Summary
Objective
A recent RCT demonstrated home-based treatment of WHO-defined severe pneumonia with oral amoxicillin was equivalent to hospital-based therapy and parenteral antibiotics. We aimed to determine whether this finding is generalizable across four countries.
Methods
Multi-centre observational study in Bangladesh, Egypt, Ghana and Vietnam between November 2005 and May 2008. Children aged 3 to 59 months with WHO-defined severe pneumonia were enrolled at participating health centers and managed at home with oral amoxicillin (80–90 mg/kg/day) for 5 days. Children were followed-up at home on days 1, 2, 3 and 6 and at a facility on day 14 to look for cumulative treatment failure through day 6 and relapse between days 6–14.
Results
Of 6,582 children screened, 873 were included, of whom 823 had an outcome ascertained. There was substantial variation in presenting characteristics by site. Bangladesh and Ghana had fever (97%) as a more common symptom than Egypt (74%) and Vietnam (66%), while in Vietnam audible wheeze was more common (49%) than at other sites (range 2%–16%). Treatment failure by day 6 was 9.2% (95% CI: 7.3%–11.2%) across all sites, varying from 6.4% (95% CI: 3.1%–9.8%) in Ghana to 13.2% (95% CI: 8.4%–18.0%) in Vietnam. 2.7% (95% CI: 1.5%–3.9%) of the 733 children well on day 6 relapsed by day 14. The most common causes of treatment failure were persistence of LCI at day 6 (3.8%; 95% CI: 2.6%–5.2%), abnormal sleepy or difficult to wake (1.3%; 95% CI: 0.7%–2.3%), and central cyanosis (1.3%; 95% CI: 0.7%–2.3%). All children survived and only one adverse drug reaction occurred. Treatment was more frequent in young infants and those presenting with rapid respiratory rates.
Conclusions
Clinical treatment failure and adverse event rates among children with severe pneumonia treated at home with oral amoxicillin did not substantially differ across geographic areas. Thus home-based therapy of severe pneumonia can be applied to a wide variety of settings.
Keywords: pneumonia, developing countries, integrated management of childhood illness, amoxicillin, effectiveness
INTRODUCTION
Pneumonia is an important cause of morbidity and the leading cause of mortality in young children in developing countries.(Black et al. 2010) Every year approximately 1.6 million children under five years of age die from acute lower respiratory illness, predominantly pneumonia. Acute respiratory infections are also a major cause of outpatient visits to health facilities and admissions to hospital (Qazi et al. 1996; Singhi et al. 2003; Pepin et al. 2001; Craig et al. 2010). To reduce child mortality from pneumonia and rationalize antibiotic treatment, WHO developed standardized case management guidelines (IMCI), which recommend that children with severe pneumonia defined by the presence of lower chest wall indrawing (LCI) be admitted to hospital and treated with parenteral antibiotics (penicillin G or ampicillin) (World Health Organization 2005a).
The results of two recent trials have demonstrated the equivalence of oral amoxicillin and parenteral penicillin for the treatment of severe pneumonia in both hospital and home-based settings (Addo-Yobo et al. 2004; Hazir et al. 2008). The NO-SHOTS study by Hazir et al. demonstrated that home-based treatment of severe pneumonia was equivalent to the standard of care consisting of hospital-based supportive therapy and parenteral antibiotics. However, despite its importance, enrolment into this study occurred in the casualty or outpatient departments of tertiary care facilities at seven sites in Pakistan, thus limiting its generalizability across varied geographic areas and health care settings. It is therefore necessary to determine if home-based treatment of WHO-defined severe pneumonia with oral amoxicillin is effective beyond the limited disease ecologies and health service circumstances found in the Pakistan study.
We conducted the current study to determine if home-based therapy has similar rates of treatment success in five settings across four geographically dispersed countries using methods similar to the NO-SHOTS study.
