you sell it. A regular person would think, ‘that doesn't add up; this is you guys messing around with people's lives again.’ … That whole thing is suspect because there's historical documentation of the White medical institution using Black bodies for physiological experiments.” (African/Caribbean key informant) | |
| “Our people don't trust the government anymore because we've been cheated so many times. The Whites brought polio to our people, other diseases. It's the government telling us, ‘take it, it will not hurt you.’” (HIV-positive Aboriginal man) | ||
| “They [IDUs] hate them [medical providers]; they feel persecuted by them; they feel belittled and judged, and I don't blame them because they are… . There has to be some repair done. I think they've widened the gap of mistrust. I might feel like I was lied to.” (IDU key informant) | ||
| Informed consent | Focus groups (3 Toronto, 2 Ottawa): HIV-negative Aboriginal women,a HIV-negative African/Caribbean women,a HIV-positive African/Caribbean women, female sex workers, HIV-positive MSM | “We can talk about saturated fats and unsaturated fats; people can understand that. But the language for communicating about a vaccine is not in most people's vocabulary.” (African/Caribbean key informant) |
| “There are a lot of very educated sex workers and there's a lot who have only made it to grade 7 or 8; so explaining what research is and making sure that the language is at their level.” (female sex worker key informant) | ||
| Key informants: Aboriginal peoples, African/Caribbean women, clinical researcher, female sex workers, IDUs, MSM | “Make sure they really, really know what they're doing; it's not just sit down in a group and explain—individually they need some counseling.” (female sex worker) | |
| “We would spend about an hour going over the consent process; we would clearly say that we don't know whether this vaccine is going to be beneficial or no help or cause harm. We really don't know; we're going into this completely blind.” (clinical researcher key informant) | ||
| Community engagement | Focus groups (3 Toronto, 1 Ottawa): HIV-negative Aboriginal women, HIV-positive African/Caribbean women, female IDUs, HIV-negative MSM | [Sex workers] “have their own subculture … speak the dialect of the population.” (female sex worker key informant) |
| “There has to be openness; it needs to be a public dialogue. It needs to be on the agenda. There needs to be people debating about it. It needs to be part of public discourse.” (African/Caribbean key informant) | ||
| Key informants: Aboriginal peoples, African/Caribbean women, clinical researcher, female sex workers, IDUs, MSM | “My community wants to feel empowered; they want to feel engaged. Engage them in the actual setting up of the trial, in recruiting for the trial. Include them in every aspect.” (IDU key informant) | |
| “One thing about First Nations: when we educate, we educate the whole community. They might start off with the youth, later we educate the elders. It affects everybody, HIV.” (Aboriginal key informant) | ||
| Altruism | Focus groups (2 Toronto, 1 Ottawa,): HIV-negative Aboriginal women,a HIV-positive Aboriginal men, HIV-positive African/Caribbean women | “I care about the future, I care about society, and I care about culture. I care especially about Indian folk; I care for my people. I want to help the women. What's a needle? If it's gonna save lives, tell me more.” (HIV-negative Aboriginal woman) |
| Key informants: female sex workers, MSM | “I want to be part of a cure.” (HIV-positive Aboriginal man) | |
| “I'm HIV-positive. If there is a vaccine or there is a trial that is going to help the next generation behind us, I'm willing to take that trial for the people of Africa, especially for the African women.” (HIV-positive African/Caribbean woman) | ||
| “Somebody says, ‘I'm participating in a trial’; immediately, people are going to say, ‘Why would you be participating in a trial; what have you been doing?’ The challenge is to flip that and say, ‘I'm doing this because this is important for the community; I want to be a part a cure for HIV and AIDS because this is a huge issue that's affecting millions and millions of people across the planet.” (MSM key informant) | ||
| Fear of vaccine-induced infection | Focus groups (Toronto): female IDUs,a HIV-negative Aboriginal women, HIV-negative African/Caribbean women | “‘Susceptible,' what does that mean? Unfortunately that further plays into this notion that you've injected me with something that's got HIV in it.” (MSM key informant) |
| Key informants: Aboriginal peoples, African/Caribbean women, female sex workers, IDUs, MSM | “There's a lot of misconceptions about what a vaccine actually is; people will think, ‘Oh my god, you want to poke me with HIV to make me immune; you're fucking nuts.'” (IDU key informant) | |
| “Whatever the disease is, they just put a teeny, teeny bit in your body; it's the disease itself that you're injecting.” (HIV-negative Aboriginal woman) | ||
| “Not everyone is going to be lining up, especially sex workers, due to the possibility of getting HIV from a vaccine.” (female sex worker key informant) |
Note. IDU = injection drug user; MSM = men who have sex with men.
