Abstract
The tumor suppressor protein Par-4 (prostate apoptosis response-4) is spontaneously secreted by normal and cancer cells. Extracellular Par-4 induces caspase-dependent apoptosis in cancer cell cultures by binding, via its effector SAC domain, to cell surface GRP78 receptor. However, the functional significance of extracellular Par-4/SAC has not been validated in animal models. We show that Par-4/SAC-transgenic mice express systemic Par-4/SAC protein and are resistant to the growth of non-autochthonous tumors. Consistently, secretory Par-4/SAC pro-apoptotic activity can be transferred from these cancer-resistant transgenic mice to cancer-susceptible mice by bone marrow transplantation. Moreover, intravenous injection of recombinant Par-4 or SAC protein inhibits metastasis of cancer cells. Collectively, our findings indicate that extracellular Par-4/SAC is systemically functional in inhibition of tumor growth and metastasis progression, and may merit investigation as a therapy.
Key words: Par-4, metastasis, systemic protein
Introduction
Spontaneous metastasis of tumors from the primary site to distant tissues via the blood circulatory route ultimately results in mortality in advanced cancer patients.1 Moreover, perturbation of tumors during surgical manipulations results in the iatrogenic shedding of cancer cells in circulation and increases the risk of metastasis.2 There is a critical need for cancer therapeutics that can target not only the primary tumors but also metastasis, while keeping the normal healthy cells unharmed. Prostate apoptosis response-4 (Par-4) is a tumor suppressor protein expressed ubiquitously among the various tissue types.3–5 Consistent with its tumor suppressor function, Par-4 is mutated or silenced in diverse cancers;3,6,7 or is in a non-functional state due to inactivation by the cell survival kinase, Akt1, as in prostate cancer.8
Par-4 overexpression is sufficient to induce apoptosis in most cancer cells, but not in normal or immortalized/non-transformed cells.9 The core domain (aa137–195) of Par-4, designated SAC (selective for apoptosis in cancer cells), which is 100% conserved in rat, mouse and human Par-4, serves as the effector domain of Par-4.9,10 Recent studies have suggested that Par-4 is secreted by a brefeldin A sensitive pathway.11 Extracellular Par-4, acting via its SAC domain, binds to cell surface receptor GRP78 to induce apoptosis by triggering endoplasmic reticulum (ER)-stress and the caspase 8/caspase 3 pathway.11 Interestingly, extracellular Par-4/SAC induces apoptosis selectively in cancer cells.11 Normal/immortalized cells fail to respond to extracellular Par-4/SAC owing to lower GRP78 levels at the cell surface and lack of a robust ER-stress response.11 Consequently, although Par-4 is secreted by both normal and cancer cells, it induces apoptosis only in cancer cell cultures. However, the functional relevance of extracellular Par-4 or its SAC domain has not been tested in animal tumor models.
Results
SAC-transgenic mice secrete SAC apoptotic-activity in their serum to inhibit the growth of non-autochthonous tumors.
