Abstract
We performed a retrospective review of 281 hepatocellular carcinoma (HCC) patients with portal vein tumor thrombosis (PVTT) treated with radiation therapy (RT) between 1998 and 2008 to develop a prognostic model for those patients. Of the 281 patients, PVTT and intrahepatic main masses completely disappeared in 10 patients (3.6%), and shown a partial response in 141 patients (50.2%). The median survival was 11.6 months. Patients who had more than PR have shown significantly longer survival than the others (22.0 months vs 5.0 months, P < 0.001). On the multivariate analysis, pre-treatment poor prognosticators for overall survival were ECOG performance status, Child-Pugh class, multiple tumors, main PVTT, complete portal vein occlusion, lymph node metastasis, and primary tumor size. Prognostic index of RT for PVTT of HCC (PITH) scores were defined as the number of pre-treatment poor prognostic factors. PITH scores correlated well with overall survival. In the analysis of 1 and 2 yr overall survival rate, patients who had PITH scores of 3 or greater showed a significantly lower rate of overall survival than the others (33.0%, 17.3% vs 70.1%, 40.8%, respectively, P < 0.001). The PITH scoring model, proposed in the current study in HCC patients with PVTT, reliably predict overall survival.
Keywords: Carcinoma, Hepatocellular; Portal Vein Tumor Thrombosis; Radiotherapy; Prognostic Index
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide and especially in East Asia, and sub-Saharan Africa, where hepatitis B virus in endemic (1). Surgical resection is the first choice of treatment for HCC, but approximately 80% of cases are unresectable because of poor hepatic functional reserve associated with cirrhosis and multifocality of its presentation (2). Especially, portal vein tumor thrombosis (PVTT), which is a common complication in patients with advanced-stage HCC, with a reported incidence of 34% to 50% of these patients (3, 4), is a major determinant of patients' prognosis (5, 6). Because of a decrease in portal blood flow, consensus treatments for HCC, including surgical resection, and transcatheter arterial chemo-embolization (TACE) lack efficacy. Sometimes major treatment-related complications are inevitable in patients with PVTT.
Recently, several studies have shown that radiation therapy (RT), particularly, three-dimensional conformal radiation therapy (3D-CRT) is effective not only for tumor responses but also for survival in HCC patients with PVTT (7, 8). Median survival time on those patients was reported as long as 6 to 13 months. But, large differences were shown in the post-RT prognosis between individual HCC patients who were treated with RT. Some patients have shown sustained local control without further treatment measures after RT. Sometimes, RT provides good results to successive additional local treatment, like TACE, radiofrequency ablation or surgical resection. But there are no established guideline which divides patient group on which RT provides good treatment response and better prognosis.
The purpose of this study is to design prognostic model for HCC patients with PVTT who are treated with RT, and to divide those patients into prognostic groups.
MATERIALS AND METHODS
Diagnosis and clinical data
We conducted a retrospective study of all HCC patients with PVTT who were treated with RT at Samsung Medical Center between January 1998 and December 2008. During this period, total 742 patients were received RT because of primary liver cancer in our institution. Of these patients, 710 patients had been diagnosed as HCC. And 320 patients (45.1%) had vascular invasion from HCC. Without PVTT, inferior vena cava or hepatic vein was involved in 19 (2.7%). And 20 patients (2.8%) had PVTT in small branches of portal vein (segmental/section branches except main, left or right hemiliver portal vein). If the PVTT is confined to small branches only, the other local modality (surgery, radiofrequency ablation, TACE, etc) should be considered. In the current study, we analyzed 281 patients (39.6%) who had tumor thrombosis in the main, right or left hemiliver portal vein.
The diagnosis of HCC was made based on the guidelines proposed by the Korea Liver Cancer Study Group (9). Using these criteria, a patient is diagnosed as HCC if who has one or more risk factors (hepatitis B or C virus infection, and/or cirrhosis) and one of the following: a serum α-fetoprotein (AFP) level of > 400 ng/mL and a positive result with at least one of the three typical imaging techniques (triple phase computerized tomography [CT], contrast enhanced dynamic magnetic resonance imaging [MRI] or hepatic angiography); or a serum AFP level of < 400 ng/mL and positive findings with at least two of three imaging techniques. A positive finding for typical HCC with dynamic CT or MRI is indicative of arterial enhancement followed by venous washout in the delayed portal/venous phase.
