Abstract
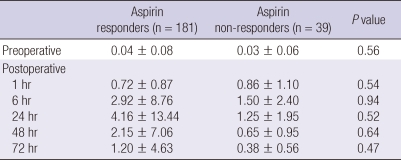
We performed a prospective cohort trial on 220 patients undergoing elective off-pump coronary artery bypass surgery and taking aspirin to evaluate the effect of aspirin resistance on myocardial injury. The patients were divided into aspirin responders and aspirin non-responders by the value of the aspirin reaction units obtained preoperatively using the VerifyNow™ Aspirin Assay. The serum levels of troponin I were measured before surgery and 1, 6, 24, 48 and 72 hr after surgery. In-hospital major adverse cardiac and cerebrovascular events, graft occlusion, the postoperative blood loss and reexploration for bleeding were recorded. Of the 220 patients, 181 aspirin responders (82.3%) and 39 aspirin non-responders (17.7%) were defined. There were no significant differences in troponin I levels (ng/mL) between aspirin responders and aspirin non-responders: preoperative (0.04 ± 0.08 vs 0.03 ± 0.06; P = 0.56), postoperative 1 hr (0.72 ± 0.87 vs 0.86 ± 1.10; P = 0.54), 6 hr (2.92 ± 8.76 vs 1.50 ± 2.40; P = 0.94), 24 hr (4.16 ± 13.44 vs 1.25 ± 1.95; P = 0.52), 48 hr (2.15 ± 7.06 vs 0.65 ± 0.95; P = 0.64) and 72 hr (1.20 ± 4.63 vs 0.38 ± 0.56; P = 0.47). Moreover, no significant differences were observed with regard to in-hospital outcomes. In conclusion, preoperative aspirin resistance does not increase myocardial injury in patients undergoing off-pump coronary artery bypass surgery. Postoperative dual antiplatelet therapy might have protected aspirin resistant patients.
Keywords: Aspirin; Coronary Artery Bypass, Off-Pump; Drug Resistance; Hemorrhage; Troponin I
INTRODUCTION
Aspirin has been one of the most popular drugs in modern medicine. Aspirin induces platelet dysfunction by irreversible inhibition of the cyclooxygenase-1 enzyme in platelets (1). This platelet inhibitor has been successfully used to prevent cardiovascular complications for high risk patients. Moreover, aspirin is also useful for treating coronary artery bypass graft surgery (CABG) patients. First, perioperative use of aspirin decreases vein graft failure (2, 3). Second, aspirin has also been proven to decrease perioperative cardiovascular complications and consequently, the morbidity and mortality (4).
However, in some patients, their platelets are still active even when taking aspirin medication and in these aspirin resistant patients, aspirin medication is not effective to prevent cardiovascular complications (5, 6). In patients undergoing CABG, aspirin resistance is known to be related with graft failure (7, 8). However, the effect of aspirin resistance on the early outcome of patients who are undergoing off-pump coronary artery bypass surgery (OPCAB) has rarely been studied.
The aim of this study was to evaluate the effect of aspirin resistance on myocardial injury in patients undergoing OPCAB. So, aspirin resistance was preoperatively evaluated in the patients undergoing OPCAB, and perioperative myocardial injury was measured by assessing the postoperative myocardial enzyme levels. The early clinical outcomes, including the in-hospital major adverse cardiac and cerebrovascular events (MACCEs), postoperative blood loss and reexploration for bleeding, were also recorded.
MATERIALS AND METHODS
Patients
This study is a prospective cohort trial of patients who were undergoing elective OPCAB and the medical records were stored in a fully-automated electronic chart system. After receiving institutional review board approval on 21 November 2008 and informed written consent from all the patients was obtained, we enrolled the elective OPCAB patients who were taking aspirin (Aspirin Protect®, Bayer AG, Leverkusen, Germany). The exclusion criteria were co-medication of any other anticoagulation medication such as antiplatelet agents, glycoprotein IIb/IIIa inhibitors, non-steroidal anti-inflammatory drugs, major combined operations such as mitral valve replacement, the preoperative use of a mechanical heart assist device and an emergency operation. The patients who had a hematocrit under 30% were also excluded according to the manufacturer's instructions for the VerifyNow™ Aspirin Assay (Accumetrics Inc., San Diego, CA, USA). The patients received aspirin (100 mg/day) until the day before surgery and they resumed taking aspirin (100 mg/day) together with taking ticlopidine (200 mg/day) on the first postoperative day.
