Abstract
Taccalonolide A is a microtubule stabilizer that has cellular effects almost identical to paclitaxel. However, biochemical studies show that, unlike paclitaxel, taccalonolide A does not enhance purified tubulin polymerization or bind tubulin/microtubules. Mechanistic studies aimed at understanding the nature of the differences between taccalonolide A and paclitaxel were conducted. Our results show that taccalonolide A causes bundling of interphase microtubules at concentrations that cause antiproliferative effects. In contrast, the concentration of paclitaxel that initiates microtubule bundling is 31-fold higher than its IC50. Taccalonolide A's effects are further differentiated from paclitaxel in that it is unable to enhance the polymerization of tubulin in cellular extracts. This finding extends previous biochemical results with purified brain tubulin to demonstrate that taccalonolide A requires more than tubulin and a full complement of cytosolic proteins to cause microtubule stabilization. Reversibility studies were conducted and show that the cellular effects of taccalonolide A persist after drug washout. In contrast, other microtubule stabilizers, including paclitaxel and laulimalide, demonstrate a much higher degree of cellular reversibility in both short-term proliferation and long-term clonogenic assays. The propensity of taccalonolide A to alter interphase microtubules at antiproliferative concentrations as well as its high degree of cellular persistence may explain why taccalonolide A is more potent in vivo than would be expected from cellular studies. The close linkage between the microtubule bundling and antiproliferative effects of taccalonolide A is of interest given the recent hypothesis that the effects of microtubule targeting agents on interphase microtubules might play a prominent role in their clinical anticancer efficacy.
Key words: taccalonolide, paclitaxel, microtubule stabilizer, microtubule targeted agent, tubulin, microtubule, laulimalide, antimitotic agent, drug persistence
Introduction
Several microtubule targeting agents have excellent utility in the treatment of cancer. These drugs are classified as microtubule stabilizers or destabilizers based on their effects on interphase microtubules at relatively high concentrations. Microtubule stabilizers, including the taxanes and laulimalide, stimulate the formation of intracellular microtubule polymer, resulting in an increased density of cellular microtubules. In contrast, microtubule destabilizers, including the vinca alkaloids, inhibit microtubule polymerization, resulting in a loss of cellular microtubules. At lower concentrations, both classes of drugs inhibit microtubule dynamics and cause mitotic arrest.1In spite of the clinical successes of the taxanes paclitaxel (Taxol) and docetaxel (Taxotere), acquired and innate drug resistance and dose-limiting toxicities prompted the development of new classes of microtubule stabilizing drugs.2,3 The epothilone ixabepilone (Ixempra™) and a new taxane cabazitaxel (Jevtana™), were recently approved for clinical use in the US and several other microtubule stabilizers are in preclinical and clinical development.4,5
Two binding sites for microtubule stabilizers have been identified: the taxane site and the laulimalide/peloruside site. The taxanes, epothilones, discodermolide and dictyostatin bind to β-tubulin within the taxane site, which is located in the interior lumen of the microtubule.6,7 Occupation of this site alters the conformation of tubulin within the intact microtubule so that it resembles the more stable GTP bound form.8 This con-formational change decreases microtubule dynamics and causes stabilization of microtubules formed from purified tubulin or in intact cells. The laulimalide/peloruside binding site was recently mapped to the β subunit of tubulin on the exterior of the microtubule.9 Although the taxane and laulimalide binding sites are completely non-overlapping and exist on different surfaces of the microtubule, drug occupation at either site causes a structurally identical state of microtubule stability.9
The taccalonolides are a new class of microtubule stabilizers that are isolated from the tropical plant, Tacca chantrieri. The taccalonolides A and E, cause an increase in cellular microtubule density, microtubule bundling and the formation of multiple aberrant mitotic spindles that lead to mitotic arrest.10 While these effects are similar to all other microtubule stabilizers, biochemical studies show that taccalonolides A and E do not bind directly to purified tubulin/microtubules and do not promote the polymerization of purified bovine brain tubulin, even at super stoichiometric concentrations.11 Taccalonolides A and E are therefore the first microtubule stabilizers identified that do not bind directly to tubulin. Likely due to this unique property, taccalonolides A and E overcome drug resistance mediated by the expression of β-III tubulin.12
Taccalonolide A also differs from other microtubule stabilizers in that it is substantially less potent in vitro. The IC50 of taccalonolide A is 594 nM in HeLa cells.12 In comparison, paclitaxel, docetaxel and epothilone B are much more potent, with IC50 values of 1.6 nM, 0.6 nM and 0.5 nM, respectively.12 In murine in vivo models, however, taccalonolide A is more potent than paclitaxel, with a maximum tolerated total dose of 45–50 mg/kg, which is half of the maximum tolerated dose of paclitaxel.12 In addition, taccalonolide A provides superior antitumor efficacy when compared to paclitaxel or doxorubicin in a multidrug resistant breast tumor model, which is likely due in part to the ability of taccalonolide A to overcome P-glycoprotein-mediated drug resistance.12 The nature of the differences between the in vitro and in vivo potencies of the taccalonolides is not yet known.
The goal of these studies was to begin to decipher the mechanistic differences between the taccalonolides and other microtubule stabilizers, most notably paclitaxel. We show three mechanistic differences between taccalonolide A and paclitaxel. First, the antiproliferative and interphase microtubule stabilization effects of taccalonolide A occur at similar concentrations, while concentrations of paclitaxel substantially higher than its IC50 are required to observe interphase microtubule bundling. Additionally, unlike paclitaxel, taccalonolide A is unable to polymerize tubulin in cellular lysates. Finally, the cellular effects of taccalonolide A persist even after a short incubation with the drug, while paclitaxel's effects are reversible. These findings demonstrate a plausible rationale for the discrepancies between the biochemical, cellular and in vivo activities of taccalonolide A, including possible explanations for the differences between its in vivo and in vitro potencies.