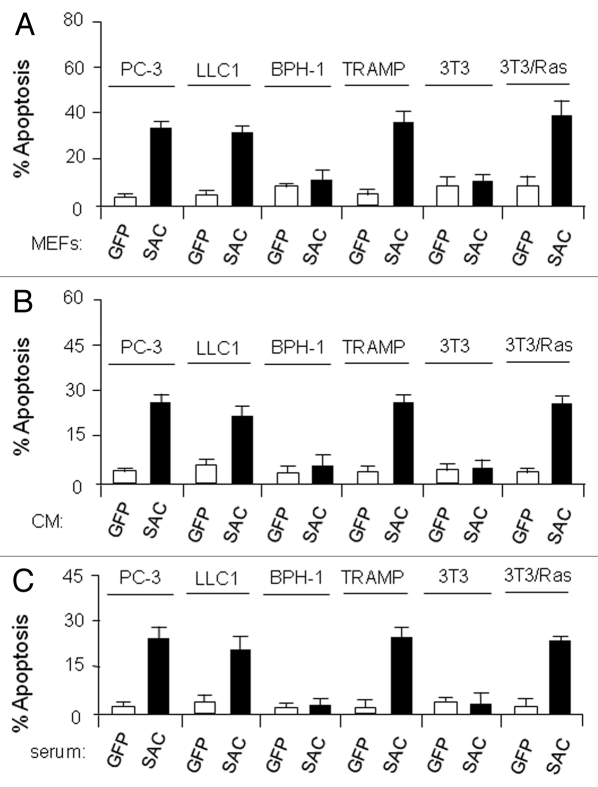
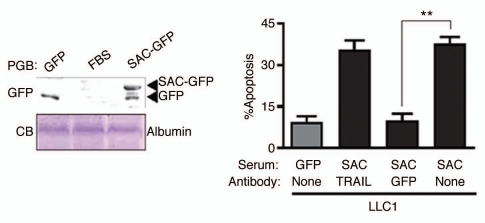
Transgenic mice ubiquitously expressing either full-length Par-4 or the SAC effector domain of Par-4 are resistant to the growth of spontaneous and inducible tumors.11,12 When MEFs from GFP- or SAC-GFP-transgenic mice were co-cultured with immortalized or transformed cells, the SAC-GFP MEFs but not the GFP control MEFs induced apoptosis in transformed cells (Fig. 1A). Similarly, conditioned medium (CM) from the SAC-GFP MEFs but not from GFP control MEFs induced apoptosis in the transformed cells (Fig. 1B). Neither the co-culture experiments nor the CM induced apoptosis in non-transformed cells (Fig. 1). In the light of these observations, we tested serum samples from the transgenic mice for apoptosis of several cell lines. Interestingly, aliquots of serum from SAC-GFP mice but not from GFP mice induced apoptosis of the transformed cells, not non-transformed cells (Fig. 1C). Protein gel blot analysis confirmed that the serum of SAC-GFP transgenic mice contained SAC-GFP protein (Fig. 2, left). Importantly, the apoptotic activity of the serum of SAC-GFP mice was neutralized by GFP antibody but not by TRAIL antibody (Fig. 2, right). These findings suggested that SAC protein secreted in serum can cause apoptosis of transformed cells.
Figure 1.
Cells from SAC-transgenic mice secrete SAC-activity. (A) SAC-MEFs induce apoptosis of co-cultured tumor cells. MEFs from SAC-or GFP-transgenic mice were cocultured with the indicated cell lines for 24 h and apoptosis was examined by ICC for active caspase 3. (B) SAC-protein in the CM from MEFs of SAC-transgenic mice induces apoptosis of cancer cells in culture. CM from the MEFs of SAC- or GFP-transgenic mice was incubated with various cell lines for 24 h and apoptosis was examined by ICC for active caspase 3. (C) SAC-protein in the serum of SAC-transgenic mice induces apoptosis of cancer cells in culture. Aliquots of serum samples from the SAC- or GFP-transgenic mice was incubated with various cell lines for 24 h and apoptosis was examined by ICC for active caspase 3.
Figure 2.
Tumor cell growth is inhibited by secreted SAC domain of Par-4. Aliquots of serum samples from the SAC-GFP-or GFP-transgenic animals were pre-incubated with GFP antibody or TRAIL antibody and then applied to LLC1 cells in culture, to score apoptosis by immunocytochemical anaysis for active caspase 3 (right). The serum samples were subjected to protein gel blot (PGB) analysis using the GFP antibody (left, top); the serum samples were also subjected to SDS-PA GE and Coomassie blue (CB) staining to reveal serum albumin for loading control (left, bottom). FBS, fetal bovine serum, was used as control serum. Mean values (±standard deviation bars) are shown. Asterisk (**) indicates the difference is statistically significant (p < 0.001) by Student's t-test.