PVTT was demonstrated using helical CT scans with contrast enhancement, MRI scans, or angiography. On contrast-enhanced CT scans, PVTT was identified by the presence of a low-attenuation intra-luminal filling defect adjacent to the primary tumor. If thrombi were located in both hemiliver branches and the main trunk, we categorized the thrombi as main PVTT because of the retrograde invasion of portal vein thrombosis. Differentiating malignant PVTT and benign PVTT may be difficult. Intrathrombus vascularity, observed in the arterial phase of imaging studies after the administration of contrast, has been reported to be a sign that is specific for PVTT on both CT and MRI (10). We assessed malignant PVTT based on these enhancing and/or growing pattern, response to treatment, and relationship with primary tumor.
The following clinical data were collected from the medical records: patient demographics; complete blood count; chemistry profiles; liver function test; AFP; size of the tumor and PVTT; number of the tumor; involved side of the liver by the tumor; involved site of the portal vein by the PVTT; length of the PVTT; completeness of the portal vein occlusion; presence of lymph nodes or distant metastasis; Eastern Cooperative Oncology Group (ECOG) performance status; Child-Pugh score and class; date of diagnosis; type of treatment; treatment response; date of last follow-up; live status; and cause of death.
Previous treatment
Two hundred forty two patients of 281 in the current series had received one or more treatments with surgical resection (19, 6.8%), radiofrequency ablation (35, 12.5%), percutaneous ethanol injection (3, 1.1%), TACE (232, 82.6%), RT (0.7%), chemotherapy (0.4%) before they were diagnosed as HCC with PVTT.
Radiation therapy
Before simulation, shallow-breathing during the procedure was asked and all patients got education and training. Then, respirespiratory motion of the liver was checked by fluoroscopy, and these data were used for determination of the margin. After that, all patients underwent contrast enhanced CT scans in a supine position for RT planning, with both arms raised above the head to facilitate use of lateral RT portals. CT scan data were transferred to a 3D-CRT treatment planning system (from 1998 to 2003, PROWESS, Alliant Medical Technology, Chico, CA, USA; from 2004 to 2008, PINNACLE, The Philips Medical System, Best, Netherland).
The PVTT, main mass, the normal liver, kidneys, duodenum and spinal cord were contoured and reconstructed to form a 3D representation. The RT beam angles were designed to minimize critical organ injury. A dose-volume histogram (DVH) was also generated.
The gross tumor volumes (GTV) were defined as the radiographically abnormal areas (main mass and PVTT, usually) noted on the CT images refer to the diagnostic CT and/or MRI. But, in several cases (large tumor [more than 2/3 of the liver] with severe liver cirrhosis, Child-Pugh class C, huge bilateral intrahepatic tumor with main PVTT, numerous intrahepatic metastasis, etc), only PVTT was delineated as GTV. The clinical target volumes (CTV) were regarded as same as GTV. In designing the PTV, the margins were individualized by observing liver position as well as liver movement at the time of simulation. In determining the cranial-caudal margins, the distance of diaphragmatic excursion by respiration, which was observed fluoroscopically, was added to the cranial-caudal margins (minimum 1.5 cm + motion). In the same way, anterio-posterior, and lateral margins (minimum 1.0 cm + motion) were decided.
A median daily dose of 3 gray (Gy, range 1.8 to 4.5 Gy) was administered using 6, 10 or 15 MV X-rays, at 5 fractions per week, to deliver a total dose of 30-54 Gy, which translates to a biologic effective dose (BED) of 39-70.2 Gy10 as the α/β = 10. BED was calculated using a linear quadratic model to be equivalent to 3 Gy fraction treatments in respect of acute effects on normal tissues and tumors. 3D-CRT planning was designed under tentative guidelines so that the normal liver volume irradiated with more than one-half of the prescription dose should not be 50% of the total liver volume.
Four-dimensional gated CT with RPM signal and gated verification in the treatment room were conducted after March 2008, and 44 patients were enrolled in this study.
Post-RT treatment
Additional treatment, mostly TACE, was planned and administered after the RT without considering of RT response after 2004. In 202 patients who received RT after 2004, TACE in 122 patients (60.4%), re-irradiation in 7 patients, radiofrequency ablation in 4 patients, chemotherapy (sorafenib) in 4 patients, and hepatectomy in 2 patients was conducted. By contrast, before 2004, TACE was considered mainly in patients who showed good response to RT. In 79 patients, 23 patients (29.1%) received TACE.
Assessment of response and toxicity
PVTT and/or tumor response (according to RT target) was assessed using World Health Organization criteria by serial CT scans, 4-8 weeks after completion of treatment and then every 2-3 months. Complete disappearance of PVTT and tumor was defined as a complete response (CR), a more than 50% reduction of thrombus in the greatest cross-sectional area and/or perfusion normalization of the area involved by tumor thrombosis was defined as a partial response (PR), a less than 50% reduction of thrombus and tumor (including no change) was defined as stable disease (SD), and tumor growth was defined as progressive disease (PD). Responders were patients with CR or PR, whereas non-responders were patients with SD or PD.