METHODS
Study sites
This multi-centre observational study was undertaken in 5 sites in Bangladesh, Egypt, Ghana and Vietnam (two) to assess the effectiveness of treating children under 5 years old with severe pneumonia with oral amoxicillin for 5 days. The study was a planned follow-on to the previously mentioned NO-SHOTS study (Hazir et al.2008). The two Vietnamese sites were Paediatric Hospital #1 in Ho Chi Minh City, an urban tertiary care facility, and Haiphong Children’s Hospital, a second-level facility in a less urbanized area. At both sites paediatricians enrolled and evaluated study subjects and managed them at home. In Egypt subjects were enrolled at 7 public primary care facilities in Ismalia governorate, a semi-rural community located 100 km from Cairo. Study physicians initially examined patients at the facility and home-based follow-up was done by trained nursing staff. Dhaka Shishu Hospital (DSH) in Bangladesh is a national referral paediatric hospital providing both primary and tertiary care. Children were brought directly to the hospital or were referred by general practitioners, hospitals and clinics. In Bangladesh, home-based study evaluations were performed by community members with a Masters degree in social science and up to three years prior experience in community health work. Home evaluators received one week of IMCI training on pneumonia case management and study procedures (Zeitz et al. 1993; World Health Organization 2005b) The Komfo Anokye Teaching Hospital in Kumasi, Ghana is primarily a tertiary care facility providing referral service for the northern sector of Ghana which also provides primary care to the surrounding subdistricts of Kumasi. Study subjects in Ghana were enrolled by study physicians at the primary health facility and were followed-up at home by nurses trained in IMCI.
Enrolment
Participants were recruited from November 2005 to May 2008. Children aged 3–59 months with WHO-defined severe pneumonia were eligible for enrolment (for detailed inclusion/exclusion criteria see Panel 1). Children presenting to a participating centre with a history of cough or difficult breathing were pre-screened and those with LCI were referred to a study physician to evaluate whether they met the eligibility criteria for outpatient home therapy. Study physicians were trained in management of such cases in accordance with WHO guidelines (World Health Organization 2005a). Children with LCI that resolved after three doses of nebulized salbutamol, given at 15 minutes intervals, were excluded. Informed consent by a legal guardian was obtained prior to enrolment. Enrolment occurred throughout the day and night in Vietnam and Egypt, but only during morning hours in Ghana and Bangladesh.
Panel 1: Definitions of inclusions and exclusions criteria in a multicenter study of outpatient treatment of children with WHO defined severe pneumonia in Bangladesh, Egypt, Ghana and Vietnam (the MASS Study).
Inclusion criteria
Children aged 3 to 59 months with severe pneumonia defined as lower chest indrawing in children with cough and/or difficult breathing, without any danger signs*
Exclusion criteria
We excluded children with any of the following:
Very severe pneumonia/disease*
Prior episodes of asthma or three or more prior episodes of wheezing
Lower chest indrawing (LCI) resolved after three doses of bronchodilator therapy
Severe malnutrition (visible severe wasting or edema)
Known anaphylactic reaction to penicillin or amoxicillin
Hospitalization in the last two weeks
Other diseases requiring antibiotic therapy at presentation, such as meningitis, dysentery, osteomyelitis, septic arthritis, evident tuberculosis, etc.
Persistent vomiting†
Previous inclusion in the study or inclusion in another study
Living outside study catchment area
Parental or caretaker refusal to participate in the study
Known chronic condition, judgment that child won’t be able to complete study
Severe pneumonia with measles
Kerosene ingestion
Near drowning
*Developing any sign of very severe disease such as central cyanosis, abnormally sleepy or difficult to wake, inability to drink, convulsions, or death.
†Persistent vomiting defined as vomiting three repeated doses of oral amoxicillin within ½ hour of administration.
Patient evaluation
After obtaining consent, a detailed history was taken and clinical examination performed by the study physician with findings recorded on standardized case report forms (CRF). Clinical information included history of present illness, physical examination findings including axillary temperature, respiratory rate, and presence of LCI and wheezing.
Laboratory investigations
In all sites except Ghana, urine samples were obtained when possible at baseline from children to assess recent antibiotic usage. Urine was centrifuged and the supernatant was sterilized by passage through a 0·22 µm filter (Millipore, Billerica, MA, USA). Samples were immediately frozen at −70°C, and thawed in batch at a later time, then cultured on a nutrient agar plate streaked with pan-sensitive Micrococcus luteus. Urine antibacterial activity was determined by interpretation of the zone of inhibition (Liu et al. 1999).
Patient management
Caretakers of enrolled patients administered the first dose of amoxicillin (80–90 mg/kg/day in two divided doses) in the health facility under supervision of study personnel. Caretakers were counselled to continue with the oral treatment prescribed for five days and about the importance of adhering to the specified dosage, timing and duration. General supportive care included oral salbutamol and antipyretics, when indicated. The caretaker was advised to return to the healthcare facility at any time if symptoms recurred or if the child developed danger signs. Each danger sign was clearly explained to the caregiver. Screening, enrolment clinical assessment, urine sampling and provision of study medication took place within one hour of enrolment.