More than 50% of participants in focus group endorsed the theme.
Pretrial Stage
Community engagement.
Community engagement emerged in narratives about mechanisms for inclusion of community representatives in trial planning and broader processes of ongoing public discourse. Participants depicted community engagement as physical involvement, such as engaging community liaisons and representatives from the early stages of trial planning, as well as a dialectical process. Several key informant narratives used metaphors of language—word of mouth, speak the dialect, and public discourse—to illustrate the importance of meaningful communication.
Altruism.
The social meaning of HIV vaccine trials as a vital community undertaking emerged as an element of continued support for HIV vaccine trials, more so than individual motivations for protection against HIV infection. Participants invoked altruism and giving back to one's community under the rubric of this communitarian construction of an HIV vaccine trial. They also discussed how a public discourse of HIV vaccine trials as a communitarian venture may contribute to transforming the stigma directed at populations associated with HIV infection, which might otherwise act as a disincentive to involvement in or even discussion about HIV vaccine trials.
Fear of vaccine-induced infection.
Preexisting mental models of vaccines led some participants to negative conclusions about the Step Study results and inferences of disingenuousness on the part of medical researchers. A mental model of vaccines as including a small dose of a pathogen to induce an immune response informed participants’ understanding of the difficult concept of increased susceptibility to HIV infection. As a result, participants conflated susceptibility with vaccine-induced infection. Several participants and key informants revealed a mental model of live virus vaccines among their communities. The shared understanding among scientists and public health authorities that live virus HIV vaccines will not be developed and tested may not have broadly penetrated a public discourse of vaccines as carrying a small dose of HIV.
Trial Implementation Stage
Targeted recruitment of vulnerable participants.
Concerns about targeted recruitment and the vulnerability of volunteers in clinical trials stemmed from perceptions of justice and fairness rather than from the scientific basis for trial eligibility criteria. Participants articulated mixed perspectives on whether certain populations might be too vulnerable to involve in clinical trials. Injection drug users were portrayed as too vulnerable because active addiction might render financial incentives tantamount to coercion.
Participant narratives revealed a mental model of social justice in articulating the importance of engaging research volunteers from the general public as well as populations at high risk. From the perspective of medical eligibility criteria to power a trial, such concerns might be read as unfounded and as evidence of misunderstanding. However, in light of social representations of ongoing and widespread health disparities, and past examples of medical research that may not have been conducted in the best interests of marginalized communities, concern with justice forms a coherent narrative. From this standpoint, participation by individuals from key populations at high risk is justified to the extent that the end result would directly benefit their communities.
Informed consent.
Participant narratives about the informed consent process belied a belief in the omniscience of medical professionals. Challenges of explaining complicated clinical trial and vaccine concepts reflected the wide range of educational levels and comprehension among potential volunteers. Furthermore, participants revealed a belief that medical professionals should be able to anticipate every contingency and trial risk. Paradoxically, doubts about the integrity of informed consent may be furthered by a mental model that presumes omniscience on the part of medical researchers, thus precluding an appreciation of the potential fallibility of medical expertise and science.
Preventive misconception.
Participants across all groups articulated concerns about a widespread preventive misconception among trial volunteers. Although most participants did not suggest that volunteers in clinical trials would intentionally enroll in a trial to enable greater sexual risk behavior, many attributed HIV infections that occurred among volunteers in the Step Study to increased risk behaviors triggered by perceived protection conferred by the trial vaccine. The image of a Superman suit in several focus group narratives invoked a mental model of invincibility conferred by HIV vaccine trial participation. By contrast, a key informant characterized a subgroup of trial volunteers as high risk before, during, and after the trial.
Posttrial Stage
Dissemination of HIV vaccine trial results.
Participants used the Step Study as an example to illustrate the importance of providing comprehensive and transparent information about outcomes of previous HIV vaccine trials to populations at risk and potential participants in subsequent trials. Participants advocated providing broad, lay-language information to support informed decision-making about trial participation. Furthermore, participants and key informants alike expressed a desire for information on the history of and rationale for launching particular experimental vaccines in clinical trials and clear explication of trial results.