Acute morbidity was evaluated weekly during treatment and 1 month after the treatment. Late morbidity was defined as occurring after 3 months. RT induced liver disease (RILD) was defined as anicteric ascites and elevation of alkaline phosphatase (ALP) levels > 2 times the pre-treatment values in the absence of PD.
If patients complained of melena or upper abdominal pain persisting for longer than 2 weeks during the follow-up period, fiberoptic gastroduodenoscopy was conducted for clarifying the gastrointestinal (GI) toxicity. Toxicity was scored using the Common Terminology Criteria for Adverse Events (CTCAE; version 3.0) at every visit.
Statistical analysis
Overall survival (OS) and PVTT progression free survival (PVTT PFS) were estimated using Kaplan-Meier product-limit method. OS was measured from the RT start date to the date of death or the last follow-up visit, and PVTT PFS from the RT start date to the date of PVTT progression or the last follow-up visit.
Univariate logistic regression analysis was used to evaluate the association between OS and various parameters. For multivariate analysis to evaluate the relation between the OS and various parameters, the stepwise procedure was performed using a logistic regression model containing all variables that attained or had a trend toward statistical significance on univariate analysis. P < 0.05 was considered statistically significant. All calculations were performed with PASW 17.0 for Windows (SPSS, Chicago, IL, USA).
Ethics statement
This study was approved of permission by the institutional review board of Samsung Medical Center, Sungkyunkwan University (IRB No. 2011-03-033). Informed consent was waived by the board.
RESULTS
Clinical characteristics
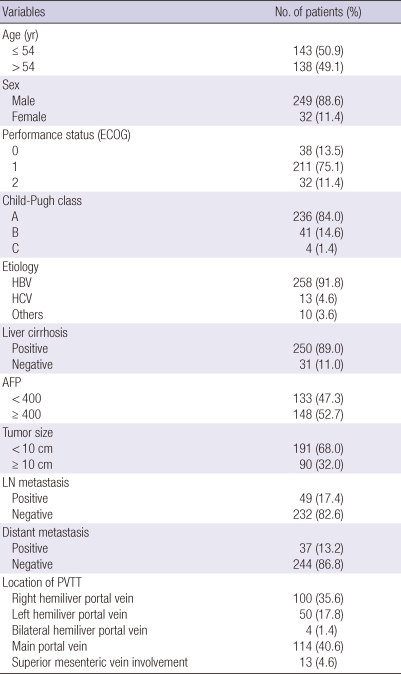
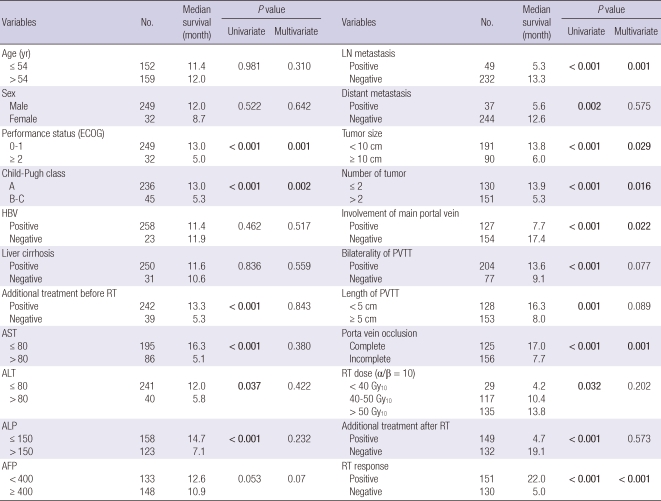
Table 1 describes the tumor characteristics of 281 patients. The median age of patients was 54 yr (range, 26-80 yr). Among the patients, 249 (88.6%) were male, and the male-to-female ratio was 7.8:1. The median size of tumor plus PVTT was 7 cm (range, 2-18 cm). Serum hepatitis B virus antigen markers were positive in 258 patients (91.8%) and hepatitis C virus antigen in 13 patients (4.6%). Specific sites of PVTT were as follows: left hemiliver portal vein (n = 50, 17.8%), right hemiliver portal vein (n = 100, 35.6%), bilateral hemiliver branch portal vein (n = 4, 1.4%), main portal vein (n = 114, 40.6%), extended to superior mesenteric vein (n = 13, 4.6%).
Table 1.
Characteristics of patients and tumor
ECOG, Eastern Cooperative Oncology Group; AFP, alpha-feto protein; LN, lymph node; PVTT, portal vein tumor thrombosis.