Follow-up
Enrolled patients were monitored for a total of 14 days. Baseline assessment (day 0) was done in hospital. Follow-up observations were conducted at home on days 1, 2, 3 and 6 and the final follow-up was given on day 14 at the facility. Adherence was evaluated at each follow-up visit by asking the caregiver how the drug was administered and the total volume of medicine used was measured on day 6. During the home visit study personnel clinically evaluated the children. If the study personnel suspected treatment failure, s/he contacted the site coordinator or a senior paediatrician to confirm the treatment failure, and referred the patient to the health facility. Children failing treatment were managed according to the usual practices of the participating site with details of the management recorded on CRFs. If parents were concerned the child was not improving or had deteriorated, patients were brought back to the health facility for unscheduled visits, and were clinically assessed and managed by the study physician. Adverse drug reactions were monitored actively by each site and recorded in the dataset.
Study outcomes
The primary endpoint for this study was treatment failure at any time through day 6. A secondary endpoint was treatment failure between day 6 and day 14, also defined as relapse. Both outcomes are defined in detail in panel 2.
Panel 2: Definitions of study endpoints in a multicenter study of outpatient treatment of children with WHO defined severe pneumonia in Bangladesh, Egypt, Ghana and Vietnam (the MASS Study).
Primary outcome (treatment failure within the first 6 days)
Defined as a child with any of the following:
Secondary outcome (treatment failure between day 6 and 14)
Any of the following:
Change of Antibiotic‡
Developing a co-morbid condition
Persistence of fever > 38°C with lower chest indrawing on day 3 (after 72 hours).
Either fever or lower chest indrawing alone at day 6 or later
*Developing any sign of very severe disease such as central cyanosis, abnormally sleepy or difficult to wake, inability to drink, convulsions, or death.
†Persisting vomiting defined as vomiting three repeated doses of oral amoxicillin within ½ hour of administration.
⌂Wheezing children were given a trial of nebulised salbutamol (0.5ml plus 2.0 ml of sterile water or 2 puffs using a metered-dose inhaler with a spacer device) and re-evaluated after 15 minutes. If the LCI persisted, a second trial of nebulisation was given, failing which a third was given.
Ethical Clearance
Caregivers of eligible children were informed about the study in their own language by study staff and a written witnessed consent was obtained. The study was approved by Ethical Review Committee/Institutional Review Boards of the participating institutions and the sponsoring organizations.
Sample size and statistical methods
The sample size for the study was estimated to be able to determine the proportion of treatment failures in children receiving oral amoxicillin with reasonable precision. We assumed, based on previous studies with similar design(Hazir et al.2008) that the treatment failure rate would be 14%. We estimated a sample size of 186 children per site would allow us to estimate a 95% confidence interval around the failure rate of +/−5% for site specific analyses (Cochrane 1977). Expecting protocol violations and loss to follow-up of 5%, we enrolled over 207 patients at each site. Our analysis began with stratifying baseline characteristics by study site to look for differences in the severity and characteristics of presenting illness across study sites. Study variables were summarized using proportions and means and are presented with 95% confidence intervals. The proportion of subjects who were treatment failures at day 6, the reasons for treatment failure, and the day 14 outcomes are presented with 95% confidence intervals (CI) stratified by study site. Comparisons between sites were made using relative risks (RR) and 95% CIs. To determine the impact of removing subjects from the dataset who did not follow the study protocol we conducted a conservative sensitivity analysis by assuming that all protocol violations were treatment failures. Finally, we identified baseline characteristics of subjects associated with treatment failure at day 6 by calculating relative risks (RR) and 95% CIs and difference in means and 95% CI for continuous characteristics. We identified variables for inclusion in adjusted models as those that were plausible predictors of treatment failure and that had a univariate pvalue < 0.25. All analyses were adjusted for age and sex regardless of the pvalue. Because antibiotic resistance testing was not conducted on all subjects we did not include this in final models despite its pvalue in univariate analyses. Adjusted analyses were conducted using log-linear regression to estimate adjusted relative risks of treatment failure. Finally, to adjust for clustering of outcomes by site, we further adjusted models for the clustering using generalized estimating equations. Analyses were conducted in SAS version 9.1.
