Abstract
Keloids are pathologic proliferations of the dermal layer of the skin resulting from excessive collagen production and deposition. Hepatocyte growth factor (HGF) increases the expression of matrix metalloproteinase (MMP)-1 and suppresses collagen synthesis to modulate extracellular matrix turnover. To investigate the anti-fibrotic effects of HGF, we examine the mRNA expression of collagen types I and III and matrix metalloproteinase (MMP-1, MMP-3) on human dermal fibroblast (HDF) cell lines and keloid fibroblasts (KFs, n = 5) after adding various amount of HGF protein. We also evaluated the enzymatic activity of MMP-2, MMP-9 by zymograghy. In HDFs treated with TGF-β1 and HGF protein simultaneously, both type I and III collagen mRNA expression significantly decreased (P < 0.05). Expression of MMP-1, MMP-3 mRNA also decreased. However, the mRNA expression of MMP-1, MMP-3 significantly increased in KFs with increasing amount of HGF in dose dependent manner (P < 0.05). The enzymatic activities of MMP-2 increased with increasing HGF protein in a dose-dependent manner. However, the enzymatic activity of MMP-9 did not change. These results suggest that the anti-fibrotic effects of HGF may have therapeutic effects on keloids by reversing pathologic fibrosis.
Keywords: Hepatocyte Growth Factor, Keloid Fibroblast, Dermal Fibroblast, Collagen, Matrix Metalloproteinase
INTRODUCTION
Keloids and hypertrophic scars are pathologic conditions resulting from excessive collagen deposition in the dermis due to skin injuries including surgery and trauma. They can lead to functional problems such as limited joint motion and aesthetic and social issues for patients. At present, the demand for keloid treatment is increasing.
Keloid fibroblasts (KFs) are similar to human normal dermal fibroblasts (HDFs) in size and shape, but show a great capacity to proliferate and produce high levels of extracellular matrix such as collagen, fibronectin, elastin, and proteoglycans (1-5). Keloids show an abnormal balance between proliferative and apoptotic cell death (6) and an excessive synthesis of extracellular matrix due to a failure to maintain homeostasis in the wound healing process. Many investigators have found that the excessive extracellular matrix (ECM) production is related to an increase or decrease of matrix metalloproteinases (MMP-1, -2, -3, -9, -13) and an increase of tissue inhibitors of metalloproteinases (TIMPs) (7-10).
Gelatinases (MMP-2, 9) digest denatured collagens (types I and III) fibrils, type IV collagen, fibronectin and elastin; additionally, these enzymes can potentiate the degradation of ECM components by activating collagenase-3 (MMP-13) and neutrophil collagenase (MMP-8). Therefore, gelatinases are crucial in physiological processes such as tissue remodeling, and in pathological processes such as wound healing and the invasion of malignant tumors (11-13). MMP-9 activity is crucial during the epithelization process and early repair events, whereas the gelatinolytic activity of MMP-2 is important during the prolonged remodeling phase (14-16). The gelatinases act on cleaved collagen more effectively than other MMPs (11).
Hepatocyte growth factor (HGF), initially discovered to be a potent mitogen for hepatocytes (17, 18) and also known as a "scatter factor", acts as a multifunctional mediator on many kinds of cells. It is secreted by mesenchymal cells and regulates cell growth, cell motility, apoptotic cell death (19), and the morphogenesis of hepatocytes, fibroblasts, and keratinocytes (20) primarily by activating a tyrosine kinase signaling cascade after binding to the proto-oncogenic c-Met receptor (21-23). It is secreted as a single, inactive polypeptide and is cleaved by serine proteases into a 69-kDa α-chain and a 34-kDa β-chain.
Recent studies have found a reverse anti-fibrotic effect in which HGF prevents fibrosis after damage to the liver (24), lungs, and kidneys (20, 25). HGF not only protects against fibrosis through inhibiting ECM deposition such as collagen but also reduces the previously formed ECM by the induction of MMP-1 (26). However, these studies show these anti-fibrotic effects only in animal models and with HGF gene therapy, and the actions of HGF on human keloid fibroblasts have yet to be studied.