Most patients had Child-Pugh classification A (84.0%) or B (14.0%), and only 2.0% had Child-Pugh class C. Thirty two patients (11.4%) presented poor performance status (ECOG 2). Eighty-four patients (29.9%) who showed one or more of these characteristics (large tumor [more than 2/3 of the liver] with severe liver cirrhosis, Child-Pugh class C, huge bilateral intrahepatic tumor with main PVTT, numerous intrahepatic metastasis, etc.) received RT only on PVTT.
Treatment responses
Four to eight weeks after the completion of the RT, an objective response was observed in 151 patients (53.8%). Of these patients, CR was observed in 10 patients (3.6%), PR in 141 patients (50.2%), and SD in 72 patients (25.6%), and PD in 37 patients (13.2%). There were 21 unevaluable patients (7.5%), who were lost to follow-up. AFP decrement was observed in 163 patients (58.0%) at 1 month follow up after RT.
Complication
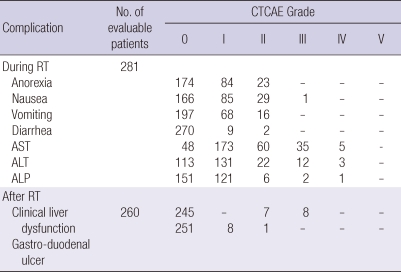
Table 2 shows the complication profile. During the RT, the most common adverse effects were anorexia and nausea (152 patients, 54.1%), but only 1 patient showed grade III nausea, the others were grade I or II. Elevation of liver enzymes was also common, but generally it was less than a five-fold of the upper normal limit. And these elevations were self-limited in most cases. After the RT, 260 patients (92.5%) were evaluable for toxicities, except 21 patients who were lost to follow up. In 15 patients (5.3%), grade II or III clinical hepatic dysfunction was observed after the RT. Among them, PD was observed in 9 of 15 patients (3.2%), and there was no classic RILD. But, in 2 patients (0.7%), transaminases (aspatate aminotransferase [AST], alanine aminotransferase [ALT]) were elevated more than five times the upper limit of the normal or of pre-treatment level. For gastrointestinal complications, 8 patients (2.8%) were scored as grade II and 1 patient (0.4%) as grade III resulting from gastric or duodenal ulcers inside the RT field.
Table 2.
Acute and chronic complication after radiation therapy
CTCAE, common terminology criteria for adverse events; RT, radiation therapy; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase.
Survival outcomes
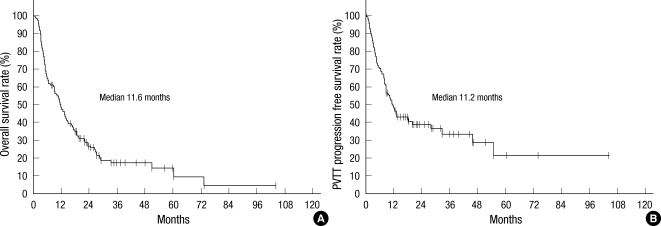
The follow-up period ranged from 0 to 103.2 (median 8.0, mean 11.1) months and 111 of 281 (39.5%) patients were still alive at the time of last follow-up. The median survival was 11.6 (range 1.0 to 103.2) months and the actuarial 6-month, 12-month, 18-month, and 24-month overall survival rates were 65.2%, 48.1%, 35.1%, and 26.9%, respectively (Fig. 1A). In the cases of PVTT, the median PVTT PFS was 11.2 (range 0.2 to 103.2) months (Fig. 1B).
Fig. 1.
Overall survival and portal vein tumor thrombosis progression free survival of all patients: Kaplan-Meier curves for overall survival (A) and portal vein tumor thrombosis (PVTT) progression free survival (B) rate of all patients with hepatocellular carcinoma (HCC) and PVTT treated with radiation therapy (RT).
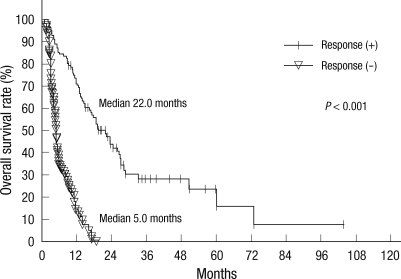
In responders, the median survival duration was 22.0 months and the 6-month, 12-month, 18-month, and 24-month overall survival rates were 85.3%, 70.0%, 53.9% and 41.4%, respectively. In non-responders, the median survival duration was 5.0 months and the 6-month, 12-month, 18-month, and 24-month overall survival rates were 34.3%, 13.8%, 2.9%, and 0.0%, respectively (Fig. 2A). The responders had a significantly higher overall survival rate than the non-responders (P < 0.001).
Fig. 2.
Overall survival according to the RT response: Kaplan-Meier curves for overall survival rate according to the RT response.