RESULTS
Screening
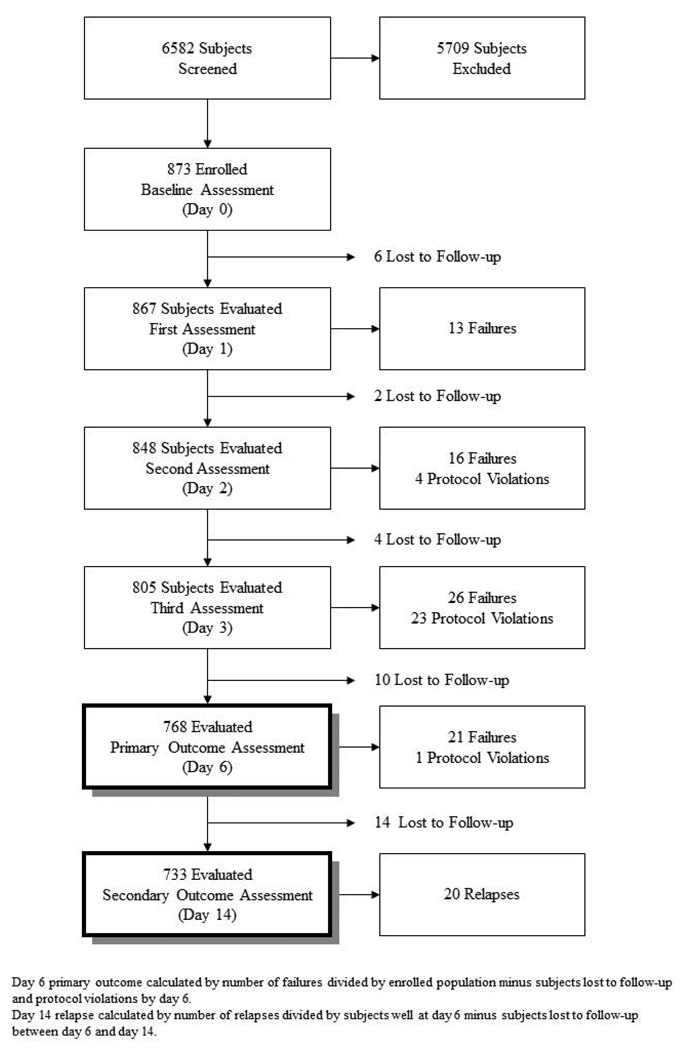
A total 6582 children presenting to the health facilities were screened of whom 873 met the eligibility criteria and were enrolled (Table 1). Three or more episodes of wheezing (27%), LCI that resolved with bronchodilator therapy (20%) and very severe disease (17%) were the most common medical reasons for exclusion. Living outside of the study catchment area (83%, 25% of all exclusions) was the most common reason for administrative exclusion. Reasons for exclusion varied by site with very severe disease being the most common reason in Ghana (26%) and also prominent in Bangladesh (30%), while history of three episodes of wheezing was most common in Vietnam (24%) and living outside the study area was most common in Egypt (30%) and Bangladesh (37%).
Table 1.
Study screening and reasons for exclusion from a multicenter study of outpatient treatment of WHO defined severe pneumonia in Bangladesh, Egypt, Ghana and Vietnam (the MASS Study)
| Bangladesh | Egypt | Ghana | Vietnam | All Sites | |
|---|---|---|---|---|---|
| Screened | 2004 | 357 | 529 | 3692 | 6582 |
| Enrolled | 209 (10%) | 237 (66%) | 212 (40%) | 215 (6%) | 873 (13%) |
| Excluded | 1795 (90%) | 120 (34%) | 317 (60%) | 3476 (94%) | 5708 (87%) |
| Exclusion Criteria, Number (%) | Bangladesh | Egypt | Ghana | Vietnam | All Sites |
| Three or more episodes of wheezing | 92 (8%) | 14 (12%) | 20 (6%) | 1527 (33%) | 1653 (27%) |
| LCI that resolves after up to 3 doses of rapid acting bronchodilator | 125 (11%) | 7 (6%) | 31 (9%) | 1066 (23%) | 1229 (20%) |
| Very severe disease | 574 (51%) | 11 (10%) | 111 (32%) | 394 (8%) | 1090 (17%) |
| Known prior episodes of asthma | 28 (2%) | 13 (11%) | 9 (3%) | 854 (18%) | 904 (14%) |
| Hospitalization in the last two weeks | 27 (2%) | 10 (9%) | 19 (6%) | 489 (11%) | 545 (9%) |
| Other diseases requiring antibiotics | 88 (8%) | 39 (34%) | 98 (29%) | 49 (1%) | 274 (4%) |
| Known chronic condition | 20 (2%) | 0 (0%) | 28 (8%) | 178 (4%) | 226 (4%) |
| Severe malnutrition | 95 (8%) | 1 (1%) | 16 (5%) | 51 (1%) | 163 (3%) |
| Persistent vomiting | 71 (6%) | 18 (16%) | 4 (1%) | 21 (0%) | 114 (2%) |
| Anaphylactic reaction | 2 (0%) | 1 (1%) | 1 (0%) | 11 (0%) | 15 (0%) |
| Severe pneumonia with measles | 9 (1%) | 0 (0%) | 1 (0%) | 0 (0%) | 10 (0%) |
| Kerosene ingestion | 3 (0%) | 0 (0%) | 5 (1%) | 1 (0%) | 9 (0%) |
| Near drowning | 0 (0%) | 0 (0%) | 0 (0%) | 4 (0%) | 4 (0%) |
| Total exclusions for medical reasons | 1134 | 114 | 343 | 4645 | 6236 |
| Living outside study area | 716 (91%) | 60 (67%) | 64 (78%) | 1390 (80%) | 2230 (83%) |
| Refusal to participate in study | 40 (5%) | 15 (17%) | 1 (1%) | 339 (20%) | 395 (15%) |
| Previous inclusion in the study or other study | 27 (3%) | 12 (13%) | 2 (2%) | 4 (0%) | 45 (2%) |
| Judgment that the child won't be able to complete study | 2 (0%) | 2 (2%) | 15 (18%) | 1 (0%) | 20 (1%) |