Results
Paclitaxel and taccalonolide A cause interphase microtubule bundling at similar concentrations.
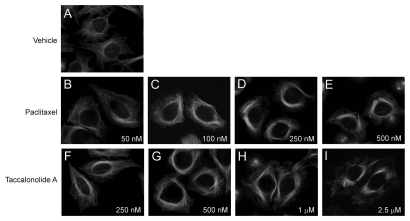
Microtubule stabilizers are well known for their ability to increase the density of interphase microtubules and to cause the formation of thick microtubule bundles in treated cells. The effects of paclitaxel and taccalonolide A on interphase microtubules were studied in HeLa cells and compared to the interphase microtubule network observed in vehicle treated cells (Fig. 1A). The first appearance of interphase microtubule bundles was observed with 50 nM paclitaxel (Fig. 1B) and the extent of bundling increased slightly at 100 nM (Fig. 1C). A concentration of 250 nM paclitaxel caused the formation of extensive microtubule bundles (Fig. 1D) and with 500 nM paclitaxel the majority of microtubules formed long thick bundles (Fig. 1E). The microtubule bundles in paclitaxeltreated cells are long, surround the nucleus and appear to emanate from the central region, possibly from the microtubule organizing center.
Figure 1.
Effects of paclitaxel or taccalonolide A on interphase microtubules in HeLa cells. Cells were incubated with vehicle (top row) or the indicated concentrations of paclitaxel (middle row) or taccalonolide A (bottom row) for 18 h. Microtubules were visualized by indirect immunofluorescence of β-tubulin.
The concentration dependent effects of taccalonolide A on interphase microtubules were also evaluated. Taccalonolide A begins to cause interphase microtubule bundles at 250 nM (Fig. 1F) and a noticeable accumulation of microtubule bundles around the nucleus was observed with 500 nM taccalonolide A (Fig. 1G). The formation of extensive short, thick microtubule bundles was evident in cells treated with 1 µM taccalonolide A (Fig. 1H) and the number and thickness of the bundles increased with 2.5 µM taccalonolide A, where the vast majority of interphase microtubules were found in tightly bundled structures (Fig. 1I). Consistent with the appearance of microtubules in paclitaxel-treated cells, the interphase microtubule bundles in taccalonolide A-treated cells are denser around the nucleus. However, unlike paclitaxel, taccalonolide A also causes the microtubules at the cell periphery to appear bundled with a short, compact, tuft-like appearance. These phenotypic effects of taccalonolide A and paclitaxel on microtubule bundling are similar to the effects observed previously in A-10 cells.10
The images in Figure 1 show that the effects of taccalonolide A and paclitaxel on interphase microtubules are similar, but not identical, suggesting subtle mechanistic differences between these stabilizers. What is striking, however, is the relative difference in the concentrations of these agents required to initiate microtubule bundling; a 5-fold difference in bundling propensity between taccalonolide A and paclitaxel was observed as compared to the 360-fold difference in IC50 values for inhibition of proliferation of these agents in HeLa cells (Fig. 1).12 The initiation of interphase microtubule effects is observed with 250 nM taccalonolide A, which is less than its IC50 value of 594 nM in this same cell line. In comparison, the first noticeable effects of paclitaxel on microtubule density in HeLa cells were observed at 50 nM, a concentration 31-fold greater than its IC50 value of 1.6 nM. These findings demonstrate that taccalonolide A causes significant alterations in interphase microtubule structures at antiproliferative concentrations, whereas paclitaxel-initiated microtubule bundling requires concentrations significantly higher than its IC50.
Taccalonolide A induced microtubule stabilization requires an intact cell.
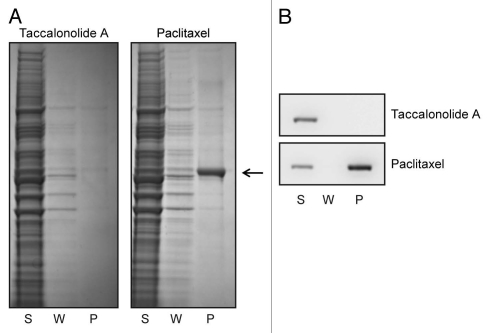
Although taccalonolide A readily causes interphase microtubule bundling at nanomolar concentrations (Fig. 1), biochemical studies with purified bovine brain tubulin showed that taccalonolide A does not promote the assembly of tubulin in the presence or absence of microtubule associated proteins.11 We conducted further studies to explore the similarities and differences between taccalonolide A and paclitaxel's effects on microtubules using whole-cell lysates. A well documented effect of paclitaxel is its ability to enhance the formation of cold-stable microtubules from soluble tubulin.13 The ability of taccalonolide A to form cold-stable microtubules from tubulin in cellular lysates was evaluated. Whole-cell lysates were collected and then chilled to depolymerize all pre-existing microtubules into soluble tubulin heterodimers. Paclitaxel or taccalonolide A (each at 20 µM) was added to the cell lysates and warmed to 37°C in the presence of GTP to stimulate microtubule polymerization. The ability of taccalonolide A and paclitaxel to support the formation of cold-stable microtubules was evaluated by then re-chilling the lysates and separating intact microtubules from soluble tubulin by centrifugation. The supernatant and pellet fractions were separated by SDS-PAGE and tubulin detected by total protein staining (Fig. 2A) or western blot using a β-tubulin antibody (Fig. 2B). When paclitaxel was present, cold stable microtubules were formed as indicated by the appearance of tubulin in the pellet (P) fraction (Fig. 2A, arrow and B). However, no tubulin was found in the pellet (P) fraction of lysates treated with taccalonolide A, indicating that taccalonolide A was unable to promote the formation of cold-stable microtubules. The lack of tubulin in the pellet after taccalonolide A treatment confirms that the chilling process used in this assay was sufficient to depolymerize all preexisting cellular microtubules and that any tubulin found in the pellet was a result of de novo microtubule polymerization in the lysates. These data show that unlike paclitaxel, taccalonolide A cannot support the formation of cold-stable microtubules from whole-cell lysates.