Prognostic factors for overall survival
The results of univariate and multivariate analyses to evaluate the relation between OS and various parameters are summarized in Table 3. Of the pre-treatment parameters, significant prognostic factors for OS in univariate analysis were performance status (ECOG), Child-Pugh class, tumor size, multiple tumor, main PVTT involvement, bilateral involvement of PVTT, size of the PVTT, completeness of obstruction, lymph node metastasis, distant metastasis, pre-treatment AST/ALT/ALP/PIVKA-II level, and so on, while age, sex, hemoglobin, and pre-treatment AFP level (P = 0.053) were not. On the multivariate analysis, performance status (ECOG, P = 0.001), Child-Pugh class (P = 0.002), tumor size (P = 0.029), multiple tumors (more than 2, P = 0.016), main PVTT involvement (P = 0.022), completeness of obstruction (P = 0.001), and lymph node metastasis (P = 0.001) showed statistically significant effects on OS.
Table 3.
Prognostic factors for overall survival rate
ECOG, Eastern Cooperative Oncology Group; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AFP, alpha-feto protein; LN, lymph node; PVTT, portal vein tumor thrombosis; RT, radiation therapy; 4D, four-dimensional; TACE, transcatheter arterial chemo-embolization.
Most significant prognostic factor after treatment was radiation response as discussed above. AFP decrement after RT was also important post-treatment prognostic factor. The patients who achieved AFP decrement (163 patients, 58.0%) showed superior median survival duration than the others (14.7 months vs 5.5 months, P = 0.009). And the other significant prognostic factors in post-treatment parameters were decrement of the PIVKA-II level (P < 0.001) and total dose of the radiation delivered (median survival duration; less than 40 Gy10 4.2 months, 40 Gy10 to 50 Gy10 10.4 months, more than 50 Gy10 13.8 months, P = 0.031) in univariate analysis. Additional treatments before and/or after RT showed a statistically significant effect on OS. But, those differences might be related with selection bias of the patients who live longer could receive additional treatments. It is biased on that additional treatments showed no differences to OS in multivariate analysis and closely related with RT response (P < 0.001).
A Predictive model for overall survival
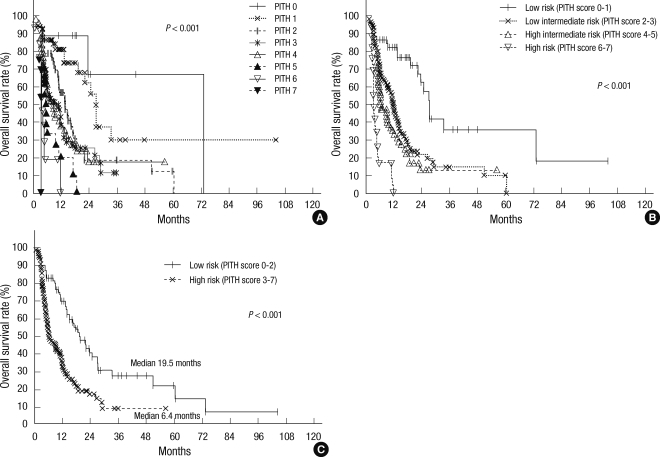
Based on clinical relevance and availability of the information, we used seven pre-treatment factors that showed statistical significance in both univariate and multivariate analysis to construct a Predictive Index for PVTT of the HCC (PITH) and calculate a PITH score. The included factors were grade II or more of performance status (ECOG), Child-Pugh classification B or C, 10 cm or more of tumor size, multiplicity of the tumor, main PVTT involvement, complete occlusion of portal flow and lymph node metastasis. PITH score is defined as the number of the above factors. We estimated OS from the PITH score, and statistically significant differences were detected in each group (P < 0.001, Fig. 3A). We divided the patients into low, low intermediate, high intermediate and high risk groups according to the PITH score. The low risk group with score 0 or 1 consisted of 54 patients (19.2%) and had 82.5% OS at one year, 27.2 months median survival. The low intermediate risk group with score 2 or 3 consisted of 133 patients (47.3%) and had 45.9% OS at one year, 11.4 months median survival. The high intermediate risk group with score 4 or 5 consisted of 76 patients (27.0%) and high risk group who had 6 or more score are made up of 18 patients (6.4%), and 33.0%, 0.0% OS at one year, and 6.4 months, 3.3 months median survival, respectively (P < 0.001, Fig. 3B). And also, when divided them into two groups by the score 0 to 2 and 3 or more (low risk group 110 patients, high risk group 171 patients), OS showed difference (70.1% vs 33.0% at one year, P < 0.001, Fig. 3C).
Fig. 3.