Anesthesia and surgical technique
All the anesthesia procedures were supervised by a single anesthesiologist with using the same anesthetic protocol. Anesthesia was induced with intravenous midazolam (0.15 mg/kg), vecuronium (0.1 mg/kg) and sufentanil (1 µg/kg), and it was maintained with inhaled sevoflurane (1-2 MAC vol%) and intravenous remifentanil (0.8 µg/kg/min) and vecuronium (1.5 µg/kg/min).
All the surgical procedures were performed by one surgeon with the same surgical technique. After a median sternotomy, the left internal thoracic artery was harvested as a bypass conduit. The saphenous vein or right gastroepiploic artery was used to make Y-grafts or I-grafts, if necessary. The conduits were immersed in warm diluted papaverine saline solution (1 mg/mL). Systemic heparinization was achieved with an initial dose of 1.5 mg/kg heparin, and this was maintained with additional heparin doses to reach an activated clotting time above 300 sec. After the pericardium was opened, two deep pericardial sutures were placed for pericardial retraction and exposure. To facilitate anastomosis, the heart was stabilized with a compression-type stabilizer (Ultima Stabilizer; Guidant, Cupertino, CA, USA) or a suction-type mechanical stabilizer (Octopus; Medtronic, Minneapolis, MN, USA). The most critical vessel, which was the left anterior descending artery in the majority of the patients, was revascularized first. After the completion of anastomosis, the effect of heparin was reversed with protamine.
Assays for platelet function
Assays for platelet function were performed using the VerifyNow™ Aspirin Assay (Accumetrics Inc.) the day before surgery, which was when the patients had been administered aspirin medication, to evaluate for aspirin resistance. The VerifyNow™ Aspirin Assay contains fibrinogen-coated microparticles of arachidonic acid. If aspirin produces the antiplatelet effect, then the aggregation of the fibrinogen-coated microparticles does not occur, and the light transmission does not increase. The instrument measures the change of light transmission caused by platelet aggregation. This test's results were expressed as aspirin reaction units (ARU), and aspirin resistance was defined as an ARU value ≥ 550 in spite of continuing aspirin medication according to the manufacturer's reference values. The patients were divided into two groups by the ARU value: aspirin responders (ARU < 550) and aspirin non-responders (ARU ≥ 550).
Data collection
The preoperative data, including demographic data, co-morbidities, medications and ARU values, were recorded. The intraoperative data such as duration of surgery and type of graft were recorded. The serum concentration of troponin I was measured before surgery and 1, 6, 24, 48, and 72 hr after surgery. The postoperative blood loss during the 12 hr after surgery was recorded as the volume of the mediastinal and pleural chest tube drainage. The total number of units of packed red blood cells given during the intraoperative and postoperative hospitalization period was recorded. Reexploration for bleeding was defined as surgical reoperation that was performed to control the bleeding that occurred after the OPCAB. MACCEs, graft occlusion, the length of stay in the intensive care unit (ICU) and the length of hospitalization after surgery were recorded. A MACCE was defined as postoperative myocardial infarction, postoperative stroke, repeated revascularization and death during the same hospital admission period. Postoperative myocardial infarction was defined by serum levels of troponin I greater than 8.35 ng/mL at 24 hr after surgery (9, 10). Postoperative stroke was defined as a new neurological deficit, as determined by a neurologist, that occurred postoperatively and it persisted for more than 24 hr. Repeat revascularization was defined as an intervention on the grafts or the grafted native vessels. Death was defined as death regardless of cause. Coronary angiography was performed on days 1 to 3 and at 1 yr after surgery to determine the patency of grafts. Graft occlusion was defined as no flow through one or more grafts.
Statistical analysis
The primary endpoint of this study was to evaluate the effect of preoperative aspirin resistance on the serum levels of troponin I during the first postoperative day after OPCAB. In our pilot study, 16% of the patients had aspirin resistance. This was compatible with the results (6.7%-23%) of the previous studies that used the VerifyNow™ Aspirin Assay (11). In our previous data, the troponin I level was 1.60 ± 1.15 ng/mL at the first postoperative day. We determined that 28 patients and 144 patients would be required in each group to achieve 80% power and an alpha level of 0.05 to detect a 0.6 ng/mL difference in the troponin I levels, which we assumed to be a clinically significant value with an standard deviation of 1.15 ng/mL.