Figure 2.
Taccalonolide A is unable to form cold stable microtubules in cell lysates. HeLa cell lysates were collected, chilled to depolymerize microtubules and then treated with 20 µM paclitaxel or 20 µM taccalonolide A for 5 min at 37°C to reform microtubules. Lysates were then re-chilled to evaluate the ability of the stabilizers to initiate the formation of cold-stable microtubules. Microtubule polymer was pelleted by centrifugation and soluble tubulin heterodimers remained in the supernatant. The total protein (A) and β-tubulin (B) levels present in the supernatant (S), wash (W) and pellet (P) fractions were evaluated by total protein staining or β-tubulin immunoblotting, respectively. The location of tubulin in the total protein stained gel is indicated with an arrow.
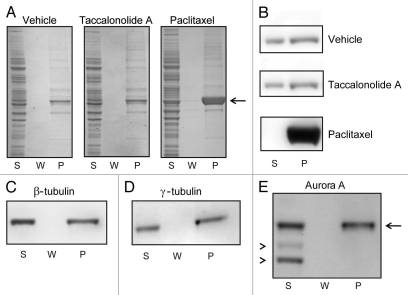
The ability of taccalonolide A to enhance the formation of microtubule polymers in cell lysates at 37°C was also evaluated using the assay system described above. Cell lysates were collected, microtubules depolymerized by chilling and then either vehicle, 20 µM taccalonolide A or 20 µM paclitaxel was added and incubated at 37°C to stimulate microtubule polymerization. In contrast to the previous experiment, lysates were not re-chilled after microtubule polymerization to allow detection of microtubules formed during the incubation period regardless of their cold stability. Microtubule polymers were formed even in the absence of any drug as is indicated by tubulin in the pellet (P) after treatment with vehicle (Fig. 3A and B). However, no additional tubulin was incorporated into microtubules in the taccalonolide A-treated lysates (Fig. 3A and B). In contrast, paclitaxel caused a significant increase in microtubule polymer, resulting in a complete shift of soluble tubulin into the polymerized form (Fig. 3A and B). To take into account the 5-fold higher concentration of taccalonolide A required to cause interphase microtubule bundling in intact HeLa cells as compared to paclitaxel, we repeated the experiment in the presence of 100 µM taccalonolide A. Treatment of lysates with 100 µM taccalonolide A did not increase the amount of β-tubulin found in the pellet fraction (P) (Fig. 3C) as compared to vehicle-treated controls (Fig. 3B).
Figure 3.
Effects of paclitaxel or taccalonolide A on tubulin polymer formation in cytosolic extracts. HeLa cell lysates were collected, chilled to depolymerize pre-existing microtubules and then incubated with vehicle, 20 µM paclitaxel or 20 µM taccalonolide A for 30 min at 37°C. Microtubule polymer was separated from soluble tubulin by centrifugation at room temperature. The total protein (A) and β-tubulin (B) levels present in the supernatant (S), wash (W) and pellet (P) were determined by total protein staining or β-tubulin immunoblotting, respectively. The location of tubulin in the total protein stained gel is indicated with an arrow (3A). β-tubulin (C) and the microtubule associated proteins γ-tubulin (D) and Aurora A (E, arrow), were detected in the microtubule containing pellet (P) of samples treated with 100 µM taccalonolide A as compared to non-specific background bands, which were retained in the supernatant (E, arrowheads).
The supernatant (S) and pellet (P) fractions of taccalonolide A-treated lysates were subjected to immunoblotting to analyze the composition of the microtubules formed in this assay. In addition to β-tubulin, the microtubule associated proteins γ-tubulin and Aurora A were also found in the microtubule pellet (P) (Fig. 3D and E). This finding demonstrates that the microtubules formed in this assay contain microtubule associated proteins, suggesting that these microtubules have a more physiological composition than those formed with only purified tubulin. The enrichment of microtubule associated proteins associated with these polymerized microtubules was noted by an absence of non-specific proteins in the pellet fraction through detection of total protein (Fig. 3A) or the background bands from Aurora A immunoblotting (Fig. 3E, arrowheads). These data show that, although microtubules containing microtubule associated proteins are able to be formed in cell lysates treated with taccalonolide A, the extent of microtubule polymerization in these extracts is not enhanced above levels that occur in vehicle-treated lysates. Thus, in contrast to intact HeLa cells, taccalonolide A is not able to enhance polymerization of tubulin in biochemical extracts even in the presence of a full complement of cytosolic proteins from these same cells, expanding on previous reports that the biochemical and cellular effects of taccalonolide A are not equivalent.
The cellular effects of taccalonolide A are highly persistent.