Overall survival according to PITH scoring (A, all groups; B, 4 groups; C, 2 groups): Kaplan-Meier curves for overall survival rate according to PITH scoring. PITH score was calculated by the number of prognostic factors; ECOG performance status (≥ 2), Child-Pugh class (B or C), multiple tumor (more than 2), main PVTT, complete portal vein occlusion, lymph node metastasis, and primary tumor size (≥ 10 cm). PITH scores correlated well with OS (A). Patients were divided into four groups (B) and two groups (C) for statistical analyses.
Comparison of the other stage system
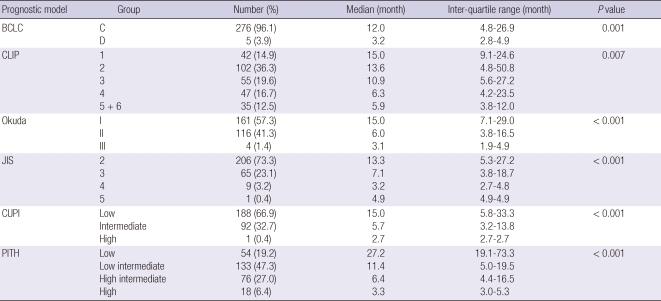
The prognostic ability of the PITH score was compared with Barcelona Clinic Liver Cancer (BCLC), Cancer of the Liver Italian Program (CLIP), Okuda, Japan Integrated Staging (JIS), and Chinese University Prognostic Index (CUPI) staging systems. For each score, this performance was evaluated by comparing by log rank test the survival curves of the single categories, calculated using the Kaplan-Meier method. In Table 4, median values and interquartile ranges of the OS for the six prognostic systems are reported. In spite of there was a significant correlation between OS and stage assigned using all six systems (BCLC P = 0.001, CLIP P = 0.007, Okuda P < 0.001; JIS P < 0.001; CUPI P < 0.001), and trend in), the PITH score performs better than the other staging systems, allowing a better characterization of the extremely good and intermediate categories of patients.
Table 4.
Comparison of survivals according to prognostic models
BCLC, Barcelona Clinic Liver Cancer (BCLC) staging classification; CLIP, Cancer of the Liver Italian Program (CLIP) scoring system; JIS, Japan Integrated Staging (JIS) scoring system; CUPI, The Chinese University Prognostic Index; PITH, Predictive Index for portal vein tumor thrombosis of the hepatocellular carcinoma.
DISCUSSION
The presence of PVTT in patients with HCC is one of the most significant prognostic factors for poor prognosis. In several reports, it is the clinic-pathologic parameters that mostly influences on survival in multivariate analysis (3-5). It can lead to conditions not only a widespread dissemination of tumors throughout the liver, but marked deterioration of hepatic function. Therefore, the prognosis of HCC patients with PVTT is extremely poor. Without treatment, their survival is less than 3 months (5). Standard treatment regimens have not been established in these patients, especially in AsiaPacific region.
In the recent large scale randomized, double blind, placebo-controlled trial, sorafenib, an oral multi-kinase inhibitor of the vascular endothelial growth factor receptor, the platelet-derived growth factor receptor and Raf showed about 2 to 3 months survival benefit in patients with advanced (unresectable or metastatic) HCC who had not received previous systemic treatment in the AsiaPacific region as well as Western (11, 12). The median survival of the experimental group was 10.7 months in Western, and 6.5 months in Asian-Pacific region. But, the results from loco-regional therapies such as TACE and/or RT have been reported better than above results especially in patients with locally advanced disease without distant metastases (13, 14). For locally advanced disease such as HCC with PVTT, TACE and/or RT is the most commonly utilized treatment in Korea. Treatment algorithm for hepatocellular carcinoma utilized in Korea supports not only sorafenib but also TACE and RT as a standard therapy for HCC with PVTT (15).
Recently, the major progress in RT for HCC has been made with deeper understanding of the liver, HCC, normal organ radiobiology (16, 17) and more elaborated radiation technique of 3D-CRT, image-guided RT (IGRT) and stereotactic body RT (SBRT). In the treatment of the HCC patients with PVTT, RT was carried out critical role. RT has an advantage that it could be performed safely without being limited by the presence of PVTT (7, 18, 19). When patients with HCC and PVTT were treated with RT and/or other treatment modalities, the response rates and median survival approximated 40%-75% and 5.3-13.1 months, respectively. Hata et al. (8) reported on 35 patients with PVTT at the main trunk or major branches, and 46% of progression free survival at 2 yr (median 21 months) suggesting that RT was a good alternative treatment approach for such patients. Kim et al. (7) reported that RT showed 45.8% objective response rate, and median survival duration in responders were 10.7 months compared with 5.3 months in the non-responders. And dose-response relationship in patients receiving less than 58 Gy10 and 58 Gy10 or more were 20% and 54.6% in the 59 patients with HCC and PVTT. A combined radiation therapy after TACE showed better clinical outcomes for HCC invading the main portal vein, when compared to TACE mono-therapy (20, 21). These results suggest that RT for HCC with PVTT is also effective and may prolong the survival of these patients.