| Total exclusions for administrative reasons | 785 | 89 | 82 | 1734 | 2690 |
| Total Reasons for Exclusion* | 1919 | 203 | 425 | 6379 | 8926 |
Note that children could have more than one reason for exclusions and therefore the total in this column may be more than the total number excluded above.
Baseline
Table 2 shows the baseline demographic and clinical characteristics of the enrolled children in the four countries. Median age of enrolled children was 8 months overall with 64% between 3–11 months of age; however children enrolled in Bangladesh were considerably younger than at the other sites with a median age of 4 months and 64% were between 3–5 months of age. Vaccination status, as assessed by caregiver report, was uniformly high at all sites (>93%). There was substantial variation in presenting characteristics by site, suggesting different spectrums of disease in each. In Bangladesh and Ghana fever was a common symptom at presentation (97%) while less so in Egypt (74%) and Vietnam (66%). In Vietnam audible wheeze was present at baseline far more often (49%) than at other sites (range 2%–16%) and is consistent with our finding that a common exclusion criteria at screening in Vietnam was a prior history of three or more episodes of wheezing (24%), or known prior asthma (13%). Reported antibiotic use in the seven days prior to enrolment also varied substantially by site: it was common in Bangladesh (44%) and Vietnam (23%) while rare in Ghana (4%) and Egypt (5%).
Table 2.
Baseline characteristics of 873 children enrolled in a multicenter study of outpatient treatment of WHO defined severe pneumonia in Bangladesh, Egypt, Ghana and Vietnam (the MASS Study)
| Parameter | Bangladesh | Egypt | Ghana | Vietnam | All Sites |
|---|---|---|---|---|---|
| Male (%) | 65.6% (137/209) | 59.1% (140/237) | 53.8% (114/212) | 60.9% (131/215) | 59.8% (522/873) |
| Age of Children | |||||
| Mean age (months)* | 4.0 (3.0 to 7.0) | 9.0 (5.0 to 16.0) | 11.0 (6.0 to 23.0) | 11.0 (6.0 to 19.0) | 8.0 (5.0 to 16.0) |
| Subjects 3–5 months (%) | 64.6% (135/209) | 27.4% (65/237) | 21.7% (46/212) | 20.5% (44/215) | 33.2% (290/873) |
| Infants (3–11 months old) (%) | 89.5% (187/209) | 66.7% (158/237) | 50.5% (107/212) | 51.2% (110/215) | 64.4% (562/873) |
| Children (12–59 months old) (%) | 10.5% (22/209) | 33.3% (79/237) | 49.5% (105/212) | 48.4% (104/215) | 35.5% (310/873) |
| Breastfeeding (%) | 96.6% (198/205) | 84.6% (176/208) | 73.2% (120/164) | 69.9% (128/183) | 81.8% (622/760) |
| Relationship of Respondent to Child | |||||
| Mother (%) | 98.6% (206/209) | 94.9% (225/237) | 93.9% (199/212) | 83.7% (180/215) | 92.8% (810/873) |
| History of: | |||||
| Fever (%) | 97.1% (203/209) | 73.8% (175/237) | 97.2% (206/212) | 66.0% (142/215) | 83.2% (726/873) |
| Cough (%) | 100.0% (209/209) | 96.6% (229/237) | 99.1% (210/212) | 99.1% (213/215) | 98.6% (861/873) |