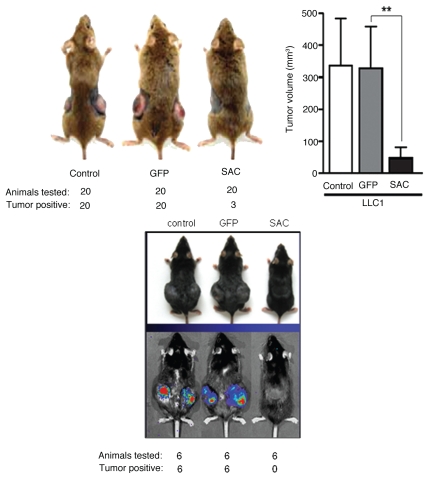
To address the relevance of Par-4/SAC secretion in the cancer-resistant phenotype of the transgenic mice, we injected LLC1 cells into both flanks of the GFP or SAC-GFP transgenic mice or littermate mice for control. Interestingly, the GFP- and littermate control mice allowed growth of tumors from the LLC1 cells, whereas the SAC-GFP transgenic mice were significantly resistant to the growth of LLC1 tumors (Fig. 3, top). These findings were further corroborated by luciferase imaging studies (Fig. 3, bottom). Together, these observations indicate that Par-4/SAC apoptotic activity is systemically present in the cancer-resistant transgenic mice, and it is associated with inhibition of non-autochthonous tumors.
Figure 3.
SAC-transgenic mice inhibit the growth of non-autochthonous tumors. LLC1 cells (5 × 106 cells/flank) were injected into both flanks of B6C3HF1 mice that ubiquitously expressed the SAC- or GFP-transgene or B6C3HF1 littermate control mice. After 28 days, the tumors were measured and the volume of the tumors (top, right) or growth of the tumors in representative animals (top, left) is shown. Mean values (±standard deviation bars) are shown. Asterisk (**) indicates the difference between tumor volume in GFP-and SAC-GFP transgenic mice is statistically significant (p < 0.001) by Student's t-test. For luciferase imaging of non-authochthonous tumor growth inhibition in SAC-transgenic mice, LLC1/luc cells that stably expressed the luciferase reporter gene driven by the CMV promoter were injected subcutaneously into C57/BL6 mice that expressed the SAC- or GFP-transgene or C57/BL6 littermate control mice, and 28 days later the animals were photographed or injected luciferin (the substrate for luciferase) for tumor-imaging (bottom).
SAC-activity is transferable from SAC-transgenic mice by bone marrow transplantation.
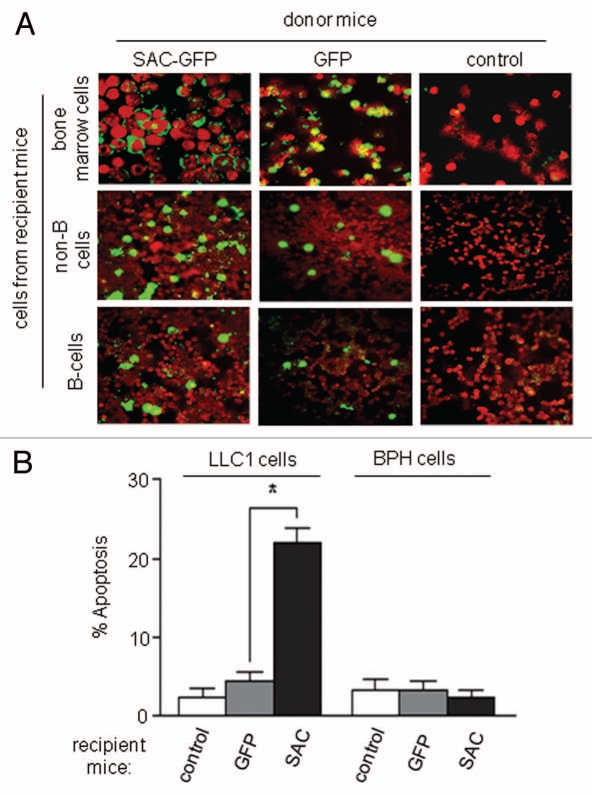
To further confirm that transgenic Par-4/SAC secretion in mice is functionally active, the bone marrow of SAC-GFP-transgenic mice, littermate control mice or GFP-transgenic control mice was transferred to irradiated littermate control mice. Colonization of the bone marrow and spleen of recipient mice with GFP+ donor cells was confirmed by immunohistochemical analysis (Fig. 4A). Moreover, the serum samples from mice that received bone marrow cells from SAC-GFP-transgenic mice, but not from control mice (GFP-transgenic or littermate), induced apoptosis of LLC1 cells in culture, implying that bone marrow transplantation had effectively transferred cells capable of secreting transgenic SAC-GFP activity into recipient mice (Fig. 4B). Together, these findings imply that SAC-killer activity is secreted and, therefore, transferable between mice by bone marrow transplantation.