Some investigators have studied an anti-fibrogenic mechanism of HGF in which increasing MMP expression promotes collagen degradation (26, 27). Adding HGF protein to fibroblast cultures of scleroderma patients significantly decreased collagen production and increased MMP-1 expression and activity (26). This means that HGF may have therapeutic effect on keloids that are similar to scleroderma with pathologic dermal fibrosis.
In this study, we investigated the mRNA levels of type I and III collagen synthesis, MMP-1, 3 expression, and MMP-2, 9 enzymatic activities in HDFs and KFs after administration of various amounts of HGF protein with or without TGF-β1.
MATERIALS AND METHODS
Keloid-derived fibroblast cultures and the human dermal fibroblast cell line
Keloid-derived fibroblasts (KFs, n = 5) were obtained from tissues excised during surgical procedures. Cells were obtained from the central dermal layer of active stage keloid patients (Table 1) after having obtained informed consent according to a protocol approved by the Yonsei University College of Medicine Institutional Review Board. All experiments involving humans were performed in adherence to the Helsinki Guidelines. Keloids were identified by trained clinicians and pathologists. The separated cells were cultured in Dulbecco's modified Eagle's medium (DMEM; GIBCO, Grand Island, NY, USA) supplemented with heat-inactivated 10% fetal bovine serum (FBS) and penicillin (30 U/mL), streptomycin (300 mg/mL), and actinomysin. The culture medium was changed in 2-3 day intervals. All cells used in this study were taken before passage 5. The human normal dermal fibroblasts (HDFs) cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The HDFs were cultured in DMEM (Gibco BRL) supplemented with 10% FBS (Gibco BRL), penicillin (30 IU/mL), and streptomycin (300 µg/mL).
Table 1.
Profiles of the keloid scar fibroblasts used in the study
Hepatocyte growth factor (HGF) and TGF-β1 treatment
The HDFs and KFs were treated with different amounts of HGF protein (1, 10, 20, and 40 ng). Three days after adding the HGF protein, the mRNA levels of collagen type I, III, MMP-1, 3 were examined by RT-PCR. Additionally, MMP-2 and -9 enzymatic activities were examined by zymography. TGF-β1 (5 ng/mL) was added simultaneously with the HGF protein.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted from samples using a Trizol reagent (Invitrogen Corp., Carlsbad, CA, USA) according to the manufacturer's protocol. One microgram of total RNA was converted into the complementary DNA using a First Strand cDNA Synthesis kit (Promega Corp., Madison, WI, USA) with random primers. A polymerase chain reaction was performed using 2× Taq Premix 2 (Solgent Co., Ltd., Seoul, Korea) and the synthetic gene-specific primers for collagen types I and III, MMP-1, MMP-3 and β-actin (Table 2). All target sequences were separately amplified for 35 cycles. Samples of each reaction product were separated by 2% agarose gel electrophoresis, visualized by ethidium bromide staining, and photographed with 290-nm ultraviolet illumination. The density of each band was measured by Image J. Relative expression of the mRNA of collagen types I and III, MMP-1, and MMP-3 were normalized to the expression of an internal control (β-actin).
Table 2.
List of primers used in our study
MMP zymography
The enzyme activities of MMP-2 and MMP-9 were measured using zymography. Culture supernatants were collected and analyzed by gel substrate zymography. Briefly, 10 mL of non-reducing sample buffer (125 mM Tris-HCl [pH 6.8], 10%[v/v] glycerol, 0.1%[w/v] BPB) was added to each sample. Samples were then loaded onto 12.5% SDS-PAGE gels containing 0.2% gelatin (Sigma, St. Louis, MO, USA). The gels were incubated with renaturing buffer (2.5% Triton X-100, 50 mM Tris-HCl, pH 7.5, and 0.1 M NaCl) for 1 hour at room temperature, and then with developing buffer (50 mM Tris-pH (pH 7.5), 10 mM CaCl2, and 0.02% NaN3) for 18 hr at 37℃. The gels were then stained with 1% Coomassie brilliant blue R-250, and gelatinolytic activity was detected as unstained bands on a blue background.