There were several prognostic models in the HCC patients (22), like BCLC (23), CLIP (24), Okuda (25), JIS (26), and CUPI (27) staging system. Above systems are regarded as reliable prognostic model, because verified by several studies. But, those models are not specified in patients with PVTT. Until recently, there was no applicable prognostic system in patients with HCC with PVTT treated with RT, to our knowledge. Therefore, it is difficult to make treatment recommendation according to a reliable estimate of life expectancy in cirrhotic patients with HCC and PVTT. This may induce unfavorable effects. Due to underestimation of life expectancy, patients are referred to supportive care only without any therapy which might have potentially positive effects on patients' outcome. On the other hand, an overestimation of life expectancy may expose patients to treatments that will result in no benefit or even harmful effects on patients' prognosis.
Recently, Huang et al. (18) published article about prognostic model. In the study, the authors conducted retrospective review for 326 HCC patients with PVTT who were treated with RT. Despite their meaningfulness, this study also has some limitations to generalize their model in patients with HCC and PVTT treated with RT. Firstly, the median survival was only 3.8 months. As stated above, most published articles with similar protocols, the median survival was 5 to 10 months. Possibility the enrolled patients had poor liver function, and radiation delivery was too delayed. Secondly, there were large missing data, 155 patients (47.5%). These limitations need to be considered while applying their rule in the treatment.
In this article, we proposed a new scoring system (PITH score) that accounts for both tumor and PVTT characteristics useful in prognostic assessment of patients with HCC and PVTT treated with RT. The PITH score is easy to calculate and based on variables that are routinely assessed during clinical examination, biochemical staging, and CT of the liver. Classification according to PITH score was well correlated with the OS after RT. Those results of the PITH score, compared with other staging systems, showed more predictable power in the patients with PVTT when treated with RT, especially in the extremely good and intermediate prognostic categories. We look forward to PITH score system might be allowed the patient with HCC and PVTT to make important decisions, and help physician for risk stratification in patients treated with RT. It could be also the guidance of additional combined treatment modalities in those patients.
Our study does have some limitations. First, as in all retrospective studies, a selection bias could have occurred, with a reduced prevalence of patients with a worse prognosis. However, this possible bias should be less important than in other series because of the relatively short and recent period of recruitment. Second, rather small number of cases might not be enough to derive concrete conclusion. For solving these problems, large, prospective validation study would be required.
In conclusion, our data demonstrates that ECOG performance status, Child-Pugh class, tumor size, tumor multiplicity, main PVTT involvement, completeness of portal vein obstruction, and lymph node metastasis are important prognostic factors for OS in HCC patients with PVTT treated with RT. From those seven prognostic factors, we designed prognostic model by the number of prognostic factors, PITH score. Our data confirm that PITH score is significantly correlated with OS. The PITH scoring model proposed in the current study in HCC patients with PVTT reliably predict OS. Our institution will consider conducting a prospective validation trial of PITH score system. This trial will permit us to resolve questions and also to confirm our conclusions and might help make a treatment decision according to risk stratification in patient with HCC and PVTT treated with RT.
Footnotes
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0011107).
AUTHOR SUMMARY
Prognostic Index for Portal Vein Tumor Thrombosis in Patients with Hepatocellular Carcinoma Treated with Radiation Therapy
Jeong Il Yu, Hee Chul Park, Do Hoon Lim, Won Park, Byung Chul Yoo, Seung Woon Paik, Kwang Cheol Koh and Joon Hyuk Lee
While radiation therapy (RT) is often applied for hepatocellular carcinoma (HCC), all previous studies dealt simply with treatment results. In this study, we analyzed the indices of the HCC patients with portal vein tumor thrombosis, which could be potentially helpful to identify the beneficial prognositic factors in the similar patients.