In addition to the finding that taccalonolide A causes dramatic microtubule bundling in intact cells despite its inability to enhance the polymerization of tubulin in cellular extracts, taccalonolide A also surprisingly shows much greater in vivo activity than would be expected from its potency in cellular assays. One possibility is that taccalonolide A binds very tightly to its target and/or rapidly sets in motion downstream events that have a low degree of reversibility. To test the persistence of taccalonolide A's cellular effects, we evaluated its effects on cell cycle distribution, cell proliferation and clonogenicity following short-term drug exposure.
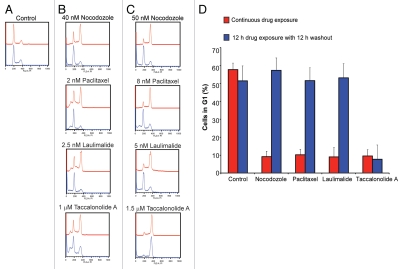
Microtubule disrupting agents are also known as antimitotics because they initiate mitotic arrest caused by multiple mitotic spindle defects. The propensity of these drugs to interrupt mitotic progression and cause a shift from the G1 population to the G2/M population is readily measured by flow cytometry, which was used to evaluate the cellular persistence of the effects of microtubule disrupting agents. Cells were incubated with the microtubule disrupting compounds for 12 h followed by removal of drug from the media for an additional 12 h. In the absence of drug, the majority of HeLa cells are in the G1 phase of the cell cycle, with approximately 20% in S phase and 20% in G2/M (Fig. 4A, red trace). Treatment of the cells with microtubule targeted agents, including the microtubule destabilizer nocodazole or the microtubule stabilizers paclitaxel, laulimalide or taccalonolide A for 12 h, caused the G1 population of cells to decrease with a concomitant increase in the G2/M population (Fig. 4B and C, red traces). This shift from G1 to a G2/M is dose dependent; higher concentrations of any microtubule disrupting agent cause a higher proportion of cells to accumulate in G2/M, which allowed identification of concentrations of each drug that caused an intermediate phenotype where the G1 and G2/M populations are approximately equal. In HeLa cells these concentrations are 40 nM nocodazole, 2 nM paclitaxel, 2.5 nM laulimalide or 1 µM taccalonolide A (Fig. 4B, red traces). Higher concentrations that cause an almost complete shift from the G1 to the G2/M population were 50 nM nocodazole, 8 nM paclitaxel, 5 nM laulimalide or 1.5 µM taccalonolide A (Fig. 4C, red traces). At these higher concentrations, the G1 population decreased from 57% to approximately 10% for all drugs (Fig. 4D).
Figure 4.
Reversibility of the cell cycle block induced by microtubule disrupting agents. Cell cycle profile of HeLa cells 12 h after drug addition (upper, red tracing in each part) or after an additional 12 h of drug washout (lower, blue tracing in each part). (A) Normal cell cycle distribution of HeLa cells. (B) Concentrations of each drug that caused significant but incomplete G2/M arrest. (C) Concentrations of each drug that caused G2/M accumulation of the majority of cells. (D) Quantitation of the percentage of cells from (C) that were in G1 after 12 h drug exposure or subsequent washout depicted in red and blue, respectively.
To determine the reversibility of the G2/M block caused by these agents, cell cycle analysis was performed 12 h after the drug was removed from the media. Measuring the change in G1 population gave the clearest indication of the cell cycle dependent effects of these drugs, as full G2/M accumulation requires longer periods of drug treatment. Cells that were incubated with either concentration of nocodazole, paclitaxel or laulimalide showed an almost complete recovery of the G1 population of cells when the drug was washed out of the media (Fig. 4B and C, blue traces). This is shown by a complete recovery of the G1 population to control levels after drug washout for all three compounds (Fig. 4D). However, cells treated with taccalonolide A were unable to fully recover the G1 population of cells after washout. Although the G1 population recovers slightly after 1 µM taccalonolide A is washed out, cells are unable to completely overcome this mitotic blockade after drug washout (Fig. 4B, blue trace). The G2/M arrest observed with 1.5 µM taccalonolide A is completely persistent (Fig. 4C, blue trace), with the G1 population remaining at 10% even after drug washout (Fig. 4D).
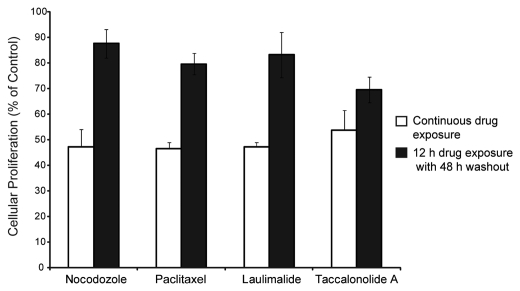
The persistence of taccalonolide A's effects on cell proliferation was monitored using the SRB assay. Dose response curves were generated for each drug to determine the concentration that causes a 50% decrease in cell proliferation during a continuous, 60 h drug exposure (Fig. 5, white bars). These concentrations were determined to be 30 nM for nocodazole, 1.5 nM for paclitaxel, 1 nM for laulimalide and 350 nM for taccalonolide A. The persistence of these drugs was determined by measuring the effects on cellular proliferation when the drug was removed following 12 h of drug treatment and the cells allowed to recover and grow for an additional 48 h. Nocodazole, paclitaxel and laulimalide-treated cells were able to recover 80–90% proliferative capacity upon drug washout (Fig. 5, black bars). However, taccalonolide A treated cells were more sensitive to this 12 h drug treatment, recovering to only 70% proliferative capacity after drug washout (Fig. 5, black bars). These data further suggest that the antiproliferative effects of taccalonolide A are more persistent and less reversible than the other microtubule disrupting agents evaluated.
Figure 5.
