Abstract
Lateral neck node metastasis is an important prognostic factor in thyroid carcinoma. We developed a scoring system for use in prediction of lateral neck node metastasis from papillary thyroid cancer. In this study, 161 consecutive patients were included in the training data set. This scoring system, named the Yonsei Estimated Value (YEV) for lymph node metastasis in papillary thyroid cancer, was developed on the basis of results from multivariate logistic regression analysis of preoperative clinical and radiologic data. Sixty eight consecutive patients were included for testing of the validity of the scoring system. The equation for prediction of lateral neck node metastasis was follows:
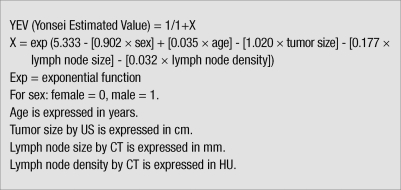
YEV (Yonsei Estimated Value) = 1/(1+X)
X = Exp (5.333-[0.902 × sex]+[0.036 × age]-[1.020 × tumor size]-[0.177 × lymph node size]-[0.032 × lymph node density])
When the YEV was 0.3 or more, the probability of lateral neck node metastasis was 79.0%, with sensitivity of 76.3%, specificity of 69.8%, positive predictive value of 56.7%, and negative predictive value of 85.1% in the training set. When fine needle aspiration biopsy for suspicious lateral neck nodes is not possible, or the results are inadequate, our scoring system for prediction of lateral neck node metastasis can be helpful in optimization of the surgical extent for each patient.
Keywords: Scoring System for Lateral Neck Node Metastasis, Predictive Factors, Papillary Thyroid Carcinoma
INTRODUCTION
Lateral neck node metastasis is an important prognostic factor, which is closely related to tumor recurrence and poor prognosis in patients with papillary thyroid carcinoma (1, 2). Precise preoperative evaluation for lateral neck node metastasis is helpful in determination of proper treatment planning and decisions regarding the extent of surgery. Among preoperative imaging methods, neck ultrasound (US) is a sensitive tool for evaluation of thyroid lesions, as well as lateral neck nodes. It can also be used in guidance of fine needle aspiration biopsy (FNAB); however, the major limitation of US is that it is primarily operator-dependent. On the other hand, computed tomography (CT) can be used for comprehensive and objective evaluation of the neck area, regardless of the operator's skill (3, 4). However, few studies have reported the diagnostic accuracy of preoperative CT (5). Based on the results of diagnostic imaging, FNAB can be performed for evaluation of the suspicious thyroid nodules as well as suspected metastatic lymph node(s) before surgery. However, the diagnostic accuracy of FNAB for evaluation of lateral neck nodes has been criticized (6, 12). In addition, even with thyroglobulin measurement by the washout method after FNAB, the accuracy could only be increased to a limited extent (14).
Therefore, there is an obvious need for a more precise method for prediction of lateral neck node metastasis. In this study, we suggest a newly developed scoring system for prediction of lateral neck node metastasis with analysis of clinical characteristics and imaging results.
MATERIALS AND METHODS
Among 582 patients who underwent operation for papillary thyroid carcinoma between January and December 2008 in Gangnam Severance Hospital, 161 consecutive patients who underwent preoperative imaging, such as US or CT, showing ambiguous results were enrolled. For selection of cases, patients who showed obvious findings of lateral neck node metastasis in imaging studies, such as necrosis, cystic change, or microcalcification on US or CT, were excluded. Those who had been previously diagnosed with lateral neck node metastasis by FNAB were also excluded. All patients underwent multidetector CT (16-detector row; Sensation 16, Siemens, Erlangen, Germany).
Preoperative US and Neck CT were performed for measurement of primary tumor size and lateral neck node size respectively. Primary tumor size and lateral neck node size were described according to maximal diameter. If vague lateral neck nodes on preoperative studies were numerous, we selected the lateral neck node with the highest CT density (Hounsfield Unit, HU).
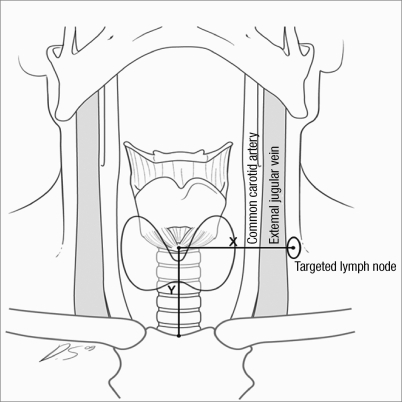
With the patient under general anesthesia in the standard operative position, on-site US-guided localization of lateral neck nodes was performed. X and Y axes and the distances were measured according to the location in the axial plane of the neck CT. The Y axis was the longitudinal distance from the sternum to the midline level of the target lateral neck node, and the X axis was the transverse distance from the midline to the target lateral neck node (Fig. 1). The shape and location of the target lateral neck node was reevaluated with on-site US, which was performed with an HDI 3000 scanner (Aloka, Wallingford, CT, USA) using a bandwidth of 7.5-12 MHz transducer. Targeting of lateral neck nodes was performed by injection of 0.1 mL of 5% vital dye using US guidance. Via the standard low neck collar incision for thyroidectomy, the stained lateral neck node was excised and sent for frozen-section histologic analysis. Surgical extent was determined according to results of frozen-section pathology.