Statistics
Results are expressed as mean ± standard error of the mean (SEM). Statistical comparisons across KFs and HDF cell lines treated with different amounts of HGF were made using paired t-tests. P values less than 0.05 were considered significant.
Ethics statement
This study was approved by the institutional review board of Yonsei Medical Center (IRB No.: 4-2011-0072). Informed consent was acquired from all patients.
RESULTS
The effects of hepatocyte growth factor on the mRNA expression of collagen types I and III in the HDFs, TGF-β1 stimulated HDFs, and keloid fibroblasts
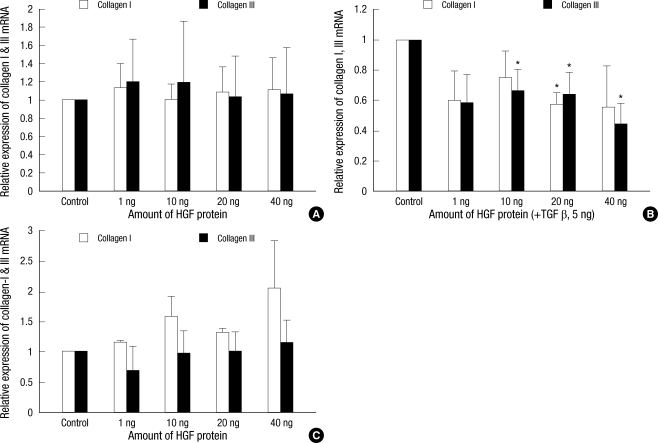
The mRNA expression of collagen types I and III were examined by RT-PCR. We found no significant differences in HDFs after adding HGF alone (Fig. 1A). However, when TGF-β1 (5 ng/mL) was added simultaneously with the HGF protein, collagen types I and III mRNA expression decreased. In HDFs treated with 40 ng HGF protein, this decrease was 57% for type I and 64% for type III collagen (P < 0.05, Fig. 1B). On the other hand, the HGF protein caused no significant change in type I collagen mRNA expression in KFs (Fig. 1C).
Fig. 1.
The effects of HGF protein on type I and III collagen mRNA expression in the human dermal fibroblast cell lines (A, B) and keloid fibroblasts (C). When TGF-β1 (5 ng/mL) was added simultaneously with HGF protein, type I and III collagen mRNA expression significantly decrease in the HDFs (P < 0.05).
The effects of hepatocyte growth factor on the mRNA expression of MMP-1 and 3 in the HDFs, TGF-β1 stimulated HDFs and keloid fibroblasts
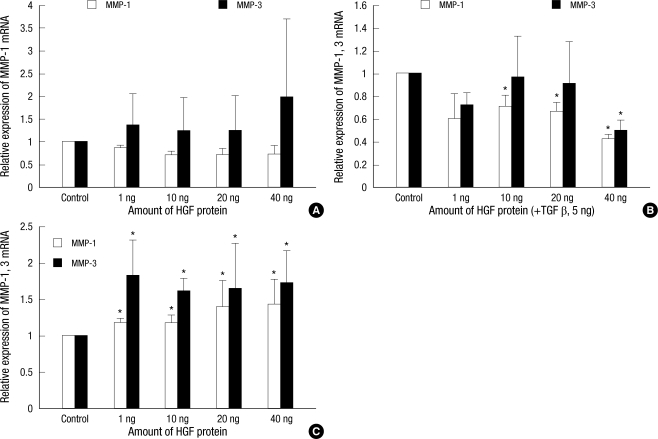
The expression of MMP-1 and MMP-3 mRNA was examined by RT-PCR. We found no significant differences in the expression of MMP-1 and MMP-3 mRNA when the HGF protein was treated alone. When TGF-β1 (5 ng/mL) was administered simultaneously with the HGF protein, both MMP-1 and MMP-3 mRNA expression decreased. This decrease was approximately 43% for MMP-1 and 49% for MMP-3 after adding 40 ng of HGF protein (Fig. 2A, B). However, mRNA expressions of MMP-1 and MMP-3 in the KFs were increased after the administration of HGF protein alone with statistical significance (P < 0.05, Fig. 2C).