Statistical analysis was conducted with SPSS 12.0 for Windows (SPSS Inc., Chicago, IL, USA). The data is presented as mean ± SD or the number of patients (percentage). Categorical data was compared with the chi-square test or Fisher's exact test. Continuous data between the groups was compared by unpaired Student's t-tests for the normally distributed variables and the Mann-Whitney rank sum test for the non-normally distributed variables. Differences were considered significant at P values < 0.05.
Ethics statement
Institutional review board of the Seoul National University Hospital approved this study (Ref: H-0811-052-262). Written informed consent was obtained from all participants.
RESULTS
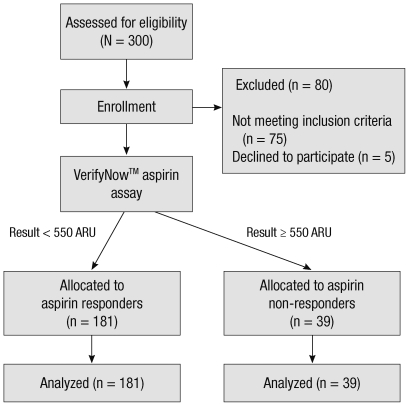
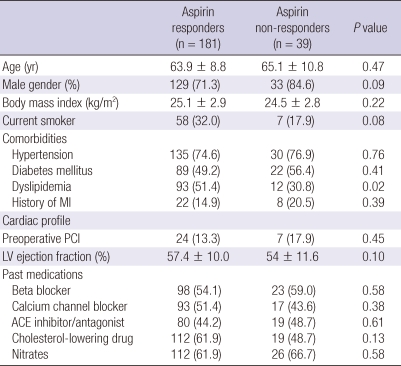
We enrolled a total of 220 patients (Fig. 1). Of the 220 patients, 39 (17.7%) had aspirin resistance. The aspirin responders had 447.8 ± 42.3 ARUs and the aspirin non-responders had 602 ± 38.2 ARUs. Table 1 displays the patients' characteristics. There were no significant differences between the two groups for age, the body mass index, preoperative comorbidities and the cardiac profile and medications except dyslipidemia. The number of anastomosis was also equivalent between the aspirin responders and non-responders (2.9 ± 0.7 vs 3.0 ± 0.8, respectively, P = 0.78).
Fig. 1.
Flow diagram. ARU, aspirin reaction units.
Table 1.
Patient characteristics
Values are either the number of patients (percentage) or mean ± standard deviation. ACE, angiotensin-converting enzyme; LV, left ventricular; MI, myocardial infarction; PCI, percutaneous coronary intervention.
The perioperative troponin I levels are presented in Table 2. There were no significant differences in the troponin I levels before surgery and 1, 6, 24, 48, and 72 hr after surgery between the two groups.
Table 2.
Troponin I levels (ng/mL)
The data is expressed as mean ± standard deviation.
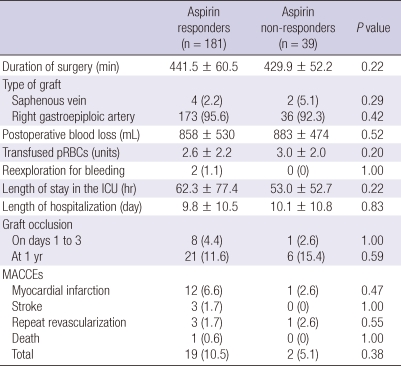
There were no significant differences of the duration of surgery, the type of graft, the postoperative blood loss, the transfused packed red blood cells and the rate of reexploration for bleeding between the two groups (Table 3). There were also no significant differences in the length of stay in the ICU, the length of hospitalization after surgery, graft occlusion and MACCEs between the two groups (Table 3).
Table 3.
In-hospital outcomes
Values are either the number of patients (percentage) or mean ± standard deviation. ICU, intensive care unit; MACCE, major adverse cardiac and cerebrovascular events; pRBCs, packed red blood cells.