Fig. 1.
Schematic figure of the X axis and Y axis. The lymph node is targeted according to the location in the axial plane of CT, with the Y axis defined as the distance from the sternum to the target lymph node, and the X axis is as the distance from the upper Y-axis point to the target lymph node.
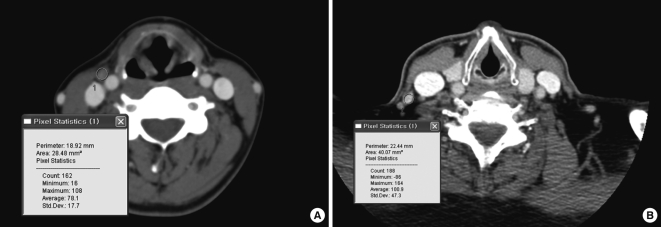
Clinical data and radiographic findings were evaluated. Clinical data included sex, age, tumor size, and coexistence of chronic thyroiditis, and findings of imaging studies for lateral neck nodes included the location, size, shape, and CT density (Hounsfield Unit, HU) after contrast enhancement of the lateral neck nodes (Fig. 2).
Fig. 2.
CT findings for lateral neck lymph nodes are CT density after contrast injection (Hounsfield Unit, HU) of the lateral neck lymph node. (A) proven nonmetastatic node, with 108 HU. (B) proven metastatic node, with 164 HU.
Data are presented as mean ± standard deviation (SD), number (percent), or range. SAS software (SAS version 9.1, SAS Institute Inc., Cary, NC, USA) was used for performance of statistical analysis. A Student's t test was performed for comparison of continuous variables. A chi-square test or Fisher exact test was used for evaluation of categorical variables. Logistic Cox regression model was used for multivariate analysis. Differences with P values of less than 0.05 were considered significant. After statistical analysis, we developed a scoring system, named the Yonsei Estimated Value (YEV), for prediction of lateral neck node metastasis. For scoring system validation, 68 patients underwent surgery for treatment of papillary thyroid carcinoma between January and June 2009 according to the same entry criteria.
Ethics statement
This study was approved by the institutional review board of Gangnam Severance Hospital, Yonsei University College of Medicine in Seoul, Korea (IRB number: 3-2008-0055). All participants gave their written informed consent.
RESULTS
Clinical characteristics
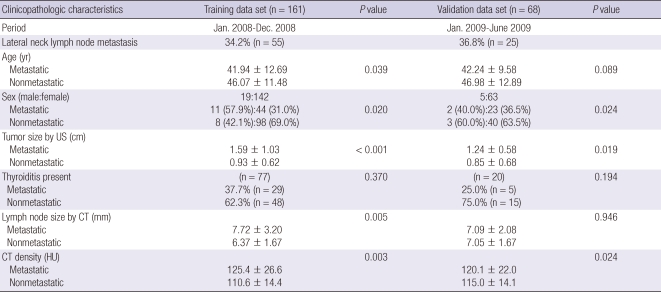
Clinical characteristics of patients in the training and validation data sets are summarized in Table 1. Training data set, included 142 female and 19 male patients (age range, 15-71 yr; mean age, 44.6 yr). Among the 161 patients, 55 patients (34.2%) were diagnosed as having lateral node metastasis by frozen section histology. Lateral neck node metastasis occurred more often in men than in women (57.9% vs 31.0%; P = 0.020). Patients with lateral neck node metastasis were younger than those without (41.94 ± 12.69 vs 46.07 ± 11.48 yr; P = 0.039). Mean size of the thyroid tumor was 1.15 cm (range, 0.2-6.0 cm). Tumor size was larger in the metastatic group than in the nonmetastatic group (1.59 ± 1.03 vs 0.93 ± 0.62 cm; P < 0.001).
Table 1.
Clinical and radiologic findings of the training and validation data sets
Data are presented as mean ± standard deviation or percent (number). CT, computed tomography; US, ultrasound; HU, Hounsfield units.