Figure 4.
SAC-activity is transferable from SAC-transgenic animals to littermate control animals by bone marrow transplantation. (A) Colonization of donor cells in recipient mice. The bone marrow of SAC- or GFP-transgenic mice was transferred by i.v. injection to irradiated littermate control B6C3HF1 mice. Three months later, bone marrow cells and splenic B and non-B cells from the recipient animals were examined for expression of the SAC-transgene by ICC for GFP. (B) Systemic expression of SAC-GFP apoptotic activity in bone marrow recipient mice. Four months after the bone marrow transplantation, aliquots of serum samples from the recipient mice (that were injected with bone marrow from the SAC-GFP- or GFP-transgenic or littermate control donor mice) were incubated for 24 h with LLC1 or non-transformed BPH -1 cell cultures and ICC was performed for active caspase 3 to score apoptotic cells. Mean values (±standard deviation bars) are shown. Asterisk (**) indicates the difference is statistically significant (p < 0.001) by Student's t-test.
Recombinant Par-4 and SAC proteins inhibit lung metastasis in mice.
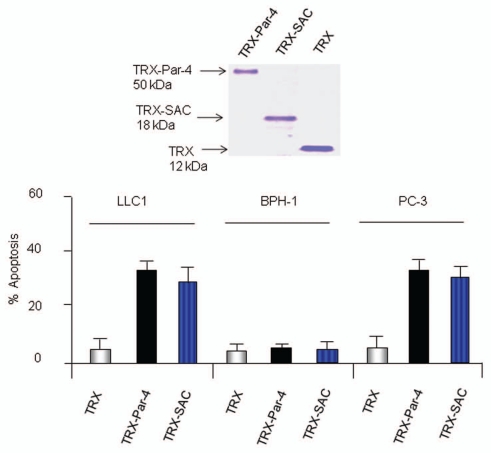
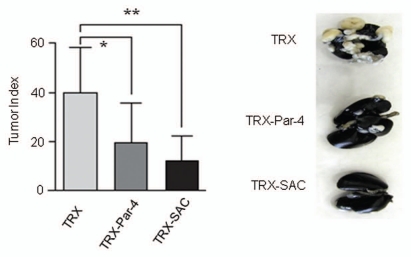
To determine whether the Par-4/SAC activity in serum of transgenic mice inhibited metastasis, we used recombinant TRX-Par-4 or TRX-SAC protein, which was previously shown to induce apoptosis of cancer cells but not non-transformed cells in culture.11 As expected, TRX-Par-4 or TRX-SAC, but not TRX control protein, induced apoptosis of LLC1 and PC-3 cells but not non-transformed BPH1 cells in culture (Fig. 5). Moreover, TRX-Par-4 or TRX-SAC protein introduced intravenously in immunocompetent C57/BL6 mice significantly inhibited lung metastasis of LLC1 cells (Fig. 6). These findings confirmed that extracellular Par-4 or SAC is functionally active in inhibition of metastasis.
Figure 5.
Recombinant Par-4 or SAC induces apoptosis in cell culture. LLC1, BPH 1 or PC-3 cells were treated with TRX-Par-4, TRX-SAC or TRX (100 nM each) for 24 h. The cells were then scored for apoptosis by ICC for caspase 3 activity (bottom). Integrity of the recombinant proteins was verified by SDS-PA GE and Coomassie blue staining of the gel (top).