Fig. 2.
The effects of HGF protein on MMP-1 and MMP-3 mRNA expression in the human dermal fibroblast cell lines (A, B) and keloid fibroblasts (C). When TGF-β1 (5 ng/mL) was added to the HDFs simultaneously with HGF protein, MMP-1 and MMP-3 mRNA expression decreased. However, in the KFs, MMP-1 and MMP-3 mRNA expression significantly increased with the administration of HGF (P < 0.05).
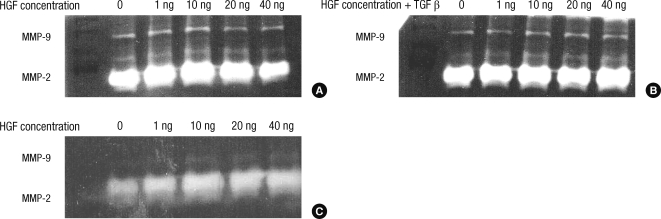
The effects of hepatocyte growth factor on MMP-2, MMP-9 enzymatic activities
The HDFs and KFs were treated with various amounts of HGF protein (1, 10, 20, and 40 ng). Three days after the administration of HGF protein, MMP-2 and MMP-9 enzymatic activities were examined by zymography.
For the HDFs, we found that MMP-2 enzymatic activity increased after adding 1, 10, and 20 ng of HGF protein (Fig. 3A). However, the enzymatic activity of MMP-9 did not change. When TGF-β1 (5 ng/mL) was added simultaneously with HGF protein, the enzymatic activities of MMP-2 and MMP-9 were unchanged (Fig. 3B). For the KFs, we found that MMP-2 activity increased with increasing amounts of HGF protein in a dose-dependent manner (Fig. 3C). However, the enzymatic activity of MMP-9 either did not change or showed only a slight increase.
Fig. 3.
Three days after adding HGF protein, the enzymatic activities of MMP-2 and MMP-9 were examined by zymography. For the HDFs, MMP-2 activity increased after adding HGF protein except 40 ng, and MMP-9 activity did not changed (A). However, after simultaneously adding TGF-β1, MMP-2 and MMP-9 activity were unchanged (B). In the KFs, (C) MMP-2 activity increased with increasing HGF protein in a dose-dependent manner.
DISCUSSION
This study investigated the anti-fibrogenic effects of hepatocyte growth factor (HGF) on the human dermal fibroblasts (HDFs) cell line and keloid fibroblasts (KFs). Fujiwara et al. (28) found that the production of type 1 collagen, MMP-1, and MMP-2 was 3-fold, 6-fold, and 2.4-fold higher, respectively, in keloid fibroblasts compared to normal dermal fibroblasts. In this study, KFs showed excessive collagen synthesis and breakdown of the extracellular matrix (ECM) due to increased MMP-1 and MMP-2 activity. TGF-β1 played a role in modulating type 1 collagen, MMP-1, and MMP-2. Since these keloids are characterized by an accumulation of collagen, it appears that the down regulation of collagen I and III expression and the upregulation of MMP-1 and MMP-2 are able to ablate the excess collagen formed by the increase in synthetic activity.
In our study, the expressions of collagen I and III mRNA induced by exogenous TGF-β1 were suppressed by adding HGF protein on the HDFs. However, these expressions were not changed by adding HGF protein alone. This means that HGF does not influence or increase ECM production of normal dermal fibroblasts, and suppresses the increased expression of collagen I and III mRNA induced by exogenous treatment with TGF-β. This suggests a high potential for HGF as therapeutics in abnormal fibrotic disease because HGF do not affect basal collagen levels in normal fibroblasts.