DISCUSSION
In this study, there were no significant differences of the postoperative troponin I levels between the aspirin responders and non-responders among the patients who were undergoing OPCAB. Moreover, there were also no significant differences of the early postoperative outcomes, including in-hospital MACCEs and postoperative bleeding.
The beneficial effects of perioperative aspirin in CABG patients have been proven in previous studies. Perioperative aspirin medication improves graft patency in CABG patients (2, 12). In a study by Dacey et al. (13), preoperative aspirin medication decreased the in-hospital mortality without a significant increase in hemorrhage, the requirement for blood products or the related morbidity. In the well known study by Mangano (4), early postoperative use of aspirin after CABG reduced the morbidity and mortality.
Although OPCAB is different from CABG for the degree of platelet activation, dysfunction, depletion and aspirin resistance, there have been only a few studies in which the clinical effects of aspirin were evaluated in patients who were undergoing OPCAB (14, 15). The beneficial effect of aspirin on the patency of vein grafts was also proved in OPCAB patients, the same as in on-pump CABG patients. In a study by Poston et al. (7), aspirin resistance was an independent predictor of vein graft thrombosis in patients who were undergoing OPCAB. During a mean follow-up period of 30 days, the incidence of early thrombotic occlusion of vein grafts was highest in the patients who had both aspirin resistance and compromised endothelial integrity. In a study by Yilmaz et al. (8), the rate of vein graft failure was much higher in the aspirin resistance patients than that in the age and gender matched control (50% vs 7.1%, respectively).
However, the effect of aspirin on the early clinical outcome has rarely been evaluated in patients who are undergoing OPCAB and any beneficial effect has not yet been proven. In a cohort study by Suwalski et al. (16), the platelet function was investigated, using a platelet function analyzer (PFA-100, Dade Behring, Germany), in 42 OPCAB patients who continued aspirin therapy until surgery. On a preoperative platelet function analysis, 13 aspirin non-responders and 29 aspirin responders were defined. There were no significant differences in troponin I, creatine kinase-MB and ST segment elevation between the aspirin responders and the aspirin non-responders. These results are in accord with our results that showed no significant differences in the troponin I levels between the aspirin responders and the non-responders. The results of Suwalski et al. (16) study may suggest that aspirin medication is not effective to prevent myocardial injury and early complications in patients who are undergoing OPCAB, like the results of our study. Consequently, this may mean that aspirin medication is useless in terms of the early outcome in patients who are undergoing OPCAB.
However, there could be other explanations for the results that aspirin resistance does not make any clinical difference, including myocardial injury, in our study. In this study, dual antiplatelet therapy with ticlopidine was used during the postoperative period. Dual antiplatelet therapy with aspirin and an ADP receptor antagonist has shown additive benefit over aspirin monotherapy in high risk patients (17, 18) and this is considered to be the most beneficial method for treating aspirin resistant patients (19). So, there is a possibility that the postoperative dual antiplatelet therapy in our study might have protected the aspirin non-responders and abolished the difference between the two groups. In another cohort study in which aspirin resistance did not increase myocardial injury, like as in our study, enoxaparin was used during the postoperative period and enoxaparin is known to be another therapeutic option for aspirin resistance (16).
Moreover, in our study, although statistical significance was not reached, troponin I levels at 6 hr, 24 hr, 48 hr, and 72 hr were 2 to 4 times higher in aspirin responders (Table 2). The worse results without statistical significance in aspirin responders were also shown in every outcome of MACCEs. So, the rate of total MACCEs was almost 3 times higher in aspirin responders (Table 3). Although there was no statistical significance, these results may be caused by type II error. Interestingly, these unexpected results without statistical significance have also been observed in other previous studies. One hundred seventy aspirin users and 170 aspirin nonusers who were undergoing OPCAB were reviewed in a retrospective study that used the propensity score matching technique (20). Although statistical significance was not reached as in our study, preoperative aspirin users showed higher incidences of stroke (0.6% vs 0%, P = 0.317), myocardial infarction (2.4% vs 1.2%, P = 0.410) and sternal wound infections (4.7% vs 1.2%, P = 0.054) after OPCAB. In a retrospective study by Gulbins et al. (21), 1,147 OPCAB patients were enrolled and the mortality was higher in continued aspirin medication group (2.1% vs 1.8%) without statistical significance. In a prospective study by Matsuura et al. (22), changes of troponin T levels from before surgery to immediately after surgery were higher in preoperative aspirin group (158% vs 205%, P = 0.31). These results may suggest that in OPCAB patients, preoperative aspirin medication might increase postoperative cardiovascular complications. However, for this topic, we may need larger scale prospective study.