Figure 6.
Recombinant Par-4 or SAC protein inhibits the growth of metastatic tumors. LLC1 cells (1.5 × 105 cells) were injected into the tail vein of B6C3H mice. After 5 h, the mice were injected via the tail vein with the indicated recombinant protein (500 µg per mouse). Four weeks later, the mice were humanely killed and the lungs were photographed (right). The lung nodules were scored and the data are expressed as tumor index (left). Mean values (±standard deviation bars) are shown. Asterisk (*) indicates the difference between TRX and TRX-Par-4 treatment is statistically significant (p = 0.0069) and (**) indicates the difference between TRX and TRX-SAC is statistically significant (p = 0.0003) by Student's t-test.
Discussion
The findings of the present study indicate that Par-4/SAC, secreted by normal cells, is systemically active in suppressing tumor growth in mice. Par-4/SAC pro-apoptotic protein activity, evident in the serum of cancer-resistant Par-4/SAC transgenic mice, was associated with inhibition of non-autochthonous tumors. Moreover, bone marrow transplantation resulted in transfer of Par-4/SAC pro-apoptotic activity from the cancer-resistant transgenic mice to cancer-susceptible mice, further corroborating the secretion of functional Par-4/SAC by normal cells. Consistent with these observations, recombinant Par-4 or SAC protein induced apoptosis of cancer cells in cell culture and inhibited experimental metastasis of lung cancer cells. All of these findings in immunocompetent mice imply that Par-4/SAC protein is systemically active over extended time periods in inducing apoptosis of cancer cells, leading to inhibition of tumor growth and metastasis. Most tumor suppressor proteins inhibit tumor growth by acting within the intracellular context of the tumor cells. By contrast, owing to its dual mode of action; i.e., intracellular, as well as extracellular apoptotic pathways;4 Par-4 protein inhibits the growth of primary tumors,4,12 and also metastasis (this study). These unique features of Par-4/SAC can be a focus of cancer-selective therapeutics, especially to inhibit metastasis associated with spontaneous or iatrogenic shedding of cancer cells.
Materials and Methods
Cell culture, recombinant proteins and mice.
Human immortalized epithelial cells BPH-1, prostate cancer cells PC-3 and mouse LLC1 cells were purchased from American Type Culture Collection, MD. TRAMP cells were from Michael Greenberg (Fred Hutchinson Cancer Center, Seattle, WA). Immortalized NIH 3T3 cells and Ras-transformed NIH (3T3/Ras) cells, and the medium for growth of the cell lines were previously described in reference 9. The prokaryotic expression constructs for thioredoxin (TRX), TRX-Par-4 or TRX-SAC, were introduced into E. coli BL21DE3 cells and the fusion proteins were purified as previously described in reference 11. Caspase 3 antibody was from Cell Signaling, Inc. The monoclonal antibody for β-actin was from Sigma Chemical Corp. All other antibodies were from SantaCruz Biotechnology, Inc. (Santa Cruz, CA).
All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Kentucky. Par-4-GFP, SAC-GFP and GFP transgenic mice generated in B6C3H strain background have been described in reference 11 and 12. Bone marrow transplantation was done using SAC-GFP or GFP-transgenic B6C3H mice or littermate B6C3H mice as donors, and littermate B6C3HF1 mice (from Jackson Laboratory, ME) as recipients. Twenty-four hours prior to bone marrow transplantation, the recipient mice were exposed to total body gamma irradiation for 3 minutes to 900 Rad, delivered by a 137Cs gamma ray source for bone marrow ablation. The mice were then transplanted with 5 × 106 bone marrow cells from donor mice. Three months later, cells from the recipient animals were examined for expression of the transgene.
Splenic-cell sorting, protein gel blot analysis, immunocytochemical and apoptosis analysis.