Mistrust and conspiracy.
Despite the best intentions of investigators focused on safe and ethical research, individuals from key populations at high risk may experience HIV vaccine trials as part of broader social phenomena. Interpretations of clinical trial processes and outcomes are subject to social representations based on historical and current experiences of exclusion and marginalization. Reactions to unexpected results from the Step Study were layered onto existing mistrust of medical research and government. In particular, African and Caribbean participants, Aboriginal participants, and injection drug users described ongoing negative experiences with medical providers that contributed to a contentious relationship with medical research. Key informant narratives suggested comprehension of the Step Study outcomes as unfortunate and unintended; however, they also revealed understanding of how the trial might exacerbate distrust among marginalized populations.
HIV vaccines as a common good.
Despite the early termination of the Step Study, participants supported the value of new HIV vaccine trials and the critical importance of a vaccine to their communities. Beyond medical studies, participants approached clinical trials for HIV vaccines as integral to advancing safety among key populations at high risk, because of their shared understanding of AIDS as a community threat. Most groups expressed tolerance for what some perceived as clinical trial failures, undergirded by a vision of HIV vaccines as a community good.
DISCUSSION
Our exploration of perspectives and reactions in the aftermath of the Step Study HIV-1 vaccine trial among key populations at high risk for HIV exposure revealed a public discourse of HIV vaccine trials underpinned by specific mental models and social meaning. Individuals constructed personal and social interpretations of HIV vaccine trials in their search for a coherent narrative to make sense of medical research. Formative research to access and understand evolving public discourse on HIV vaccine trials may provide an empirical foundation for knowledge translation and community engagement strategies to support the long-term process of HIV vaccine development.9
In general, participants interpreted the trial as a social phenomenon in the context of community disenfranchisement and historical examples of unethical medical research, rather than as an isolated medical study subject to the uncertainties of scientific discovery. Our findings further suggested specific interpretive pathways through which existing misconceptions and mistrust regarding medical research and vaccines may result in confusion and misunderstanding in the aftermath of the Step Study.
A narrative emerged in a social representation of unfair recruitment practices for HIV vaccine trials, from a perspective of social justice rather than from a framework of medical eligibility criteria. Participants construed targeting recruitment to high-risk volunteers as unfair. Furthermore, somewhat paradoxically embedded in the narrative context of mistrust was a belief in medical researchers' omniscience; unexpected trial results were construed as neglect or disingenuousness on the part of researchers. This narrative of clinical trial recruitment did not suggest inability to understand medical eligibility criteria or science; it was a parallel construction of social meaning based on equally valid and enduring principles of justice and fairness that undergird the terms of engagement in democratic society. Although clinical trials may not benefit individual volunteers, trial sponsors' guarantees of access in the event of an efficacious product may contribute to community perceptions of fairness.
HIV infections that occurred in the course of the trial were often attributed to the trial itself, either through preventive misconception on the part of trial volunteers or by vaccine-induced infection, rather than to ongoing risk behaviors. In this context, the unexpected outcome that a subset of volunteers who received the experimental vaccine may have become more susceptible to HIV infection11,13 was interpreted in light of a mental model of vaccines as conferring immunity through exposure to live virus31 and in the context of social representations of past and present experiences of injustice in interactions with medical research and health care. As a consequence, susceptibility was conflated with vaccine-induced infection, and sexual risk behaviors were unilaterally attributed to preventive misconceptions about HIV vaccine trial participation.
Several themes revealed steadfast support for HIV vaccine research. The construction of HIV vaccine trials as a communitarian and global public health endeavor incorporated altruistic intentions to contribute to HIV vaccine development as a way of giving back to and protecting one's community and humanity—a finding supported by previous research on willingness to participate in HIV vaccine trials.21,22 A counternarrative to mistrust was revealed in some participants’ demonstrated understanding of the incremental nature of scientific progress and the fallibility of science, a framework that tended to mitigate ascriptions of blame to medical researchers.