Antiproliferative effects of continuous or 12 h drug exposure. The concentration of drug required to observe a 50% inhibition in cellular proliferation following continuous 60 h incubation was determined (white bars). The antiproliferative effects of these same concentrations were evaluated after a 12 h exposure followed by drug washout for an additional 48 h (black bars).
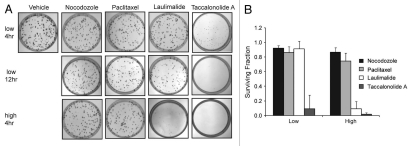
The clonogenic assay was employed to evaluate the reversibility of short-term (4 or 12 h) drug treatment, on long-term (10 day) cell viability. Clonogenic viability was determined after treatment of HeLa cells with the antiproliferative or the G2/M accumulation concentrations of each drug as identified in Figures 5 and 4, respectively. Nocodazole was used as a positive control of a rapidly reversible microtubule disrupting agent. A 4 h exposure with 30 nM nocodazole caused no effect on long term clonogenic cell survival and was essentially identical to vehicle treated controls (Fig. 6A and top row). Quantification of these effects from three experiments showed that a 4 h incubation with this concentration of nocodazole caused an 8% decrease in the fraction of surviving colony forming cells (Fig. 6B). When cells were treated with 1.5 nM paclitaxel or 1 nM laulimalide for 4 h, the majority of single cells were able to form viable colonies after drug washout (Fig. 6A, top row). The survival fraction was 86% for paclitaxel-treated cells and 91% for laulimalide (Fig. 6B). In dramatic contrast, a 4 hr treatment of cells with 350 nM taccalonolide A greatly diminished their ability to form colonies (Fig. 6A, top row) and the fraction of surviving cells was only 9%. A longer, 12 h, incubation before drug washout caused slight loss of clone viability in the paclitaxel and laulimalide treated cultures, but essentially eliminated all colonies in the taccalonolide A treated plates (Fig. 6A, middle row).
Figure 6.
Effects of 4 and 12 h drug exposure on clonogenic cell viability. (A) HeLa cells were treated with vehicle or concentrations of drug that inhibited cellular proliferation by 50% (low) or that caused G2/M arrest (high) for 4 or 12 h before media was replaced. Colony formation was determined after an additional 10 days of cell growth. (B) the surviving fraction of colony forming cells after a 4 h drug treatment and subsequent washout as compared to vehicle treated controls.
When cells were treated for 4 h with slightly higher concentrations of nocodazole and paclitaxel that caused maximal G2/M accumulation (Fig. 4C), they retained the ability to form colonies with surviving fractions of 86 and 74%, respectively (Fig. 6A, bottom row and B). In contrast, taccalonolide A treated cells had a very poor colony formation efficiency of 2% when treated with this concentration for 4 h (Fig. 6A, bottom row and B). Compared to 1 nM laulimalide, which had minimal effects on colony formation after 4 or 12 h treatment, a 4 hr exposure to 5 nM laulimalide greatly decreased the colony formation efficiency to 9% of control (Fig. 6A, bottom row and B). The clonogenic potential of cells treated for 4 h with both the antiproliferative and G2M concentrations of each drug are quantified in Figure 6B. These data demonstrate that the cellular effects of taccalonolide A are more persistent and less reversible than other classes of microtubule targeting agents when the drugs are added at the same relative concentrations. Additionally, these data show that laulimalide is intermediate between taccalonolide A and paclitaxel with regard to its reversibility. These results confirm previous reports showing that paclitaxel treatment is reversible and adds to the growing body of evidence that the taccalonolides are mechanistically distinct from other classes of microtubule stabilizing agents.
Discussion
Paclitaxel is a potent antimitotic agent with IC50 values in the low nanomolar range in a variety of cancer cell lines. At these concentrations, paclitaxel does not affect interphase microtubules and is instead thought to cause its antiproliferative effects by inhibiting microtubule dynamics, resulting in mitotic arrest and culminating in apoptotic cell death. In contrast, the concentration of paclitaxel required to cause significant interphase microtubule bundling is 31-fold greater than the IC50, making it unlikely that these gross effects on interphase microtubule structures are related to their antiproliferative effects in vitro. The taccalonolides have IC50 values in these same cell lines that are 100–500-fold higher than paclitaxel.10,12 However, changes in interphase microtubules are apparent at antiproliferative concentrations of taccalonolide A (Fig. 1), raising the possibility that these changes may be involved in the mechanism of taccalonolide-induced cell death in vitro. This finding is of interest in light of accumulating evidence that microtubule targeted agents may be effective anticancer agents in the clinic because of their ability to disrupt the diverse functions of interphase and mitotic microtubules as opposed to only their antimitotic effects.14 It is interesting to speculate that one of the reasons why taccalonolide A is so much more potent in vivo than would be anticipated from cellular studies is that its effects on interphase microtubules play an important role in its in vivo antitumor activity.
The large discrepancy between the concentrations of taccalonolide A and paclitaxel that cause interphase microtubule changes and antiproliferative effects supports the hypothesis that these two drugs have similar, but mechanistically distinct mechanisms of action. The differential potencies of taccalonolide A and paclitaxel have been observed in a wide variety of biochemical, cellular and in vivo studies. In spite of the fact that taccalonolide A causes microtubule bundling in interphase cells at concentrations only 5-fold higher than paclitaxel (Fig. 1), this propensity to cause cellular microtubule bundling does not extend to biochemical studies where taccalonolide A is unable to enhance microtubule polymerization even in the presence of a full complement of cytosolic proteins (Figs. 2 and 3). Additionally, previous reports have found that taccalonolide A is 2-fold more potent than paclitaxel in a murine model.12 These data clearly demonstrate that the relationship between these two drugs is more complicated than would be expected if taccalonolide A was simply binding to the taxane binding site with a different affinity than paclitaxel and further supports the hypothesis that taccalonolide A has a unique mechanism of action as compared to other microtubule stabilizers.