Applying HGF proteins to KFs did not significantly change the expression of collagen I and III mRNA (Fig. 1). In previous studies, collagen I and III mRNA expression was found to be more enhanced in KFs than HDFs, so that we expected the addition of HGF protein to cultured KFs to decrease collagen I and III mRNA expression since TGF-β is antagonized by HGF (20). However, contrary to our hypothesis, this did not happen. This seems to be caused by a different profibrogenic reaction with TGF-β in KFs compared with HDFs. More TGF-β is secreted in KFs than in normal fibroblasts and it plays a predominant role in the profibrogenic reaction; the profibrogenic reaction of TGF-β in KFs is more sensitive and persistent than in HDFs. Thus, the anti-fibrogenic reaction of HGF seems to be different in KFs from that in HDFs. Therefore, it appears that HGF protein decreases the expression of type I and III collagen mRNA in profibrogenic conditions (HDFs adding TGF-β), but does not influence these expression in keloid-like conditions (high TGF-β concentration). However, Jinnin et al. (26) reported that HGF decreased the deposition of type I collagen protein by C-met overexpression and MMP-1 induced collagenolysis. Therefore, further investigation of the effects of HGF on collagen metabolism of keloid-derived fibroblast would be warranted.
Applying TGF-β (5 ng) and HGF to the cultured HDFs significantly decreased the expression of MMP-1 and MMP-3 mRNA, but adding HGF alone showed no significant differences. Also, when TGF-β and HGF are both applied to cultured HDFs, the expression of collagen type I and type III mRNA decreases. Because healing of wounds such as lacerations, requires early profibrogenic conditions similar to secreted TGF-β in HDFs, HGF treatments may impair the wound healing process due to a decrease in extracellular matrix synthesis and degradation rate. In addition, we found no changes in MMP-9 activity in the HDFs, which is further evidence that HGF protein therapy will have little benefit in the wound healing process. However, other study shows that continuous high HGF protein expression by the introduction of the HGF gene promotes dermal regeneration, possibly not only by suppres sing the overgrowth of collagen due to its antifibrotic effect but also by promoting regeneration (22). Therefore we think that repetitive high doses of HGF could enhance effectively an acute wound healing. But, this approach has disadvantages, including the requirement for large amounts of HGF, the short half-life, the potential toxicity, and difficulty with appropriate delivery of HGF in the wound. Further studies are necessary for changes of collage and MMPs expression using HGF gene therapy.
Adding HGF to cultured KFs increased MMP-1 and MMP-3 mRNA expression (Fig. 2C). The enzymatic activity of MMP-2 also increased with increasing HGF protein concentration (Fig. 3). In contrast, we saw no detectable changes in MMP-9 activity in the KFs. The increase in MMP-2 activity is important in inducing the degradation of excessive ECM in keloids, which seems to enhance MMP-1 and MMP-3 expression, indicating that HGF may have therapeutic effects on keloids by reversing pathologic fibrosis. The anti-fibrogenic effects of HGF generally increased in a dose-dependent manner, and so the appropriate treatment dose remains to be determined. In addition, further studies are necessary to develop efficient continuous release vehicles for HGF such as gene therapy that has its very short half-life.
In conclusion, contrary to previously published reports which found that HGF promotes wound healing, this study showed that HGF might inhibit the profibrogenic effects in wound healing. In addition, we found that HGF may have a therapeutic effect on keloids through enhancing MMP-1 and MMP-3 mRNA expression and MMP-2 enzymatic activity.
Footnotes
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Republic of Korea Government (MEST) (No. 2009-0073055). All authors have no conflicts of interest or financial arrangements that could potentially influence the described research.