Seventy-seven patients (47.8%) were diagnosed with coexistent chronic thyroiditis. However, these factors were not associated with the likelihood of lateral neck node metastasis.
The location of lateral neck nodes was analyzed according to the classification of the American Association of Head and Neck Surgery. The most frequent location of excised lateral lymph nodes was level 3 (82.0%), followed by level 4 (8.7%), and level 2 (9.3%). The size of excised nodes ranged from 2.9 to 22.0 mm (mean, 7.21 mm). Sizes of nodes were larger in the metastatic group than in the nonmetastatic group (7.72 ± 3.20 vs 6.37 ± 1.67 mm; P = 0.005). The shape of excised nodes was more frequently oval (65.2%) than round (34.8%); however, this factor was not statistically different in the metastatic and nonmetastatic groups. CT density of all lateral neck nodes after contrast enhancement ranged from 34 HU to 185 HU (mean, 119 HU). Metastatic nodes showed higher density than nonmetastatic nodes (125.4 ± 26.6 vs 110.6 ± 14.4 HU; P = 0.003). However, in the 77 cases of coexistence with chronic thyroiditis, CT density did not differ between metastatic nodes and nonmetastatic nodes (119 ± 12.3 vs 132 ± 24.3 HU; P = 0.169).
Scoring system
Multivariate logistic regression analysis was performed for evaluation of risk factors for lateral neck node metastasis. Consequently, sex, age, tumor size, lymph node size, and HU on lymph node were statistically significant. The YEV scoring system was developed by statistical analyses based on the sum of the β-coefficient of each variable (Fig. 3). Goodness-of-fit in the scoring system was verified by the Hosmer-Lemeshow test. When the YEV was 0.3 or more the probability of lateral neck node metastasis was 79.0%, sensitivity was 76.3%, specificity was 69.8%, positive predictive value was 56.7%, and negative predictive value was 85.0%.
Fig. 3.
Scoring system. CT, computed tomography; US, ultrasound; HU, Hounsfield units.
The YEV system was applied to the validation data set (n = 68). When the YEV was 0.3, the sensitivity was 60%, specificity was 65%, positive predictive value was 50%, and negative predictive value was 74% in the validation set. Comparison of the training data set with the validation data set, showed similar results (Table 1). Therefore, our scoring system is a good discriminative method for prediction of lateral neck node metastasis.
DISCUSSION
Preoperative evaluation for prediction of lateral neck node metastasis is essential in planning of appropriate treatment of thyroid cancer. Many studies have been conducted for prediction of lateral neck node metastasis according to clinical factors and radiologic findings (5, 7, 8, 11, 12). Significant clinical factors that have been reported included male sex, age under 45 yr, tumor size more than 2 cm, and the presence of extracapsular invasion (7). In agreement with our previous report (8), the current study revealed that statistically significant factors associated with lateral neck node metastasis included male sex, younger age (41.94 ± 12.69 vs 46.07 ± 11.48 yr), and larger tumor (1.59 ± 1.03 vs 0.93 ± 0.62 cm). However, coexistence of chronic thyroiditis was not associated with lateral neck node metastasis.
In consideration of radiological findings of lateral neck nodes, the generally accepted risk factors associated with metastasis include round shape, cystic necrosis of lymph node, irregular margin, and size greater than 1 cm (9, 10). In the current study, as we excluded cases with obvious findings of metastasis, we found that larger lateral node size (7.72 ± 3.20 vs 6.37 ± 1.67 mm), and increased CT density (125.4 ± 26.6 vs 110.6 ± 14.4 HU) were statistically significant.
Reactive lymph node enlargement is very common in cases of coexistent chronic thyroditis. In this study, in cases with thyroiditis, there were no significant differences in CT density, node shape and node size between metastatic and nonmetastatic lymph nodes. Therefore, in cases with chronic thyroiditis, discrimination of metastatic lateral neck nodes by imaging is very difficult, and care must be taken in evaluation of lateral neck nodes for metastasis.
Few studies have reported on the diagnostic value of US for preoperative diagnosis of node metastasis from thyroid carcinoma. Previous studies have shown variable sensitivities (37%-84%) and relatively high specificities (89%-98%) for US in detection of metastatic nodes (11, 12). However, US has many limitations, including the fact that results are operator-dependent, and difficulty in evaluating deep lymph nodes such as retropharyngeal, mediastinal, and low level 6 nodes (12). Preoperative US of lateral neck nodes suspicious for metastasis has been reported as effective (13); however, at the same time, questions have remained which regard to how the exact lymph node detected by US can be excised during the operation.