One explanation for the ability of taccalonolide A to cause microtubule stabilization in intact cells but not in biochemical preparations is that the drug is metabolized in cells to a molecule that binds to tubulin and initiates microtubule stabilization. If this metabolism also occurs systemically when taccalonolide A is administered in vivo in murine models, then this could also explain why taccalonolide A is so much more potent in these models than would be expected from its IC50 in vitro. This is an important consideration since all evidence that the taccalonolides do not directly bind to and polymerize tubulin is based on biochemical studies that preclude cellular metabolism. There are multiple functional groups on taccalonolide A that are potentially susceptible to metabolic conversion including hydrolysis of specific acetate groups or the epoxide and/or opening of the lactone ring. The effects of these modifications on taccalonolide A activity in both cellular assays and biochemical preparations is currently being investigated. Additionally, studies to identify cellular metabolites of taccalonolide A are also underway.
Predicting in vivo activity or potential clinical efficacy from cellular studies is a continuing challenge in drug development. Numerous agents have shown promising activity in cellular experiments, but were ineffective in vivo. Conversely, other classes of agents have shown surprising in vivo efficacy with little or no activity against cancer cells in culture. This is the case for mTOR inhibitors as well as anti-angiogenic agents because disruption of the tumor microenvironment cannot be fully analyzed in ex vivo settings.15 Metabolism also plays an important role in the activation of prodrugs like CPT-11 (irinotecan) which is not effective in vitro because it requires metabolism by carboxylesterases to be converted into an active topoisomerase I inhibitor.16 There are also discrepancies between the efficacy of drugs in pre-clinical in vivo studies and clinical efficacy. 2-Methoxyestradiol and discodermolide both showed promising activities in preclinical studies, but neither advanced in clinical development due to low bioavailability or unexpected toxicities, respectively.17,18 Another example of the discrepancy between cellular and in vivo potency was reported for the microtubule destabilizer eribulin and its closely related analog ER-076349. In cytotoxicity assays ER-076349 was shown to be, on average, four times more potent than eribulin (ER-086526, E7389, Havalen™).19 However, in vivo studies showed that eribulin had superior antitumor efficacy.19 Follow-up cellular studies demonstrated that ER-076349 caused a reversible mitotic blockade while the effects of eribulin were more persistent after drug washout. Together, these data demonstrate that there is not necessarily a direct correlation between cellular activity, in vivo antitumor effects and clinical efficacy and that multiple aspects of drug action contribute to clinical efficacy.
Along with previous work, this study provides clear evidence that all microtubule targeted agents are not equal with regard to cellular persistence as defined by the reversibility of their effects after drug removal. Taken together, analysis of the relative persistence of diverse microtubule targeting agents in this and previous studies showed that the cellular effects of eribulin, vincristine, colchicine and taccalonolide A strongly persist after drug washout while the effects of nocodazole, vinblastine, paclitaxel and laulimalide are more reversible (Figs. 4–6).20 Unfortunately, there is no clear indication to what extent cellular persistence is a desirable property for a drug. The relative reversibility of a compound does not often factor into cellular assays where the cells are constantly bathed in drug-containing media. However, this property might be important in vivo where clearance and metabolism prevent continuous drug exposure. Clinically used drugs, including vincristine and eribulin, demonstrate a high degree of cellular persistence.20 In contrast, the cellular effects of both paclitaxel and vinblastine, which are also clinically valuable microtubule targeting agents, are less persistent.20 Further analysis of the relationship between in vitro reversibility and clinical efficacy may be valuable to identify whether there is a link between these factors.
There are several possible scenarios that singly or in combination could give rise to the persistence of taccalonolide A's cellular effects. First, the cellular accumulation and retention of taccalonolide A may be very high, which would allow sufficient drug to be retained in the cells to cause continued mitotic arrest and cytotoxicity even when residual drug is removed from the media. To test this hypothesis, current studies are underway to radiolabel taccalonolide A, which will allow for direct measurement of the rate and extent of intracellular taccalonolide A accumulation and retention. Another possibility is that taccalonolide A binds to its target protein with a high affinity. The distinct possibility of a tight interaction between taccalonolide A and its target protein gives promise to our future efforts to identify the intracellular binding partner of taccalonolide A by standard biochemical approaches. Other situations that could give rise to taccalonolide A's cellular persistence include the possibility that a very low intracellular concentration of the drug is required to elicit these effects or that taccalonolide A causes persistent effects downstream of the initial binding event. These scenarios are more difficult to test since the binding site of taccalonolide A, much less the signaling pathways that link this binding event to its downstream cellular effects, are not yet known. Regardless of the precise mechanism(s), it is very likely that the high persistence of taccalonolide A's cellular effects and/or the fact that taccalonolide A alters interphase microtubule structures at antiproliferative concentrations may contribute to the fact that the in vivo activity of taccalonolide A is so much greater than would be expected from its potency in cellular cytotoxicity assays.
Materials and Methods
Materials.
Paclitaxel and nocodazole were purchased from Sigma-Aldrich (#T1912 and #M1404). Taccalonolide A was purified from the roots and rhizomes of Tacca chantrieri as previously described in reference 12. Laulimalide was kindly provided by Dr. Bradley Davidson (Utah State University, Logan, UT). Ethanol was used as a vehicle for all drugs.