B lymphocytes and non-B cells from the spleen were separated using anti-B220 antibody coupled magnetic beads and Miltenyii AutoMacs cell separator. Immunocytochemical analysis procedures have been described in reference 9 and 11. Apoptotic nuclei were identified by caspase 3 immunostaining and 4,6-diamidino-2-phenylindole (DAPI) staining. A total of three independent experiments were performed; and approximately 300 cells were scored in each experiment for apoptosis under a fluorescent microscope.9,11
Statistical analysis.
Statistical analyses were carried out with the Statistical Analysis System software Version 9.2 (SAS Institute, Cary, NC). Student's t-statistic was used to calculate between-group comparison p values. For the animal experiments, group-wise comparisons were made using ANOVA where p values were calculated using Student's t-test.
Acknowledgments
This study was supported by KLCR grant, NIH/NCI grants CA060872, CA105453 and CA116658 (to V.M.R.).
References
- 1.Cooperberg MR, Broering JM, Carroll PR. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J Natl Cancer Inst. 2009;101:878–887. doi: 10.1093/jnci/djp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eschwège P, Moutereau S, Droupy S, Douard R, Gala JL, Benoit G, et al. Prognostic value of prostate circulating cells detection in prostate cancer patients: a prospective study. Br J Cancer. 2009;100:608–610. doi: 10.1038/sj.bjc.6604912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sells SF, Wood DP, Jr, Joshi-Barve SS, Muthukumar S, Jacob RJ, Crist SA, et al. Commonality of the gene programs induced by effectors of apoptosis in androgen-dependent and -independent prostate cells. Cell Growth Differ. 1994;5:457–466. [PubMed] [Google Scholar]
- 4.Shrestha-Bhattarai T, Rangnekar VM. Cancer-selective apoptotic effects of extracellular and intracellular Par-4. Oncogene. 2010;29:3873–3880. doi: 10.1038/onc.2010.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Cao I, Duran A, Collado M, Diaz-Meco MT, Serrano M, Moscat J. Tumor-suppression activity of the proapoptotic regulator Par4. EMBO Rep. 2003;6:577–583. doi: 10.1038/sj.embor.7400421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook J, Krishnan S, Ananth S, Sells SF, Shi Y, Walther MM, et al. Decreased expression of the pro-apoptotic protein Par-4 in renal cell carcinoma. Oncogene. 1999;18:1205–1208. doi: 10.1038/sj.onc.1202416. [DOI] [PubMed] [Google Scholar]
- 7.Moreno-Bueno G, Fernandez-Marcos PJ, Collado M, Tendero MJ, Rodriguez-Pinilla SM, Garcia-Cao I, et al. Inactivation of the candidate tumor suppressor par-4 in endometrial cancer. Cancer Res. 2007;67:1927–1934. doi: 10.1158/0008-5472.CAN-06-2687. [DOI] [PubMed] [Google Scholar]
- 8.Goswami A, Burikhanov R, de Thonel A, Fujita N, Goswami M, Zhao Y, et al. Binding and phosphorylation of par-4 by akt is essential for cancer cell survival. Mol Cell. 2005;20:33–44. doi: 10.1016/j.molcel.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 9.El-Guendy N, Zhao Y, Gurumurthy S, Burikhanov R, Rangnekar VM. Identification of a unique core domain of par-4 sufficient for selective apoptosis induction in cancer cells. Mol Cell Biol. 2003;23:5516–5525. doi: 10.1128/MCB.23.16.5516-5525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Guendy N, Rangnekar VM. Apoptosis by Par-4 in cancer and neurodegenerative diseases. Exp Cell Res. 2003;283:51–66. doi: 10.1016/s0014-4827(02)00016-2. [DOI] [PubMed] [Google Scholar]
- 11.Burikhanov R, Zhao Y, Goswami A, Qiu S, Schwarze SR, Rangnekar VM. The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis. Cell. 2009;138:377–388. doi: 10.1016/j.cell.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, Burikhanov R, Qiu S, Lele SM, Jennings CD, Bondada S, et al. Cancer resistance in transgenic mice expressing the SAC module of Par-4. Cancer Res. 2007;67:9276–9285. doi: 10.1158/0008-5472.CAN-07-2124. [DOI] [PubMed] [Google Scholar]