Although perioperative aspirin use increased the risk of bleeding in patients who were undergoing conventional pump CABG in several previous studies (23, 24), aspirin resistance was not correlated with postoperative blood loss and the rate of reexploration for bleeding in our study. This is consistent with a previous study that reported no significant differences of the postoperative blood loss and the rate of reexploration between the aspirin users and nonusers who were undergoing OPCAB (20). In the previous studies, dual antiplatelet therapy was shown to have a synergistic effect on the bleeding tendency. Preoperative clopidogrel exposure increases the risk for reoperation, major bleeding and the length of stay for patients who are undergoing on-pump CABG (25). Preoperative clopidogrel also induced higher blood loss in the patients who were undergoing OPCAB (26). However, early postoperative use of clopidogrel, like as in our study, did not increase the risk of bleeding complications in on-pump and off-pump CABG patients (27).
Our study has several limitations. First, aspirin resistance was evaluated one time preoperatively, although aspirin resistance has been considered to vary according to time and the clinical condition (28, 29). Moreover, OPCAB can cause platelet activation and it can attenuate the effect of aspirin during the postoperative period (16). Aspirin resistance can probably be detected more precisely using several diagnostic methods concurrently before and after the surgery. Second, this study was not performed in a randomized manner. A prospective randomized clinical trial that includes a group without aspirin medication may clear up whether perioperative aspirin medication is beneficial in patients who are undergoing OPCAB, but this kind of study may cause some ethical problems. Third, the sample size analysis was based on the troponin I levels. Therefore, this study may not be sufficiently powered with regard to other clinical outcomes, including MACCEs. Power analysis based on the results of our study determined that 3312 subjects would be required for 80% power for assessing MACCEs. Fourth, the difference of the number between two groups is large. So, the power can decrease because of increasing standard error and propensity score matching could have been tried. However, the ratio between two group was already considered on the sample size analysis and the number of aspirin resistant patient might be too small for the use of propensity score matching to be advantageous.
In this study, there were no significant differences of the postoperative troponin I levels, in hospital MACCEs, postoperative blood loss and the rate of reexploration for bleeding between the aspirin responders and non-responders among the patients who were undergoing OPCAB. In conclusion, aspirin resistance does not increase myocardial injury in patients who are undergoing OPCAB.
ACKNOWLEDGMENTS
We would like to thank the members of Medical Research Collaborating Center, Seoul National University Hospital, Seoul National University College of Medicine for statistical analysis.
AUTHOR SUMMARY
Preoperative Aspirin Resistance does not Increase Myocardial Injury during Off-pump Coronary Artery Bypass Surgery
Hyun Joo Kim, Jung-Man Lee, Jeong Hwa Seo, Jun-Hyeon Kim, Deok-Man Hong, Jae-Hyon Bahk, Ki-Bong Kim and Yunseok Jeon
Aspirin prevent cardiovascular complications not only in high risk patients, but also in the patients undergoing coronary artery bypass surgery. However, some patients are resistant to aspirin medication. Here we investigated the influence of aspirin resistance on myocardial injury in patients undergoing off-pump coronary artery bypass surgery. Perioperative myocardial injury was measured by assessing the postoperative myocardial enzyme levels. There were no significant differences between groups with regard to myocardial injury and in-hospital outcomes.
