Abstract
Genomic instability is thought to be critical for the development of cancer. Among its causes microsatellite instability (MIN) and chromosomal instability (CIN) have attracted the most attention. Cell cycle checkpoints and DNA repair mechanisms are the first line of defense against DNA damage. Among the most dangerous DNA lesions are double-strand breaks. The response to DNA double strand breaks is regulated mainly by the serine/threonine kinases ATM and Chk2 and their downstream target the tumor suppressor p53, which in turn stimulates the expression of growth-inhibitory genes like CDKN1A (encoding p21) or proapoptotic genes like Bax. The balance between these gene products determines the fate of a cell. EAPP is a nuclear phosphoprotein that is frequently upregulated in human tumors. We have recently shown that EAPP levels are critical for cellular homeostasis. DNA damage elevates EAPP levels and its overexpression results in G1 arrest and impairs apoptosis in a p21-dependent manner. EAPP binds to the p21 promoter, stimulates its activity and seems to be essential for transcription initiation.
In the present work we show that EAPP also regulates the phosphorylation status and thus the activity of Chk2. EAPP binding seems to trigger the dephosphorylation of P-Chk2 resulting in its inactivation. A newly described function of Chk2 in mitosis that secures genomic integrity might also be affected by EAPP overexpression. This might explain the abundance of EAPP in aneuploid tumor cells.
Key words: DNA damage, checkpoint, EAPP, Chk2, p21, phosphorylation
Introduction
Initiation and progression of cancer is intimately connected with genetic instability caused by DNA damage or chromosome aberrations. The primary cellular response to DNA damage is to repair, but if the damage overwhelms the repair capacity, apoptosis is initiated instead. After DNA damage alarm has been raised, a number of repair mechanisms are switched on that recognize specific DNA lesions. The phosphatidylinositol-3-kinase like kinases ATM (Ataxia Telangiectasia, mutated) and ATR (ATM- and Rad3-Related) transduce damage-specific responses together with their respective downstream targets, the checkpoint kinases Chk2 and Chk1, in a hierarchical manner.1,2
The MRN (Mre11, Rad50, Nbs1) complex is a sensor of DNA double strand breaks (DSB) that rapidly induces ATMkinase activity after the occurrence of DSBs.3 One of the main targets of ATM is the tumor suppressor p53, which becomes phosphorylated directly by ATM on Ser15 4,5 and indirectly on Ser20 via Chk2,6,7 resulting in stabilization and accumulation of p53.8 p53 is a transcription factor that controls the expression of various growth-inhibitory and pro-apoptotic genes.9–11 DNA damage-induced G1 arrest depends on the expression of the cyclin-dependent kinase inhibitor p21, a target of p53.12,13 In addition to its activation by p53, p21 can be stimulated by oncogenic stress, via its Sp1 and nuclear receptor binding sites, and by E2F transcription factors. Since it is essential for the induction of cell cycle arrest and the onset of senescence it is considered a tumor suppressor.14,15 Nevertheless, p21 has rarely been found inactivated in human cancers, possibly due to its antiapoptotic properties (reviewed in ref. 15) and its activity as a negative regulator of p53.16 Moreover, cytoplasmic p21 might act as an oncogene17 and corresponds with a poor prognosis of breast cancer patients.18 In response to DNA damage, p21 mediates cyclin B1 degradation and is required for sustained G2 cell cycle arrest.19 Accumulating evidence suggests that besides its well-characterized function as a tumor suppressor, p21 also acts as a nodal point that integrates signals from a number of factors with context-dependent opposing functions in cancer. Among these factors are KLF4, TGFβ, E2F1, Ras, Notch and Runx (reviewed in ref. 20). We have previously identified EAPP (E2F-associated phosphoprotein) that stimulates E2F-dependent transcription and is found overexpressed in many human tumor cells.21 This overexpression might be caused by aberrant ratios of the transcription factors Sp1, Sp3 and Egr-1, the main regulators of EAPP expression.22
In a recently published study we could show that EAPP is influenced by DNA damage and is itself involved in the response to this damage.23 We briefly review and discuss the implications of our findings in this extra view, and present some new data on the role of EAPP in DNA damage response.