Overall, men's and women's groups largely concurred in their responses; differences arose primarily in women's discussions of HIV infection risks that were not in their control (e.g., from violence and sexual assault), which enhanced their support for HIV vaccine trials. Women's participation in endeavors to support biomedical HIV prevention could be constructed as taking agency or empowerment, a narrative mobilized by the Global Campaign for Microbicides.32
Notably, HIV-positive and -negative groups alike voiced support for HIV vaccine research; HIV-positive participants indicated a desire to give back to their community and prevent further infections, even if a vaccine might not benefit them personally. In accordance with UNAIDS recommendations,33 this finding supports important roles for persons living with HIV (e.g., as community liaisons) in promoting research on preventive as well as therapeutic vaccines. The narrative of altruism also offers a counterpoint to the sensationalist construction of a criminalization discourse regarding HIV-positive persons and disease transmission.34 Furthermore, the notion that persons living with HIV are relevant only to the quest for therapeutic vaccines may reflect an overly narrow focus of sociobehavioral research on HIV vaccine trials in terms of willingness to participate.
Groups in Toronto and Ottawa endorsed 8 of the 9 themes. Fear of vaccine-induced infection, the least endorsed theme, was expressed as a concern only in Toronto; this theme may have reflected an important mental model underlying community interpretations of HIV vaccine trials,31 but it appeared not to be a primary factor in willingness to participate in clinical trials.
Key informants endorsed all themes raised in focus groups. Community groups largely attributed risk behaviors in the trial to preventive misconception; key informants, however, articulated that a subgroup of participants were likely to continue high-risk behaviors that were neither fueled by trial participation nor mitigated by prevention counseling. Key informants also provided broader perspectives on community engagement, reflecting their experience as community leaders and HIV prevention advocates. Focus group participants, by contrast to key informants, more extensively stressed the importance of HIV vaccines as a community good.
Our key informants largely concurred on emerging themes. Notably, mistrust and conspiracy were uniformly articulated by key informants from African and Caribbean communities and Aboriginal communities, but not by White respondents. This may reflect the prominence of concerns about trust in health care and government among Aboriginal, African, and Caribbean communities in Canada.
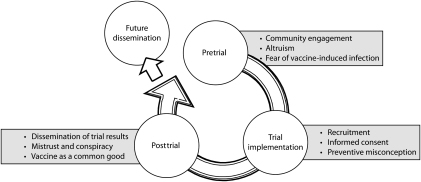
Figure 1 illustrates a conceptual framework that encompasses all themes in our analysis and their interrelationships. We categorized the 9 interpretative themes across pretrial (community engagement, altruism, fear of vaccine-induced infection), trial implementation (targeted recruitment, informed consent, therapeutic misconception), and posttrial (dissemination of trial results, mistrust and conspiracy, vaccine as a common good) dimensions.
FIGURE 1.
Themes that emerged from focus groups and key informant interviews across the trajectory of HIV vaccine trials.
Our model suggests that challenges arising at each trial stage influence later trial stages. For example, tenuous community engagement may exacerbate later mistrust; meaningful engagement may mitigate mistrust and support conceptions of trials as serving the common good. The model also depicts a cycle in which results from initial trials affect planning and implementation of future trials: for example, transparent and effective dissemination of results may support ongoing community engagement and future informed consent. Finally, we envision the full cycle of HIV vaccine trials as laying the foundation for future HIV vaccine dissemination.10 This model may prove to be a useful heuristic for future social science investigations conducted in tandem with HIV vaccine and other biomedical prevention trials; overall, it suggests the importance of incorporating social science research throughout the cycle of HIV vaccine development, from pretrial to clinical trial and posttrial stages.
Limitations
We conducted purposive sampling of participants from 1 geographical region. We did not have approval or resources to extend the investigation to US or other international Step Study sites. Generalizability is not an inherent aim of qualitative research; further studies are therefore needed to assess the representativeness of the findings among populations at high risk for HIV and key stakeholders in other locales in Canada, the United States, or elsewhere.
Future studies might involve key stakeholders who represent civil society organizations, government, and other populations not included in our study. However, we successfully recruited ethnically and sexually diverse HIV-negative and -positive men and women in 2 cities across a variety of key populations at high risk of HIV exposure, who collectively contributed to a coherent narrative about HIV vaccine trials.
Conclusions
Scientific narratives regarding HIV vaccine trials, as in many areas of biomedicine, reveal disagreements among researchers, public health officials, and clinicians.1–7,11–14 In addition to evolving and sometimes contradictory scientific discourse about HIV vaccine research, an evolving public discourse is constructed among key populations at high risk as a productive means of interpreting complex clinical trial processes and outcomes, and making sense of the larger HIV research endeavor. Formative research to explore and uncover public discourse in the social meanings and mental models of HIV vaccines and clinical trials31—speaking the dialect—may provide evidence to support systematic processes of meaningful community engagement in HIV vaccine research.9,35
Amid ongoing counternarratives offered by AIDS denialists,36 antivaccination extremists,37 and neoconservatives,38,39 it is important to actively and strategically engage in coconstruction of public discourse on HIV vaccines. The renaissance in HIV vaccine development40 is a welcome opportunity to develop an integrated research approach that builds on our best social science in partnership with biomedical science.