References
- 1.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 2.Goldman S, Copeland J, Moritz T, Henderson W, Zadina K, Ovitt T, Doherty J, Read R, Chesler E, Sako Y. Improvement in early saphenous vein graft patency after coronary artery bypass surgery with antiplatelet therapy: results of a Veterans Administration Cooperative Study. Circulation. 1988;77:1324–1332. doi: 10.1161/01.cir.77.6.1324. [DOI] [PubMed] [Google Scholar]
- 3.Goldman S, Copeland J, Moritz T, Henderson W, Zadina K, Ovitt T, Kern KB, Sethi G, Sharma GV, Khuri S. Long-term graft patency (3 years) after coronary artery surgery. Effects of aspirin: results of a VA Cooperative study. Circulation. 1994;89:1138–1143. doi: 10.1161/01.cir.89.3.1138. [DOI] [PubMed] [Google Scholar]
- 4.Mangano DT. Aspirin and mortality from coronary bypass surgery. N Engl J Med. 2002;347:1309–1317. doi: 10.1056/NEJMoa020798. [DOI] [PubMed] [Google Scholar]
- 5.Gum PA, Kottke-Marchant K, Welsh PA, White J, Topol EJ. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol. 2003;41:961–965. doi: 10.1016/s0735-1097(02)03014-0. [DOI] [PubMed] [Google Scholar]
- 6.Pamukcu B, Oflaz H, Onur I, Oncul A, Ozcan M, Umman B, Mercanoglu F, Meric M, Nisanci Y. Clinical relevance of aspirin resistance in patients with stable coronary artery disease: a prospective follow-up study (PROSPECTAR) Blood Coagul Fibrinolysis. 2007;18:187–192. doi: 10.1097/MBC.0b013e328040c115. [DOI] [PubMed] [Google Scholar]
- 7.Poston RS, Gu J, Brown JM, Gammie JS, White C, Nie L, Pierson RN, 3rd, Griffith BP. Endothelial injury and acquired aspirin resistance as promoters of regional thrombin formation and early vein graft failure after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2006;131:122–130. doi: 10.1016/j.jtcvs.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 8.Yilmaz MB, Balbay Y, Caldir V, Ayaz S, Guray Y, Guray U, Korkmaz S. Late saphenous vein graft occlusion in patients with coronary bypass: possible role of aspirin resistance. Thromb Res. 2005;115:25–29. doi: 10.1016/j.thromres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Peivandi AA, Dahm M, Hake U, Hafner G, Opfermann UT, Loos AH, Tzanova I, Oelert H. Patterns and diagnostic value of cardiac troponin I vs. troponin T and CKMB after OPCAB surgery. Thorac Cardiovasc Surg. 2001;49:137–143. doi: 10.1055/s-2001-14289. [DOI] [PubMed] [Google Scholar]
- 10.Peivandi AA, Dahm M, Opfermann UT, Peetz D, Doerr F, Loos A, Oelert H. Comparison of cardiac troponin I versus T and creatine kinase MB after coronary artery bypass grafting in patients with and without perioperative myocardial infarction. Herz. 2004;29:658–664. doi: 10.1007/s00059-004-2543-y. [DOI] [PubMed] [Google Scholar]
- 11.van Werkum JW, Harmsze AM, Elsenberg EH, Bouman HJ, ten Berg JM, Hackeng CM. The use of the VerifyNow system to monitor antiplatelet therapy: a review of the current evidence. Platelets. 2008;19:479–488. doi: 10.1080/09537100802317918. [DOI] [PubMed] [Google Scholar]
- 12.Gavaghan TP, Gebski V, Baron DW. Immediate postoperative aspirin improves vein graft patency early and late after coronary artery bypass graft surgery. A placebo-controlled, randomized study. Circulation. 1991;83:1526–1533. doi: 10.1161/01.cir.83.5.1526. [DOI] [PubMed] [Google Scholar]
- 13.Dacey LJ, Munoz JJ, Johnson ER, Leavitt BJ, Maloney CT, Morton JR, Olmstead EM, Birkmeyer JD, O'Connor GT Northern New England Cardiovascular Disease Study Group. Effect of preoperative aspirin use on mortality in coronary artery bypass grafting patients. Ann Thorac Surg. 2000;70:1986–1990. doi: 10.1016/s0003-4975(00)02133-0. [DOI] [PubMed] [Google Scholar]