EAPP Influences Cell Cycle Progression and DNA Damage Response via p21
Recently we found that DNA doublestrand breaks result in elevated EAPP and this in turn stimulates p21 expression independently of p53. Reporter gene assays demonstrated that EAPP enhances p21 promoter activity and chromatin immunoprecipitation (ChIP) assays suggested binding of EAPP in the vicinity of the TATA box. Promoter stimulation occurred via two of the six Sp1 binding sites, possibly by direct DNA binding.24 Knockdown of EAPP caused a reduction of promoter bound components of the basal transcription machinery. This suggested that EAPP is required for the assembly of the preinitiation complex. On the other hand, its knockdown or overexpression did not alter the examined chromatin modification pattern.23 EAPP-driven elevated p21 protected cells from apoptosis whereas lower EAPP levels facilitated apoptosis. RNAi-induced knockdown of p21 diminished the anti-apoptotic effect of higher EAPP levels.23 Figure 1 shows that following DNA damage, the increase of both, p21 mRNA and p21, mirror the rise of EAPP in two different series; with the build-up of p21 itself being more pronounced. Why the addition of equal amounts of etoposide resulted in different dynamics of EAPP- and p21 induction in the two series is unclear, but might have to do with slight variations of the initial cell properties like cell density. This would be in accordance with our observation that the expression of both, p21 and EAPP is sensitive to and increases with cell density.23
Figure 1.
The increase of EAPP and p21 upon DNA double strand break induction shows a similar pattern. (A) U2OS cells were treated with 13 µM etoposide and harvested after indicated time points. Protein levels were determined by western analysis. Means and standard deviations of three independent experiments of two series are shown. (B and C) The corresponding p21 protein and mRNA levels were obtained by western [p21 (187)] analysis and RT-PCR. p21 mRNA levels of the high EAPP series were determined only once. The quantification was done with ImageQuant with Actin (AC-74) for western analysis and GAPDH for RT -PCR as references.
EAPP Affects the Phosphorylation Status of Chk2
We also observed a delay in the recovery from DNA damage in U2OS cells with lowered EAPP, indicating that besides p21 it affects other facets of checkpoint control in addition. Cell cycle reentry requires checkpoint termination and a network of proteins that inactivate the G2/M DNA damage checkpoint has been proposed.25 These proteins include Cdk1, Plk1, 53BP1 and Chk2, which are normally involved in mitotic phosphorylation events. In addition, the oncogenic phosphatase Wip1 (PPM1D) maintains cells competent for cell cycle reentry by antagonizing p53 and Chk2 26 and by dephosphorylating γH2AX, which serves as a platform for components of the checkpoint signaling cascade.27
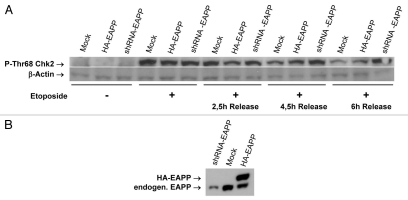
To find out whether EAPP influences other proteins involved in the DNA damage response we checked the expression and/or modification status of a number of these proteins in the presence of normal, lowered and elevated EAPP levels before and after DNA damage. Whereas the dynamics of p53 activation and deactivation following DNA damage and recovery did not seem to be affected by lower EAPP (not shown) this was clearly the case with Chk2. Figure 2 shows that the levels of P-Thr68-Chk2 were dramatically increased in all three, control cells, EAPP overexpressing and EAPP knockdown cells after treatment with etoposide, which induces DNA double strand breaks. Four and a half and even more pronounced six hours after the release from etoposide treatment, a clear difference in P-Chk2 levels became apparent. It remained high in EAPP knockdown cells but went down in control and EAPP overexpressing cells. This suggested that either phosphorylation of Chk2 was prolonged, or its dephosphorylation was impaired by reduced EAPP. To find out if EAPP also affects Chk2 phosphorylation in undamaged cells we reduced EAPP levels successively to very low levels. This resulted in the appearance of P-Thr68-Chk2 in a dose-dependent manner whereas Chk2 protein levels were not affected (Fig. 3A). Conversely, massive overexpression of EAPP in cells treated with etoposide entailed a reduction of P-Thr68-Chk2, again without influencing Chk2 protein levels (Fig. 3B). To ascertain that the Chk2 phosphorylation induced by EAPP knockdown is ATM dependent, we compared control cells and cells treated with the ATM inhibitor KU55933. Figure 4 shows that the knockdown of EAPP resulted again in the induction of P-Thr68-Chk2 and this was strongly reduced by the ATM inhibitor demonstrating that this phosphorylation is predominantly caused by ATM. In addition, Wip1 levels are also lowered in EAPP knockdown cells, indicating that the emergence of P-Chk2 is rather caused by reduced dephosphorylation than by increased phosphorylation.