| Difficulty Breathing (%) | 99.5% (208/209) | 62.4% (148/237) | 98.1% (208/212) | 78.1% (168/215) | 83.8% (732/873) |
| Reported Antibiotic Use in Past 7 Days (%) | 44.0% (92/209) | 5.1% (12/237) | 3.8% (8/212) | 23.3% (50/215) | 18.6% (162/873) |
| Past History of Wheezing (1 or 2 episodes) (%) |
9.1% (19/209) | 7.2% (17/237) | 5.7% (12/212) | 14.0% (30/215) | 8.9% (78/873) |
| Up-to-date immunization status (%) | 96.2% (201/209) | 97.9% (232/237) | 93.9% (199/212) | 96.3% (207/215) | 96.1% (839/873) |
| Examination | |||||
| Presence of Dehydration | 1.9% (4/209) | 0.4% (1/237) | 3.3% (7/212) | 0.9% (2/215) | 1.6% (14/873) |
| Weight-for-age Z-score | −1.0 (−2.04 to −0.44) | −0.2 (−0.91 to 0.66) | −0.9 (−1.66 to 0.19) | −0.6 (−1.51 to 0.31) | −0.7 (−1.56 to 0.21) |
| Temperature (C)* | 37.8 (37.2 to 37.8) | 37.5 (37.0 to 38.0) | 37.8 (37.2 to 38.2) | 37.0 (37.0 to 37.9) | 37.7 (37.0 to 38.0) |
| Respiratory rate/min (3–11 months)* | 61.0 (57.5 to 67.0) | 54.5 (49.5 to 60.0) | 63.5 (59.0 to 66.0) | 55.0 (52.0 to 56.0) | 58.0 (53.0 to 63.5) |
| Respiratory rate/min (12–59 months)* | 58.8 (51.5 to 62.5) | 44.0 (36.5 to 51.0) | 58.5 (50.5 to 64.0) | 51.0 (46.0 to 54.0) | 51.0 (45.0 to 58.0) |
| Audible Wheeze (%) | 2.9% (6/209) | 15.6% (37/237) | 1.9% (4/212) | 48.8% (105/215) | 17.4% (152/873) |
Median (IQR)
Outcomes
Of the 873 children enrolled, 50 were excluded from primary outcome analyses. Of the 50, 22 were lost to follow-up before day 6, while the remaining 28 were protocol violations (though are included in sensitivity analyses). In 25 instances subjects enrolled at the start of the study who had LCI or fever were incorrectly declared to be treatment failures (rather than LCI and fever), and 3 additional cases were deemed treatment failures because of presence of grunting – not an outcome characteristic. The proportion of children experiencing cumulative treatment failure by day 6 (Table 3 primary outcome, Panel 2) was 9.2% (95% CI: 7.3%–11.2%) across all sites, varying from 6.4% (95% CI: 3.1%–9.8%) in Ghana to 13.2% (95% CI: 8.4%–18.0%) in Vietnam. Of the total 76 cumulative failures by day 6, 13 (17%) occurred within the first 24 hours (day 0), 16 (21%) between day 1 and 2, 26 (34%) by day 3 and 21 (28%) occurred between day 3 and 6 (Figure 1). Twenty-two subjects (2.6%) were lost to follow-up during the first 6 days (97.4% retention), and 14 more (1.9%) were lost by day 14 (Table 3). When we assumed that the 22 subjects lost to follow-up and the 28 protocol violations were treatment failures, the treatment failure rate increased to 14.4% (126/876; 95% CI: 12.2%–16.9%). Among the 733 children whose pneumonia resolved by day 6 and were not lost, 2.7% (20/733; 95% CI: 1.5%–3.9%) developed relapse by day 14 (Secondary outcome, Panel 2). When those lost between day 6 and 14 were included as failures (there were no additional protocol violations) the relapse rate increases to 4.6% (34/747; 95% CI: 3.2%–6.2%).