To date, a few studies have reported on the diagnostic accuracy of CT for detection of metastatic nodes in thyroid cancer. CT has some advantages for prediction of lateral neck node metastasis from thyroid cancer. First, results of CT are not operator-dependent. Second, it can provide a comprehensive evaluation of the entire neck area. Third, when the shape of nodes on US is nonspecific or ambiguous, CT may be helpful in diagnosis of node metastasis (12). However, CT for preoperative diagnosis of node metastasis from thyroid carcinoma has some limitations, including low sensitivity and the effect of intravenous iodinated contrast agent on subsequent radioiodine ablation therapy. In our hospital, radioiodine therapies are usually performed at approximately 12 or more weeks after CT scanning. Therefore, preoperative CT using an iodinated contrast agent has no significant effect on subsequent radioiodine ablation therapy. This study was designed to maximize the advantages of both CT and US in targeting of suspicious lymph nodes.
When lateral neck node metastasis is obvious or definitively diagnosed preoperatively, the surgical strategy can be easily established. However, when conducting an evaluation for lateral neck node metastasis, there is a risk of nondiagnostic findings on imaging. And it can be a serious problem for patients, because postoperative adjuvant therapy, such as radio-iodine therapy, is well known to be ineffective for nodal metastasis from thyroid cancers, compared with remnant thyroid tissues or hematogenous metastasis.
In this study, we intended to determine how many lateral neck nodes with nonspecific or confusing findings on imaging are metastatic from thyroid cancer and what risk factors affect this. Despite a diagnosis of not definitive or not specific by preoperative imaging studies, the prevalence of lateral neck node metastases was unexpectedly high in this study. This means that attention should be paid to the possibility of lateral neck node metastasis, even when the imaging findings are not suspicious.
When performance of preoperative US with FNAB is not possible, or the results are not adequate, intraoperative selective lateral neck node biopsy can determine the extent of surgery. However, nonlocalized lymph node biopsy such as berry picking or blind biopsy will not be helpful for diagnosis of lymph node metastasis, as well as for prevention of operative morbidity. To overcome these problems, we have suggested a method for targeted lymph node sampling, based on CT imaging in a previous study (8). However, the limitation of the study design was the question of how we could excise the exact node detected by CT. Therefore, we updated the previous method to amend the predictive value of targeted lymph node sampling for diagnosis of lateral neck node metastasis. In the current study, in order to increase the accuracy of lymph node biopsy, we used on-site US-guided lateral neck lymph node targeting with vital dye.
With careful statistical analysis of all risk factors, we developed a scoring system, YEV, for prediction of lateral neck node metastasis in thyroid cancer. To the best of our knowledge, this is the first study to evaluate lateral neck node metastasis in papillary thyroid cancer using a scoring system that utilizes commonly available clinical and radiologic information. The YEV system will be helpful for preoperative prediction of lateral neck node metastasis from thyroid cancer and for use in appropriate treatment planning for patients with thyroid cancer.
In conclusion, when the results of preoperative diagnostic evaluation are not definitive or FNAB results are inadequate, the YEV system for prediction of lateral neck node metastasis can be used as an alternative diagnostic approach, and it can be helpful in optimization of the surgical extent for each patient. Based on the results, if the YEV is calculated to be 0.3 or more, sampling of the lateral neck node using intraoperative US-guided localization biopsy is highly recommended.
ACKNOWLEDGMENTS
The authors are grateful to Dong-Su Jang, (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea) for his help with the illustrations (Fig. 1).
AUTHOR SUMMARY
A Scoring System for Prediction of Lateral Neck Node Metastasis from Papillary Thyroid Cancer
Jong Ju Jeong, Yong Sang Lee, Seung Chul Lee, Sang-Wook Kang, Woong Youn Chung, Hang-Seok Chang, Won Youl Seo, Ki Jun Song and Cheong Soo Park
Lateral neck node metastasis is an important prognostic factor in thyroid carcinoma. We developed a scoring system for use in prediction of lateral neck node metastasis from papillary thyroid cancer. This scoring system for lymph node metastasis in papillary thyroid cancer was developed on the base of the results from multivariate logistic regression analysis of preoperative clinical and radiologic data. When fine needle aspiration biopsy for suspicious lateral neck nodes is not possible, or the results are inadequate, our scoring system for prediction of lateral neck node metastasis can be helpful.