Acknowledgments
This research was supported in part by the Ontario HIV Treatment Network (grant ROGB169) and the Canada Research Chairs Program.
Initial results from this study were presented at AIDS Vaccine 2010, Atlanta, GA.
The research was conducted in collaboration with AIDS Committee of Ottawa, AIDS Committee of Toronto, Streetlight Support Services, and Women's Health in Women's Hands. The lead author thanks Adrienne Chambon for methodological consultation on data analysis.
Human Participation Protection
The University of Toronto Research Ethics Board approved the study protocol.
References
- 1.Belshe R, Franchini G, Girard MP, et al. Support for the RV144 HIV vaccine trial. Science. 2004;305(5681):177–180 [DOI] [PubMed] [Google Scholar]
- 2.Burton DR, Desrosiers RC, Doms RW, et al. Public health. A sound rationale needed for phase III HIV-1 vaccine trials. Science. 2004;303(5656):316. [DOI] [PubMed] [Google Scholar]
- 3.Fauci AS, Johnston MI, Dieffenbach CW, et al. HIV vaccine research: the way forward. Science. 2008;321(5888):530–532 [DOI] [PubMed] [Google Scholar]
- 4.Khanlou H, Weinstein M. Enough is enough: instead of continuing to squander hundreds of millions of dollars on a futile quest for an HIV vaccine, focus AIDS spending on prevention, testing and treatment. AIDS Healthcare Foundation; March 23, 2008. Available at: http://www.increaseaidsfunding.ca/docs/baltimoresun_032308.pdf. Accessed February 4, 2011 [Google Scholar]
- 5.Leavy O. HIV vaccine results controversy. Nat Rev Immunol. 2009;9(11):755 [Google Scholar]
- 6.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220 [DOI] [PubMed] [Google Scholar]
- 7.Watanabe ME. Skeptical scientists skewer VaxGen statistics. Nat Med. 2003;9(4):376. [DOI] [PubMed] [Google Scholar]
- 8.Singh JA, Mills EJ. The abandoned trials of pre-exposure prophylaxis for HIV: what went wrong? PLoS Med. 2005;2(9):e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman PA. Towards a science of community engagement. Lancet. 2006;367(9507):302. [DOI] [PubMed] [Google Scholar]
- 10.Newman PA, Duan N, Kakinami L, Roberts KJ. What can HIV vaccine trials teach us about dissemination? Vaccine. 2008;26(20):2528–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corey L, McElrath MJ, Kublin JG. Post-step modifications for research on HIV vaccines. AIDS. 2009;23(1):3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med. 2008;205(1):7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watkins DI, Burton DR, Kallas EG, Moore JP, Koff WC. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat Med. 2008;14(6):617–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Dijk TA. Analyzing racism through discourse analysis. Some methodological reflections. : Stanfield JH, Dennis RM, Race and Ethnicity in Research Methods. Newbury Park, CA: Sage Publications; 1993:92–134 [Google Scholar]
- 16.Roth WD, Mehta JD. The Rashomon effect: combining positivist and interpretivist approaches in the analysis of contested events. Sociol Methods Res. 2002;31(2):131–173 [Google Scholar]
- 17.Fischhoff B, Bostrom A, Quadrel MJ. Risk perception and communication. Annu Rev Public Health. 1993;14:183–203 [DOI] [PubMed] [Google Scholar]
- 18.Morgan GM, Fischhoff B, Bostrom A, Atman CJ. Risk Communication: A Mental Models Approach. Cambridge, UK: Cambridge University Press; 2002 [Google Scholar]
- 19.Slovic P. Trust, emotion, sex, politics and science: surveying the risk-assessment battlefield. Risk Anal. 1999;19(4):689–701 [DOI] [PubMed] [Google Scholar]
- 20.Dhalla S, Nelson K, Singer J, Poole G. HIV vaccine preparedness studies in the non–Organization for Economic Co-operation and Development (non-OECD) countries. AIDS Care. 2009;21(3):335–348 [DOI] [PubMed] [Google Scholar]