AUTHOR SUMMARY
Effects of Hepatocyte Growth Factor on Collagen Synthesis and Matrix Metalloproteinase Production in Keloids
Won Jai Lee, Sang Eun Park and Dong Kyun Rah
The purpose of this study is to investigate the effects of hepatocyte growth factor (HGF) in the human dermal fibroblasts (HDFs) and keloid fibroblasts (KFs). In HDFs, treatment with TGF-β1 and HGF decreased type I and III collagen mRNA expression. However, the expressions of MMP-1, MMP-3 mRNA were also decreased. In contrast, the expressions of MMP-1 and MMP-3 mRNA were increased in KFs by HGF. Also, the enzymatic activity of MMP-2 was increased by HGF. These results suggest that the anti-fibrotic effects of HGF may have therapeutic effects on keloids by reversing pathologic fibrosis.
References
- 1.Babu M, Diegelmann R, Oliver N. Fibronectin is overproduced by keloid fibroblasts during abnormal wound healing. Mol Cell Biol. 1989;9:1642–1650. doi: 10.1128/mcb.9.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bettinger DA, Yager DR, Diegelmann RF, Cohen IK. The effect of TGF-beta on keloid fibroblast proliferation and collagen synthesis. Plast Reconstr Surg. 1996;98:827–833. doi: 10.1097/00006534-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Calderon M, Lawrence WT, Banes AJ. Increased proliferation in keloid fibroblasts wounded in vitro. J Surg Res. 1996;61:343–347. doi: 10.1006/jsre.1996.0127. [DOI] [PubMed] [Google Scholar]
- 4.Kischer CW, Hendrix MJ. Fibronectin (FN) in hypertrophic scars and keloids. Cell Tissue Res. 1983;231:29–37. doi: 10.1007/BF00215771. [DOI] [PubMed] [Google Scholar]
- 5.Russell SB, Trupin JS, Kennedy RZ, Russell JD, Davidson JM. Glucocorticoid regulation of elastin synthesis in human fibroblasts: down-regulation in fibroblasts from normal dermis but not from keloids. J Invest Dermatol. 1995;104:241–245. doi: 10.1111/1523-1747.ep12612788. [DOI] [PubMed] [Google Scholar]
- 6.Akasaka Y, Fujita K, Ishikawa Y, Asuwa N, Inuzuka K, Ishihara M, Ito M, Masuda T, Akishima Y, Zhang L, Ito K, Ishii T. Detection of apoptosis in keloids and a comparative study on apoptosis between keloids, hypertrophic scars, normal healed flat scars, and dermatofibroma. Wound Repair Regen. 2001;9:501–506. doi: 10.1046/j.1524-475x.2001.00501.x. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds JJ. Collagenases and tissue inhibitors of metalloproteinases: a functional balance in tissue degradation. Oral Dis. 1996;2:70–76. doi: 10.1111/j.1601-0825.1996.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 8.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 9.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 10.Kischer CW, Thies AC, Chvapil M. Perivascular myofibroblasts and microvascular occlusion in hypertrophic scars and keloids. Hum Pathol. 1982;13:819–824. doi: 10.1016/s0046-8177(82)80078-6. [DOI] [PubMed] [Google Scholar]
- 11.Parks WC. Matrix metalloproteinases in repair. Wound Repair Regen. 1999;7:423–432. doi: 10.1046/j.1524-475x.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- 12.Vihinen P, Ala-aho R, Kähäri VM. Matrix metalloproteinases as therapeutic targets in cancer. Curr Cancer Drug Targets. 2005;5:203–220. doi: 10.2174/1568009053765799. [DOI] [PubMed] [Google Scholar]
- 13.Santos MC, Souza AP, Gerlach RF, Tabchoury CM, Line SR. Inhibition of human gelatinases (matrix metalloproteinase-2 and matrix metalloproteinase-9) activity by zinc oxide: a possible mechanism to enhance wound healing. Br J Dermatol. 2001;145:854–855. doi: 10.1046/j.1365-2133.2001.04502.x. [DOI] [PubMed] [Google Scholar]