References
- 1.Harwood J, Clark OH, Dunphy JE. Significance of lymph node metastasis in differentiated thyroid cancer. Am J Surg. 1978;136:107–112. doi: 10.1016/0002-9610(78)90209-x. [DOI] [PubMed] [Google Scholar]
- 2.Hughes CJ, Shaha AR, Shah JP, Loree TR. Impact of lymph node metastasis in differentiated carcinoma of the thyroid: a matched-pair analysis. Head Neck. 1996;18:127–132. doi: 10.1002/(SICI)1097-0347(199603/04)18:2<127::AID-HED3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 3.King AD, Tse GM, Ahuja AT, Yuen EH, Vlantis AC, To EW, van Hasselt AC. Necrosis in metastatic neck nodes: diagnostic accuracy of CT, MR imaging, and US. Radiology. 2004;230:720–726. doi: 10.1148/radiol.2303030157. [DOI] [PubMed] [Google Scholar]
- 4.Sarvanan K, Bapuraj JR, Sharma SC, Radotra BD, Khandelwal N, Suri S. Computed tomography and ultrasonographic evaluation of metastatic cervical lymph nodes with surgicoclinicopathologic correlation. J Laryngol Otol. 2002;116:194–199. doi: 10.1258/0022215021910519. [DOI] [PubMed] [Google Scholar]
- 5.Jeong HS, Baek CH, Son YI, Choi JY, Kim HJ, Ko YH, Chung JH, Baek HJ. Integrated 18F-FDG PET/CT for the initial evaluation of cervical node level of patients with papillary thyroid carcinoma: comparison with ultrasound and contrast-enhanced CT. Clin Endocrinol (Oxf) 2006;65:402–407. doi: 10.1111/j.1365-2265.2006.02612.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee SK, Choi JH, Lim HI, Kim WW, Kim SM, Choe JH, Lee JE, Shin JH, Choi JY, Kim JH, Kim JS, Nam SJ, Yang JH. Sentinel lymph node biopsy in papillary thyroid cancer: comparison study of blue dye method and combined radioisotope and blue dye method in papillary thyroid cancer. Eur J Surg Oncol. 2009;35:974–979. doi: 10.1016/j.ejso.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Mirallié E, Sagan C, Hamy A, Paineau J, Kahn X, Le Néel JC, Auget JL, Murat A, Joubert M, Le Bodic MF, Visset J. Predictive factors for node involvement in papillary thyroid carcinoma. Univariate and multivariate analyses. Eur J Cancer. 1999;35:420–423. doi: 10.1016/s0959-8049(98)00399-2. [DOI] [PubMed] [Google Scholar]
- 8.Lim CY, Sohn EJ, Lee J, Yun JS, Nam KH, Chang HS, Chung WY, Park CS. The significant predicting factors influencing lateral neck node metastasis in papillary thyroid carcinoma. J Korean Surg Soc. 2006;71:326–330. [Google Scholar]
- 9.Mancuso AA, Harnsberg HR, Muraki AS, Stevens MA. Computed tomography of cervical and retropharyngeal lymph nodes: normal anatomy and, variants of normal, and application in stging head and neck cancer. Part II: pathology. Radiology. 1983;148:715–723. doi: 10.1148/radiology.148.3.6878692. [DOI] [PubMed] [Google Scholar]
- 10.Som PM, Brandwein M, Lidov M, Lawson W, Biller HF. The varied presentations of papillary thyroid carcinoma cervical nodal disease: CT and MR findings. AJNR Am J Neuroradiol. 1994;15:1123–1128. [PMC free article] [PubMed] [Google Scholar]
- 11.Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Kuma K, Miyauchi A. Ultrasonographically and anatomopathologically detectable node metastases in the lateral compartment as indicators of worse relapse-free survival in patients with papillary thyroid carcinoma. World J Surg. 2005;29:917–920. doi: 10.1007/s00268-005-7789-x. [DOI] [PubMed] [Google Scholar]
- 12.Kim EH, Park JS, Son KR, Kim JH, Jeon SJ, Na DG. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid. 2008;18:411–418. doi: 10.1089/thy.2007.0269. [DOI] [PubMed] [Google Scholar]
- 13.Kang TW, Shin JH, Han BK, Ko EY, Kang SS, Hahn SY, Kim JS, Oh YL. Preoperative ultrasound-guided tattooing localization of recurrences after thyroidectomy: safety and effectiveness. Ann Surg Oncol. 2009;16:1655–1659. doi: 10.1245/s10434-009-0431-7. [DOI] [PubMed] [Google Scholar]
- 14.Kim MJ, Kim EK, Kim BM, Kwak JY, Lee EJ, Park CS, Cheong WY, Nam KH. Thyroglobulin measurement in fine-needle aspirate washouts: the criteria for neck node dissection for patients with thyroid cancer. Clin Endocrinol (Oxf) 2009;70:145–151. doi: 10.1111/j.1365-2265.2008.03297.x. [DOI] [PubMed] [Google Scholar]