- 21.Mills E, Cooper C, Guyatt G, et al. Barriers to participating in an HIV vaccine trial: a systematic review. AIDS. 2004;18(17):2235–2242 [DOI] [PubMed] [Google Scholar]
- 22.Newman PA, Duan N, Roberts KJ, et al. HIV vaccine trial participation among ethnic minority communities. J Acquir Immune Defic Syndr. 2006;41(2):210–217 [DOI] [PubMed] [Google Scholar]
- 23.Strauss AL, Corbin JM. Basics of Qualitative Research: Techniques And Procedures for Developing Grounded Theory. 4th ed Thousand Oaks, CA: Sage Publications; 1998 [Google Scholar]
- 24.Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community-based research: assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19:173–202 [DOI] [PubMed] [Google Scholar]
- 25.Statistics Canada Canada's population clock. November 18, 2010. Available at: http://www.statcan.gc.ca/ig-gi/pop-ca-eng.htm. Accessed February 4, 2011
- 26.Public Health Agency of Canada, Surveillance and Risk Assessment Division, Centre for Communicable Diseases and Infection Control HIV and AIDS in Canada: surveillance report to December 31, 2008. Available at: http://www.phac-aspc.gc.ca/aids-sida/publication/survreport/2008/dec/pdf/survrepdec08.pdf. Accessed February 4, 2011
- 27.Remis RS, Swantee C, Liu J. Report on HIV/AIDS in Ontario 2008. Ontario Ministry of Health and Long-Term Care. April, 2010. Available at: http://www.phs.utoronto.ca/ohemu/doc/PHERO2008_report_final.pdf. Accessed February 4, 2011
- 28.Charmaz K. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. Thousand Oaks, CA: Sage Publications; 2006 [Google Scholar]
- 29.Glaser BG. Doing Grounded Theory: Issues and Discussions. Mill Valley, CA: Sociology Press; 1998 [Google Scholar]
- 30.Kuper A, Reeves S, Levinson W. An introduction to reading and appraising qualitative research. BMJ. 2008;337:a288. [DOI] [PubMed] [Google Scholar]
- 31.Newman PA, Seiden DS, Roberts KJ, Kakinami L, Duan N. A small dose of HIV? HIV vaccine mental models and risk communication. Health Educ Behav. 2009;36(2):321–333 [DOI] [PubMed] [Google Scholar]
- 32.Global Campaign for Microbicides About microbicides. 2009. Available at: http://www.global-campaign.org/about_microbicides.htm. Accessed February 4, 2011
- 33.UNAIDS The UN declaration of commitment and people living with HIV/AIDS PWAs. 2001. Available at: http://data.unaids.org/pub/BaseDocument/2007/gipa2001un_en.pdf. 2001. Accessed February 4, 2011
- 34.Adam BD, Elliott R, Husbands W, Murray J, Maxwell J. Effects of the criminalization of HIV transmission in Cuerrier on men reporting unprotected sex with men. Can J Law Soc. 2008;23(1–2):143–159 [Google Scholar]
- 35.Newman PA, Yim S, Daley A, et al. “Once bitten, twice shy”: volunteer perspectives in the aftermath of an early HIV vaccine trial termination. Vaccine. 2011;29(3):451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalichman SC. Denying AIDS: Conspiracy Theories, Pseudoscience, and Human Tragedy. New York, NY: Springer; 2009 [Google Scholar]
- 37.Jacobson RM, Targonski PV, Poland GA. A taxonomy of reasoning flaws in the anti-vaccine movement. Vaccine. 2007;25(16):3146–3152 [DOI] [PubMed] [Google Scholar]
- 38.Marlow LAV, Forster AS, Wardle J, Waller J. Mothers' and adolescents' beliefs about risk compensation following HPV vaccination. J Adolesc Health. 2009;44(5):446–451 [DOI] [PubMed] [Google Scholar]
- 39.Human Papillomavirus HPV Vaccination Program Update. Toronto, Ontario, Canada: Medical Officer of Health; 2008. Available at: http://www.toronto.ca/legdocs/mmis/2008/hl/bgrd/backgroundfile-15460.pdf. Accessed February 4, 2011 [Google Scholar]
- 40.Koff WC, Berkley SF. The renaissance in HIV vaccine development—future directions. N Engl J Med. 2010;363(5):e7. [DOI] [PubMed] [Google Scholar]