- 14.Gillard JA, Reed MW, Buttle D, Cross SS, Brown NJ. Matrix metalloproteinase activity and immunohistochemical profile of matrix metalloproteinase-2 and -9 and tissue inhibitor of metalloproteinase-1 during human dermal wound healing. Wound Repair Regen. 2004;12:295–304. doi: 10.1111/j.1067-1927.2004.012314.x. [DOI] [PubMed] [Google Scholar]
- 15.Inkinen K, Turakainen H, Wolff H, Ravanti L, Kähäri VM, Ahonen J. Expression and activity of matrix metalloproteinase-2 and -9 in experimental granulation tissue. APMIS. 2000;108:318–328. doi: 10.1034/j.1600-0463.2000.d01-63.x. [DOI] [PubMed] [Google Scholar]
- 16.Agren MS. Gelatinase activity during wound healing. Br J Dermatol. 1994;131:634–640. doi: 10.1111/j.1365-2133.1994.tb04974.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122:1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- 18.Asami O, Ihara I, Shimidzu N, Shimizu S, Tomita Y, Ichihara A, Nakamura T. Purification and characterization of hepatocyte growth factor from injured liver of carbon tetrachloride-treated rats. J Biochem. 1991;109:8–13. [PubMed] [Google Scholar]
- 19.Bardelli A, Longati P, Albero D, Goruppi S, Schneider C, Ponzetto C, Comoglio PM. HGF receptor associates with the anti-apoptotic protein BAG-1 and prevents cell death. EMBO J. 1996;15:6205–6212. [PMC free article] [PubMed] [Google Scholar]
- 20.Sato C, Tsuboi R, Shi CM, Rubin JS, Ogawa H. Comparative study of hepatocyte growth factor/scatter factor and keratinocyte growth factor effects on human keratinocytes. J Invest Dermatol. 1995;104:958–963. doi: 10.1111/1523-1747.ep12606221. [DOI] [PubMed] [Google Scholar]
- 21.Ono I. The effects of basic fibroblast growth factor (bFGF) on the breaking strength of acute incisional wounds. J Dermatol Sci. 2002;29:104–113. doi: 10.1016/s0923-1811(02)00019-1. [DOI] [PubMed] [Google Scholar]
- 22.Ono I, Yamashita T, Hida T, Jin HY, Ito Y, Hamada H, Akasaka Y, Ishii T, Jimbow K. Local administration of hepatocyte growth factor gene enhances the regeneration of dermis in acute incisional wounds. J Surg Res. 2004;120:47–55. doi: 10.1016/j.jss.2003.08.242. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem. 1996;119:591–600. doi: 10.1093/oxfordjournals.jbchem.a021283. [DOI] [PubMed] [Google Scholar]
- 24.Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E, Fujimoto J. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226–230. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- 25.Patijn GA, Lieber A, Schowalter DB, Schwall R, Kay MA. Hepatocyte growth factor induces hepatocyte proliferation in vivo and allows for efficient retroviral-mediated gene transfer in mice. Hepatology. 1998;28:707–716. doi: 10.1002/hep.510280317. [DOI] [PubMed] [Google Scholar]
- 26.Jinnin M, Ihn H, Mimura Y, Asano Y, Yamane K, Tamaki K. Effects of hepatocyte growth factor on the expression of type I collagen and matrix metalloproteinase-1 in normal and scleroderma dermal fibroblasts. J Invest Dermatol. 2005;124:324–330. doi: 10.1111/j.0022-202X.2004.23601.x. [DOI] [PubMed] [Google Scholar]
- 27.Esposito C, Parrilla B, Cornacchia F, Grosjean F, Mangione F, Serpieri N, Valentino R, Villa L, Arra M, Esposito V, Dal Canton A. The antifibrogenic effect of hepatocyte growth factor (HGF) on renal tubular (HK-2) cells is dependent on cell growth. Growth Factors. 2009;27:173–180. doi: 10.1080/08977190902834077. [DOI] [PubMed] [Google Scholar]
- 28.Fujiwara M, Muragaki Y, Ooshima A. Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br J Dermatol. 2005;153:295–300. doi: 10.1111/j.1365-2133.2005.06698.x. [DOI] [PubMed] [Google Scholar]