Figure 2.
Lower EAPP levels prolong the phosphorylation of Chk2 on Thr68 during checkpoint recovery after release from etoposide. (A) U2OS with stably integrated knockdown and overexpression vectors for EAPP were treated for 16 h with 10 µM etoposide. Cells were washed to remove the drug and after the indicated time points cellular extracts were prepared and western analysis was done to determine the Chk2 phosphorylation state [p-Chk2 (Thr68)-R, Actin (AC-74)]. (B) Western analysis to show the levels of EAPP in the stable cell lines.
Figure 3.

EAPP knockdown can increase and overexpression can decrease the levels Chk2 phosphorylation on Thr68. (A) U2OS cells were transfected with increasing amounts of a shRNA-EAPP vector followed by western analysis to determine protein levels. (B) U2OS cells were transfected with an HA-EAPP expression vector and treated for 16 h with 10 µM etoposide. Harvested cells were analyzed by western blotting to obtain protein levels [p-Chk2 (Thr68)-R, Chk2 (A-11), Actin (AC-74)].
Figure 4.
Chk2 phosphorylation on Thr68 induced by EAPP knockdown depends on ATM activity. U2OS cells were transfected with either an EAPP knockdown and an EAPP overexpression vector. Untreated cells and cells treated for 16 h with 10 µM of the ATM inhibitor KU55933 were harvested and protein levels were determined by western analysis (p-Chk2 (Thr68)-R, Chk2 (A-11), Wip1 [H-300), Actin (AC-74)].
EAPP Specifically Interacts with Phosphorylated Chk2
Examination of the amino acid sequence of EAPP revealed the presence of a putative Chk2 binding site suggesting that the two proteins might interact. We have therefore used extracts from U2OS cells expressing HA-Chk2 for immunoprecipitation experiments. It turned out that HA-Chk2 selectively binds to endogenous EAPP after addition of etoposide. As this interaction could have also been induced by a damage-caused modification of EAPP we carried out pull down assays with bacterially produced GST-EAPP. The binding to GST-EAPP following DNA damage shows, that it is indeed the modification of Chk2 that is required for the interaction (Fig. 5A). Immunoprecipitation assays only with the endogenous proteins confirmed the interaction (Fig. 5B).
Figure 5.
EAPP binds preferentially to phosphorylated Chk2 (A) U2OS cells were transfected with HA-Chk236 followed by treatment with 10 µM etoposide for 16 h or mock treatment. 48 hours after transfection the cells were harvested and immunoprecipitation-(IP) and GST pulldown assays were carried out. For the IP 400 µg extract was incubated for 8 hours with an anti-EAPP antibody coupled to Protein A and subsequently analyzed by western with an anti-HA antibody (16B12). Preimmune serum of the EAPP antibody was used as control. For the GST pulldown assay 400 µg extract was incubated with either 0.5 µg GST-EAPP or 0.5 µg GST bound to glutathione agarose beads and again analyzed by western with an anti-HA antibody (16B12). For the input control 20 µg of each extract were taken. (B) To examine the interaction of only the endogenous proteins U2OS cells were treated as described above and 400 µg extracts were incubated with an anti-EAPP antibody coupled to Protein A and analyzed by western with an anti-Chk2 antibody.