- 14.Møller CH, Steinbrüchel DA. Platelet function after coronary artery bypass grafting: is there a procoagulant activity after off-pump compared with on-pump surgery? Scand Cardiovasc J. 2003;37:149–153. doi: 10.1080/14017430310001456. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann N, Kurt M, Wenk A, Winter J, Gams E, Hohlfeld T. Is cardiopulmonary bypass a reason for aspirin resistance after coronary artery bypass grafting? Eur J Cardiothorac Surg. 2005;27:606–610. doi: 10.1016/j.ejcts.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Suwalski G, Suwalski P, Filipiak KJ, Postuła M, Majstrak F, Opolski G. The effect of off-pump coronary artery bypass grafting on platelet activation in patients on aspirin therapy until surgery day. Eur J Cardiothorac Surg. 2008;34:365–369. doi: 10.1016/j.ejcts.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Chen ZM, Jiang LX, Chen YP, Xie JX, Pan HC, Peto R, Collins R, Liu LS COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1607–1621. doi: 10.1016/S0140-6736(05)67660-X. [DOI] [PubMed] [Google Scholar]
- 18.Steinhubl SR, Berger PB, Mann JT, 3rd, Fry ET, DeLago A, Wilmer C, Topol EJ CREDO Investigators. Clopidogrel for the Reduction of Events During Observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 19.Gasparyan AY, Watson T, Lip GY. The role of aspirin in cardiovascular prevention: implications of aspirin resistance. J Am Coll Cardiol. 2008;51:1829–1843. doi: 10.1016/j.jacc.2007.11.080. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan AK, Grayson AD, Pullan DM, Fabri BM, Dihmis WC. Effect of preoperative aspirin use in off-pump coronary artery bypass operations. Ann Thorac Surg. 2003;76:41–45. doi: 10.1016/s0003-4975(03)00182-6. [DOI] [PubMed] [Google Scholar]
- 21.Gulbins H, Malkoc A, Ennker IC, Ennker J. Preoperative platelet inhibition with ASA does not influence postoperative blood loss following coronary artery bypass grafting. Thorac Cardiovasc Surg. 2009;57:18–21. doi: 10.1055/s-2008-1038791. [DOI] [PubMed] [Google Scholar]
- 22.Matsuura K, Imamaki M, Ishida A, Shimura H, Miyazaki M. The effect of preoperative aspirin administration on postoperative level of von Willebrand factor in off-pump coronary artery bypass surgery. Heart Vessels. 2009;24:169–174. doi: 10.1007/s00380-008-1105-2. [DOI] [PubMed] [Google Scholar]
- 23.Kallis P, Tooze JA, Talbot S, Cowans D, Bevan DH, Treasure T. Pre-operative aspirin decreases platelet aggregation and increases post-operative blood loss: a prospective, randomised, placebo controlled, double-blind clinical trial in 100 patients with chronic stable angina. Eur J Cardiothorac Surg. 1994;8:404–409. doi: 10.1016/1010-7940(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 24.Morawski W, Sanak M, Cisowski M, Szczeklik M, Szczeklik W, Dropinski J, Waclawczyk T, Ulczok R, Bochenek A. Prediction of the excessive perioperative bleeding in patients undergoing coronary artery bypass grafting: role of aspirin and platelet glycoprotein IIIa polymorphism. J Thorac Cardiovasc Surg. 2005;130:791–796. doi: 10.1016/j.jtcvs.2005.02.041. [DOI] [PubMed] [Google Scholar]
- 25.Berger JS, Frye CB, Harshaw Q, Edwards FH, Steinhubl SR, Becker RC. Impact of clopidogrel in patients with acute coronary syndromes requiring coronary artery bypass surgery: a multicenter analysis. J Am Coll Cardiol. 2008;52:1693–1701. doi: 10.1016/j.jacc.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 26.Maltais S, Perrault LP, Do QB. Effect of clopidogrel on bleeding and transfusions after off-pump coronary artery bypass graft surgery: impact of discontinuation prior to surgery. Eur J Cardiothorac Surg. 2008;34:127–131. doi: 10.1016/j.ejcts.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 27.Chan V, Kulik A, Bourke ME, Ressler L, Mesana TG, Ruel M. Clopidogrel is safe early after on- and off-pump coronary artery bypass surgery. J Card Surg. 2007;22:493–497. doi: 10.1111/j.1540-8191.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 28.Hankey GJ, Eikelboom JW. Aspirin resistance. Lancet. 2006;367:606–617. doi: 10.1016/S0140-6736(06)68040-9. [DOI] [PubMed] [Google Scholar]
- 29.Martin CP, Talbert RL. Aspirin resistance: an evaluation of current evidence and measurement methods. Pharmacotherapy. 2005;25:942–953. doi: 10.1592/phco.2005.25.7.942. [DOI] [PubMed] [Google Scholar]