References
- 1.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15:223–243. doi: 10.1016/j.cld.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int. 2008;7:237–257. [PubMed] [Google Scholar]
- 3.The Cancer of the Liver Italian Program (CLIP) Investigators. Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;31:840–845. doi: 10.1053/he.2000.5628. [DOI] [PubMed] [Google Scholar]
- 4.Park KW, Park JW, Choi JI, Kim TH, Kim SH, Park HS, Lee WJ, Park SJ, Hong EK, Kim CM. Survival analysis of 904 patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol. 2008;23:467–473. doi: 10.1111/j.1440-1746.2007.05112.x. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 6.Park W, Lim DH, Paik SW, Koh KC, Choi MS, Park CK, Yoo BC, Lee JE, Kang MK, Park YJ, Nam HR, Ahn YC, Huh SJ. Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1143–1150. doi: 10.1016/j.ijrobp.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, Kho KC, Kim TH, Ahn YC, Huh SJ. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103:2419–2426. doi: 10.1002/cncr.21043. [DOI] [PubMed] [Google Scholar]
- 8.Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Tokita M, Abei M, Shoda J, Matsuzaki Y, Thono E, Tsuboi K, Tokuuye K. Proton-beam therapy for hepatocellular carcinoma associated with portal vein tumor thrombosis. Strahlenther Onkol. 2009;185:782–788. doi: 10.1007/s00066-009-2020-x. [DOI] [PubMed] [Google Scholar]
- 9.Park JW. Practice guideline for diagnosis and treatment of hepatocellular carcinoma. Korean J Hepatol. 2004;10:88–98. [PubMed] [Google Scholar]
- 10.Piscaglia F, Gianstefani A, Ravaioli M, Golfieri R, Cappelli A, Giampalma E, Sagrini E, Imbriaco G, Pinna AD, Bolondi L Bologna Liver Transplant Group. Criteria for diagnosing benign portal vein thrombosis in the assessment of patients with cirrhosis and hepatocellular carcinoma for liver transplantation. Liver Transpl. 2010;16:658–667. doi: 10.1002/lt.22044. [DOI] [PubMed] [Google Scholar]
- 11.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomized, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 13.Liang SX, Zhu XD, Lu HJ, Pan CY, Li FX, Huang QF, Wang AY, Chen L, Fu XL, Jiang GL. Hypofractionated three-dimensional conformal radiation therapy for primary liver carcinoma. Cancer. 2005;103:2181–2188. doi: 10.1002/cncr.21012. [DOI] [PubMed] [Google Scholar]
- 14.Seong J, Park HC, Han KH, Chon CY, Chu SS, Kim GE, Suh CO. Clinical results of 3-dimensional conformal radiotherapy combined with transarterial chemoembolization for hepatocellular carcinoma in the cirrhotic patients. Hepatol Res. 2003;27:30–35. doi: 10.1016/s1386-6346(03)00162-1. [DOI] [PubMed] [Google Scholar]
- 15.Chen PJ, Furuse J, Han KH, Hsu C, Lim HY, Moon H, Qin S, Ye SL, Yeoh EM, Yeo W. Issues and controversies of hepatocellular carcinoma-targeted therapy clinical trials in Asia: experts' opinion. Liver Int. 2010;30:1427–1438. doi: 10.1111/j.1478-3231.2010.02292.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Lim DH, Paik SW, Yoo BC, Koh KG, Lee JH, Choi MS, Park W, Park HC, Huh SJ, Choi DH, Ahn YC. Predictive factors of gastroduodenal toxicity in cirrhotic patients after three-dimensional conformal radiotherapy for hepatocellular carcinoma. Radiother Oncol. 2009;93:302–306. doi: 10.1016/j.radonc.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:150–155. doi: 10.1016/s0360-3016(02)02864-x. [DOI] [PubMed] [Google Scholar]
- 18.Huang YJ, Hsu HC, Wang CY, Wang CJ, Chen HC, Huang EY, Fang FM, Lu SN. The treatment responses in cases of radiation therapy to portal vein thrombosis in advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;73:1155–1163. doi: 10.1016/j.ijrobp.2008.06.1486. [DOI] [PubMed] [Google Scholar]
- 19.Yamada K, Izaki K, Sugimoto K, Mayahara H, Morita Y, Yoden E, Matsumoto S, Soejima T, Sugimura K. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:113–119. doi: 10.1016/s0360-3016(03)00434-6. [DOI] [PubMed] [Google Scholar]
- 20.Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 21.Seong J. Challenge and hope in radiotherapy of hepatocellular carcinoma. Yonsei Med J. 2009;50:601–612. doi: 10.3349/ymj.2009.50.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29:502–510. doi: 10.1111/j.1478-3231.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 24.The Cancer of the Liver Italian Program (CLIP) investigators. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 25.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Kitai S, Kudo M, Minami Y, Haji S, Osaki Y, Oka H, Seki T, Kasugai H, Sasaki Y, Matsunaga T. Validation of a new prognostic staging system for hepatocellular carcinoma: a comparison of the biomarker-combined Japan Integrated Staging Score, the conventional Japan Integrated Staging Score and the BALAD Score. Oncology. 2008;75(Suppl 1):83–90. doi: 10.1159/000173428. [DOI] [PubMed] [Google Scholar]
- 27.Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, Lau JT, Yu SC, Johnson PJ. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–1769. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]