Conclusions
Cellular checkpoints are signaling pathways evolved in eukaryotes to maintain genomic integrity. Checkpoint activation by genotoxins results in cell cycle arrest and repair of the damage, senescence or apoptosis. Malfunctioning checkpoints lead to an accumulation of genetic alterations which is directly associated with cancerogenesis. p21 and Chk2 are important components of DNA damage checkpoints connected by the tumor suppressor p53, which is an inducer of p21 and a substrate of Chk2. p21 induces arrest in G1 by inactivating G1-specific cyclin/Cdk complexes. Chk2 phosphorylates and inactivates Cdc25C, which is required for the activation of Cdk1 at the G2/M boundary, resulting in a G2 arrest. Phosphorylation of Chk2 at Thr68 by ATM is the first step in its activation.28,29 It is followed by homodimerization and trans-phosphorylation at Thr383 and Thr387 resulting in full activation.30
EAPP is an E2F-binding protein that stimulates E2F-dependent transcription and is frequently overexpressed in human tumor cells.21 We have shown recently that EAPP is not only essential for the survival of a cell but also an important regulator of p21 activity.23 We show here that EAPP also interacts with Chk2 and seems to interfere with its activity.
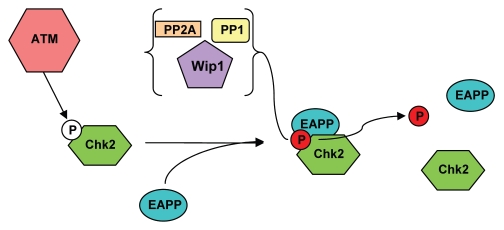
Figure 6 shows a model that could explain our observations. Following DNA damage, ATM phosphorylates Chk2 at Thr68, a step required for full activation of Chk2. Activated Chk2 phosphorylates substrates including Cdc25C, p53, E2F1 and PML, resulting in cell cycle arrest and DNA repair or apoptosis. EAPP also accumulates during DNA damage. It binds to phosphorylated Chk2 and the resulting dimeric or oligomeric complex recruits a phosphatase like Wip1, PP2A or PP1 that dephosphorylates Chk2 and this in turn could result in the disassembly of the oligomeric complex. The emergence of P-Chk2 in EAPP knockdown cells would thus be the consequence of inefficient dephosphorylation. The reduction of Wip1 (and possibly other phosphatases) levels would also contribute. In addition to the downregulation of p21, this activation of Chk2 may contribute to apoptosis observed in EAPP knockdown cells.23 Chk2 has been shown to phosphorylate E2F1 on Ser364, a modification that enhances E2F1 stability31 and seems to alter its promoter specificity32 resulting in the expression of pro-apoptotic genes like p73.33 According to this model EAPP would be required for the inactivation of Chk2 during checkpoint recovery and a certain level of EAPP would be crucial for the proper regulation of Chk2 activity. Since the accumulation of EAPP as a result of DNA damage occurs slower than Chk2 phosphorylation, premature Chk2 inactivation is avoided. Two different scenarios are conceivable: in the first one EAPP interacts only with the newly Thr68 phosphorylated, monomeric, Chk2, stimulating its dephosphorylation and thus preventing dimerization and full activation. In this case already active Chk2 would not be affected; only its replenishment would be inhibited. Inactivation of Chk2 during checkpoint recovery could be achieved by Plk1 through phosphorylation of the FHA domain of Chk2.25 In the second scenario EAPP interacts with both monomeric and dimeric Chk2, stimulating their dephosphorylation and thus contributing to their inactivation.
Figure 6.
Proposed model for the interaction of EAPP and phosphorylated Chk2. Without DNA damage a fraction of Chk2 becomes phosphorylated by the existing small amounts of active ATM. This active Chk2 is important for maintaining genomic integrity. EAPP binds to phosphorylated Chk2 and the resulting complex recruits the phosphatases PP2A, PP1 and Wip1, which constantly remove the phosphate moiety. Reduced EAPP levels prevent the removal of the phosphorylation and hence lead to an accumulation of the modified Chk2. EAPP overexpression triggers a faster displacement of the phosphate group by the phosphatases.