Cell culture.
HeLa cervical cancer cells were purchased from American Type Culture Collection (#CCL-2). Cells were cultured in Basal Medium Eagle (Sigma, #B9638) with 10% FBS (Hyclone, #SH30070.03) and 50 µg/ml gentamicin (Invitrogen, #15710-064).
Immunofluorescence.
HeLa cells were plated on glass cover-slips and allowed to adhere overnight before addition of compounds. 18 h after drug addition, the cells were fixed with methanol and stained for β-tubulin by indirect immunofluorescence as previously described in reference 10. Cells were visualized using a Nikon Eclipse 80i fluorescence microscope and NIS Elements software.
Microtubule polymerization from cellular lysates.
Microtubules were polymerized from whole-cell lysates using a method adapted from Vallee et al.13,21 HeLa cells were scraped off of the tissue culture plate, washed with chilled PEM buffer
(0.1 M PIPES, 1 mM EGTA, 1 mM MgSO4, pH 6.6) and lysed by Dounce homogenization in hypotonic buffer (1 mM EGTA, 1 mM MgSO4, pH 6.6) supplemented with protease inhibitors. After lysis, 0.1 M PIPES (pH 6.6) was added and lysates were centrifuged at 4°C for 10 min at 25,000x g to pellet cell debris and unlysed cells. The supernatant was removed and clarified by centrifugation at 4°C for 90 min at 130,000x g. These steps were conducted in the cold to depolymerize preexisting cellular micro-tubules and prevent tubulin polymerization. The supernatant was then incubated with vehicle (ethanol), 20 µM paclitaxel or 20–100 µM taccalonolide A at 37°C for 30 min in the presence of 1 mM GTP to allow microtubules to form. For the analysis of cold stable microtubules, the lysates were then returned to a 4°C ice bath for 15 min to depolymerize cold labile microtubules and each of the following steps were also carried out at 4°C. In contrast, for the analysis of total microtubule formation, lysates were kept at 25°C after microtubules were formed for the duration of the experiment. Microtubules were separated from soluble tubulin by centrifugation for 30 min at 25,000x g. The supernatant, containing soluble tubulin, was removed and added to 4x sample buffer. The pellet, which contained polymerized microtubules, was gently washed with PEM buffer and resuspended in 4x sample buffer in PEM. Protein in the supernatant (S), wash (W) and pellet (P) fractions was separated by SDS-PAGE and visualized by total protein staining (Invitrogen, #LC6065) or immunoblotting for β-tubulin (Sigma-Aldrich, #4026), γ-tubulin (Sigma-Aldrich, #3559) or Aurora A (Cell Signaling, #3092).
Flow cytometry.
HeLa cells were treated with drugs for 12 h and then harvested by cell scraping and centrifugation. Cells were washed three times with fresh media and collected by centrifugation to remove residual drug. One aliquot of cells was centrifuged a final time and resuspended in Krishan's reagent containing propidium iodide22 and cell cycle distribution evaluated on a FACS Calibur flow cytometer (BD Biosciences). Propidium iodide intensity was plotted vs. relative number of events using FlowJo software (Tree Star). The percentage of cells in G1 was measured using ModFIt LT 3.0 (Verity Software). For drug washout experiments, a second aliquot of cells was replated and allowed to grow for an additional 12 h in fresh medium before harvesting and analyzing cell cycle distribution.
Inhibition of cellular proliferation.
The sulforhodamine B (SRB) assay was used to measure inhibition of cell proliferation23 as previously described in reference 10, with minor alterations. HeLa cells were plated in 96-well plates and 24 h later drug was added in triplicate wells. For washed cells, the media was removed 24 h after drug addition, the cells rinsed three times and then incubated in the presence of fresh media for an additional 48 h. Continuous drug exposure for the full 60 h was used for another population of cells. Cell density was determined by absorbance of the SRB solution at A560 nm after fixation with TCA and staining with SRB dye. The average percent inhibition ±SD was determined in at least three independent experiments.
Clonogenic assay.
HeLa cells were plated at a density that generated approximately 150 colonies per plate. Drugs were added 24 h after plating at either the concentration that caused a 50% decrease in cell proliferation in the SRB assay or the concentration that caused accumulation of the majority of cells in the G2/M phase of the cell cycle. At 4 or 12 h after drug addition, cells were washed two times, fresh media added and colonies allowed to grow for an additional 10 days. Colonies were fixed and stained with a 20% methanol: 0.5% crystal violet solution after washing with room temperature PBS. Excess stain was removed by gently washing with PBS. GeneTools software (Syngene) was used to count colonies from images of the plates acquired using the Geliance imaging system (PerkinElmer). The survival fraction of cells subjected to short term drug treatment as compared to vehicle treated controls was calculated from three independent experiments.
Acknowledgments
We would like to thank Ms. Cristina Rohena for her thoughtful comments and editing of the manuscript. This work was supported by a grant from the National Cancer Institute of the National Institutes of Health (CA121138) to SLM, the Cancer Center support grant CA P30 (CA054174) and the DOD-CDMRP Postdoctoral Award (BC087466) to A.L.R.