A DNA damage independent function of Chk2 during mitosis has been described recently. Chk2 mediated phosphorylation of Ser988 on BRCA1 and this is required for correct formation of the mitotic spindle.34 The mitotic activity Chk2 was similar to DNA damage induced activity. This is somewhat surprising since its activator ATM seems to be only modestly more active in mitosis than in interphase. We have shown previously that EAPP levels go down during mitosis.21 Our model predicts that EAPP is required for Chk2 dephosphorylation. If it is correct, it could explain high Chk2 activity in mitosis. Reduced dephosphorylation resulting from lower EAPP and steady phosphorylation of Chk2 would lead to more active P-Chk2. Its abrogation has been shown to induce chromosomal instability (CIN) leading to gain or loss of whole chromosomes.34 The resulting aneuploidy is a hallmark of cancer and contributes to tumorigenesis and tumor progression.35 This might be one of the reasons why so many tumors have elevated EAPP levels. The concomitant reduction of active Chk2 favors aneuploidy possibly followed by cancer.
Acknowledgments
We are grateful to David Stern for Chk2 expression vectors. This work was supported by the Austrian FWF (Grant P18417) and by the Herzfelder'sche Familienstiftung. Peter Andorfer was supported by a fellowship of the University of Vienna.
References
- 1.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavin MF, Kozlov S. ATM activation and DNA damage response. Cell Cycle. 2007;6:931–942. doi: 10.4161/cc.6.8.4180. [DOI] [PubMed] [Google Scholar]
- 3.Stracker TH, Petrini JH. The MRE11 complex: Starting from the ends. Nat Rev Mol Cell Biol. 12:90–103. doi: 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 5.Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 6.Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, et al. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- 7.Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- 8.Khanna KK, Keating KE, Kozlov S, Scott S, Gatei M, Hobson K, et al. ATM associates with and phosphorylates p53: mapping the region of interaction. Nat Genet. 1998;20:398–400. doi: 10.1038/3882. [DOI] [PubMed] [Google Scholar]
- 9.Rozan LM, El-Deiry WS. p53 downstream target genes and tumor suppression: A classical view in evolution. Cell Death Differ. 2007;14:3–9. doi: 10.1038/sj.cdd.4402058. [DOI] [PubMed] [Google Scholar]
- 10.Das S, Boswell SA, Aaronson SA, Lee SW. p53 promoter selection: Choosing between life and death. Cell Cycle. 2008;7:154–157. doi: 10.4161/cc.7.2.5236. [DOI] [PubMed] [Google Scholar]
- 11.Efeyan A, Serrano M. p53: Guardian of the genome and policeman of the oncogenes. Cell Cycle. 2007;6:1006–1010. doi: 10.4161/cc.6.9.4211. [DOI] [PubMed] [Google Scholar]
- 12.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 13.Gu Y, Turck CW, Morgan DO. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993;366:707–710. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- 14.Jung YS, Qian Y, Chen X. Examination of the expanding pathways for the regulation of p21 expression and activity. Cell Signal. 22:1003–1012. doi: 10.1016/j.cellsig.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbas T, Dutta A. p21 in cancer: Intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broude EV, Demidenko ZN, Vivo C, Swift ME, Davis BM, Blagosklonny MV, et al. p21 (CDKN1A) is a negative regulator of p53 stability. Cell Cycle. 2007;6:1468–1471. [PubMed] [Google Scholar]
- 17.Blagosklonny MV. Are p27 and p21 cytoplasmic oncoproteins? Cell Cycle. 2002;1:391–393. doi: 10.4161/cc.1.6.262. [DOI] [PubMed] [Google Scholar]
- 18.Winters ZE, Leek RD, Bradburn MJ, Norbury CJ, Harris AL. Cytoplasmic p21WAF1/CIP1 expression is correlated with HER-2/neu in breast cancer and is an independent predictor of prognosis. Breast Cancer Res. 2003;5:242–249. doi: 10.1186/bcr654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillis LD, Leidal AM, Hill R, Lee PW. p21Cip1/WAF1 mediates cyclin B1 degradation in response to DNA damage. Cell Cycle. 2009;8:253–256. doi: 10.4161/cc.8.2.7550. [DOI] [PubMed] [Google Scholar]
- 20.Rowland BD, Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer. 2006;6:11–23. doi: 10.1038/nrc1780. [DOI] [PubMed] [Google Scholar]
- 21.Novy M, Pohn R, Andorfer P, Novy-Weiland T, Galos B, Schwarzmayr L, et al. EAPP, a Novel E2F binding protein that modulates E2F-dependent transcription. Mol Biol Cell. 2005;16:2181–2190. doi: 10.1091/mbc.E04-11-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarzmayr L, Andorfer P, Novy M, Rotheneder H. Regulation of the E2F-associated phosphoprotein promoter by GC-box binding proteins. Int J Biochem Cell Biol. 2008;40:2845–2853. doi: 10.1016/j.biocel.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Andorfer P, Rotheneder H. EAPP: Gatekeeper at the crossroad of apoptosis and p21-mediated cell cycle arrest. Oncogene. 2011;9:62. doi: 10.1038/onc.2010.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen K, Ou XM, Wu JB, Shih JC. Transcription factor E2F-associated phosphoprotein (EAPP), RAM2/CDCA7L/JPO2 (R1) and simian virus 40 promoter factor 1 (Sp1) cooperatively regulate glucocorticoid activation of monoamine oxidase B. Molecular pharmacology. 79:308–317. doi: 10.1124/mol.110.067439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Vugt MA, Gardino AK, Linding R, Ostheimer GJ, Reinhardt HC, Ong SE, et al. A mitotic phosphorylation feedback network connects Cdk1, Plk1, 53BP1 and Chk2 to inactivate the G(2)/M DNA damage checkpoint. PLoS Biol. 8:1000287. doi: 10.1371/journal.pbio.1000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindqvist A, de Bruijn M, Macurek L, Bras A, Mensinga A, Bruinsma W, et al. Wip1 confers G2 checkpoint recovery competence by counteracting p53-dependent transcriptional repression. EMBO J. 2009;28:3196–3206. doi: 10.1038/emboj.2009.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macurek L, Lindqvist A, Voets O, Kool J, Vos HR, Medema RH. Wip1 phosphatase is associated with chromatin and dephosphorylates gammaH2AX to promote checkpoint inhibition. Oncogene. 29:2281–2291. doi: 10.1038/onc.2009.501. [DOI] [PubMed] [Google Scholar]
- 28.Ahn JY, Schwarz JK, Piwnica-Worms H, Canman CE. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 2000;60:5934–5936. [PubMed] [Google Scholar]
- 29.Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci USA. 2000;97:10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CH, Chung JH. The hCds1 (Chk2)-FHA domain is essential for a chain of phosphorylation events on hCds1 that is induced by ionizing radiation. J Biol Chem. 2001;276:30537–30541. doi: 10.1074/jbc.M104414200. [DOI] [PubMed] [Google Scholar]
- 31.Stevens C, Smith L, La Thangue NB. Chk2 activates E2F-1 in response to DNA damage. Nat Cell Biol. 2003;5:401–409. doi: 10.1038/ncb974. [DOI] [PubMed] [Google Scholar]
- 32.Pediconi N, Ianari A, Costanzo A, Belloni L, Gallo R, Cimino L, et al. Differential regulation of E2F1 apoptotic target genes in response to DNA damage. Nat Cell Biol. 2003;5:552–558. doi: 10.1038/ncb998. [DOI] [PubMed] [Google Scholar]
- 33.Stiewe T, Putzer BM. Role of the p53-homologue p73 in E2F1-induced apoptosis. Nat Genet. 2000;26:464–469. doi: 10.1038/82617. [DOI] [PubMed] [Google Scholar]
- 34.Stolz A, Ertych N, Kienitz A, Vogel C, Schneider V, Fritz B, et al. The CHK2-BRCA1 tumor suppressor pathway ensures chromosomal stability in human somatic cells. Nat Cell Biol. 12:492–499. doi: 10.1038/ncb2051. [DOI] [PubMed] [Google Scholar]
- 35.Holland AJ, Cleveland DW. Boveri revisited: Chromosomal instability, aneuploidy and tumorigenesis. Nat Rev Mol Cell Biol. 2009;10:478–487. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X, Tsvetkov LM, Stern DF. Chk2 activation and phosphorylation-dependent oligomerization. Mol Cell Biol. 2002;22:4419–4432. doi: 10.1128/MCB.22.12.4419-4432.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]