Abbreviations
- IC50
concentration that causes 50% inhibition of proliferation
- eribulin
ER-086526, E7389, Havalen™
References
- 1.Dumontet C, Jordan MA. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov. 2010;9:790–803. doi: 10.1038/nrd3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fojo T, Menefee M. Mechanisms of multidrug resistance: the potential role of microtubule-stabilizing agents. Ann Oncol. 2007;18(Suppl. 5):v3–v8. doi: 10.1093/annonc/mdm172. [DOI] [PubMed] [Google Scholar]
- 3.Pusztai L. Markers predicting clinical benefit in breast cancer from microtubule-targeting agents. Ann Oncol. 2007;18(Suppl. 12):xii15–xii20. doi: 10.1093/annonc/mdm534. [DOI] [PubMed] [Google Scholar]
- 4.Galsky MD, Dritselis A, Kirkpatrick P, Oh WK. Cabazitaxel. Nat Rev Drug Discov. 2010;9:677–678. doi: 10.1038/nrd3254. [DOI] [PubMed] [Google Scholar]
- 5.Morris PG, Fornier MN. Microtubule active agents: beyond the taxane frontier. Clin Cancer Res. 2008;14:7167–7172. doi: 10.1158/1078-0432.CCR-08-0169. [DOI] [PubMed] [Google Scholar]
- 6.Nogales E, Wolf SG, Khan IA, Luduena RF, Downing KH. Structure of tubulin at 6.5 A and location of the taxol-binding site. Nature. 1995;375:424–427. doi: 10.1038/375424a0. [DOI] [PubMed] [Google Scholar]
- 7.Ojima I, Chakravarty S, Inoue T, Lin S, He L, Horwitz SB, et al. A common pharmacophore for cytotoxic natural products that stabilize microtubules. Proc Natl Acad Sci USA. 1999;96:4256–4261. doi: 10.1073/pnas.96.8.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elie-Caille C, Severin F, Helenius J, Howard J, Muller DJ, Hyman AA. Straight GDP-tubulin protofilaments form in the presence of taxol. Curr Biol. 2007;17:1765–1770. doi: 10.1016/j.cub.2007.08.063. [DOI] [PubMed] [Google Scholar]
- 9.Bennett MJ, Barakat K, Huzil JT, Tuszynski J, Schriemer DC. Discovery and characterization of the laulimalide-microtubule binding mode by mass shift perturbation mapping. Chem Biol. 2010;17:725–734. doi: 10.1016/j.chembiol.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Tinley TL, Randall-Hlubek DA, Leal RM, Jackson EM, Cessac JW, Quada J, Jr, et al. Taccalonolides E and A: Plant-derived steroids with microtubule-stabilizing activity. Cancer Res. 2003;63:3211–3220. [PubMed] [Google Scholar]
- 11.Buey RM, Barasoain I, Jackson E, Meyer A, Giannakakou P, Paterson I, et al. Microtubule interactions with chemically diverse stabilizing agents: thermodynamics of binding to the paclitaxel site predicts cytotoxicity. Chem Biol. 2005;12:1269–1279. doi: 10.1016/j.chembiol.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Risinger AL, Jackson EM, Polin LA, Helms GL, LeBoeuf DA, Joe PA, et al. The taccalonolides: microtubule stabilizers that circumvent clinically relevant taxane resistance mechanisms. Cancer Res. 2008;68:8881–8888. doi: 10.1158/0008-5472.CAN-08-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallee RB. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs) J Cell Biol. 1982;92:435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komlodi-Pasztor E, Sackett D, Wilkerson J, Fojo T. Mitosis is not a key target of microtubule agents in patient tumors. Nat Rev Clin Oncol. 2011;8:244–250. doi: 10.1038/nrclinonc.2010.228. [DOI] [PubMed] [Google Scholar]
- 15.Lane HA, Wood JM, McSheehy PM, Allegrini PR, Boulay A, Brueggen J, et al. mTOR inhibitor RAD001 (everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitor. Clin Cancer Res. 2009;15:1612–1622. doi: 10.1158/1078-0432.CCR-08-2057. [DOI] [PubMed] [Google Scholar]
- 16.Smith NF, Figg WD, Sparreboom A. Pharmacogenetics of irinotecan metabolism and transport: an update. Toxicol In Vitro. 2006;20:163–175. doi: 10.1016/j.tiv.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 17.Dahut WL, Lakhani NJ, Gulley JL, Arlen PM, Kohn EC, Kotz H, et al. Phase I clinical trial of oral 2-methoxyestradiol, an antiangiogenic and apoptotic agent, in patients with solid tumors. Cancer Biol Ther. 2006;5:22–27. doi: 10.4161/cbt.5.1.2349. [DOI] [PubMed] [Google Scholar]
- 18.Mita A, Lockhart A, Chen TL, Bochinski K, Curtright J, Cooper W, et al. A phase I pharmacokinetic (PK) trial of XAA296A (discodermolide) administered every 3 wks to adult patients with advance solid malignancies. J Clin Oncol. 2004;22:2025. [Google Scholar]
- 19.Towle MJ, Salvato KA, Budrow J, Wels BF, Kuznetsov G, Aalfs KK, et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001;61:1013–1021. [PubMed] [Google Scholar]
- 20.Towle MJ, Salvato KA, Wels BF, Aalfs KK, Zheng W, Seletsky BM, et al. Eribulin induces irreversible mitotic blockade: implications of cell-based pharmacodynamics for in vivo efficacy under intermittent dosing conditions. Cancer Res. 2011;71:496–505. doi: 10.1158/0008-5472.CAN-10-1874. [DOI] [PubMed] [Google Scholar]
- 21.Vallee RB, Collins CA. Purification of microtubules and microtubule-associated proteins from sea urchin eggs and cultured mammalian cells using taxol, and use of exogenous taxol-stabilized brain microtubules for purifying microtubule-associated proteins. Methods Enzymol. 1986;134:116–127. doi: 10.1016/0076-6879(86)34080-1. [DOI] [PubMed] [Google Scholar]
- 22.Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975;66:188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]