Table 3.
Treatment outcomes in 873 children enrolled in a multicenter study of outpatient treatment of WHO defined severe pneumonia in Bangladesh, Egypt, Ghana and Vietnam (the MASS Study)
| Study Site | |||||
|---|---|---|---|---|---|
| Outcome | Bangladesh | Egypt | Ghana | Vietnam | Total‡ |
| Day 6 Outcomes | |||||
| Cumulative failure (day 6) | 18/199 (9.0%) | 20/232 (8.6%) | 13/202 (6.4%) | 25/190 (13.2%) | 76/823*** (9.2%) |
| Reasons for Failure by Day 6 | |||||
| Inability to Drink | 0/199 (0.0%) | 5/232 (2.2%) | 2/202 (1.0%) | 1/190 (0.5%) | 8/823 (1.0%) |
| Abnormally Sleepy/Difficult to Wake | 1/199 (0.5%) | 6/232 (2.6%) | 0/202 (0.0%) | 4/190 (2.1%) | 11/823 (1.3%) |
| Central Cyanosis | 3/199 (1.5%) | 1/232 (0.4%) | 0/202 (0.0%) | 7/190 (3.7%) | 11/823 (1.3%) |
| Persistent Vomiting | 0/199 (0.0%) | 1/232 (0.4%) | 0/202 (0%) | 1/190 (0.5%) | 2/823 (0.2%) |
| Persistence of Fever and LCI* at Day 3 | 1/199 (0.5%) | 1/232 (0.4%) | 3/202 (1.5%) | 3/190 (1.6%) | 8/823 (1.0%) |
| Persistence of LCI* at Day 6 | 13/199 (6.5%) | 6/232 (2.6%) | 5/202 (2.5%) | 7/190 (3.7%) | 31/823 (3.8%) |
| Newly Diagnosed Co-morbid Condition | 0/199 (0.0%) | 0/232 (0.0%) | 2/202 (1.0%) | 0/190 (0.0%) | 2/823 (0.2%) |
| Adverse Drug Reaction | 0/199 (0.0%) | 0/232 (0.0%) | 0/202 (0.0%) | 1/190 (0.5%) | 1/823 (0.1%) |
| Received Another Antibiotic | 0/199 (0.0%) | 0/232 (0.0%) | 1/202 (0.5%) | 1/190 (0.5%) | 2/823 (0.2%) |
| Lost to Follow-up by Day 6 | 9/208 (4.3%) | 1/233 (0.4%) | 10/212 (4.7%) | 2/192 (1.0%) | 22/845 (2.6%) |
| Day 14 Outcomes | |||||
| Lost to Follow-up between Day 6–14** | 9/181 (5.0%) | 1/212 (0.5%) | 1/189 (0.5%) | 3/165 (1.8%) | 14/747 (1.9%) |
| Relapse by Day 14# | 10/172 (5.8%) | 7/211 (3.3%) | 0/188 (0.0%) | 3/162 (1.9%) | 20/733 (2.7%) |
LCI-Lower Chest Wall In-drawing
Includes only subjects well on day 6 and not lost to follow-up before day 6
Denominator excludes subjects loss to follow-up (22) and protocol violations (28) by day 6.
Includes only subjects well on day 6 and not lost to follow-up between day 6 and 14
note that the number of children with outcomes is 823 as 22 subjects were lost to follow-up before day 6 and 28 were excluded from outcome analyses as they were protocol violations
Figure 1.
Study profile of a multicenter study of outpatient treatment of WHO defined severe pneumonia in Bangladesh, Egypt, Ghana and Vietnam (the MASS Study)
The most common causes of treatment failure were persistence of LCI at day 6 (3.8%; 95% CI: 2.6%–5.2%), being abnormally sleepy or difficult to wake, and central cyanosis (both 1.3%; 95% CI: 0.7%–2.3%), shown in Table 3. Taken together, danger signs accounted for 42% (32/76) of all failures and persistence of LCI at day 6 for a further 41%. Persistence of LCI at day 6 was more than twice as common in Bangladesh as in all the other sites combined (RR 2.3; 95% CI: 1.1–4.5) while central cyanosis was substantially more common in Vietnam than at any other site (RR 5.8; 95% CI: 1.7–19.7). There were no deaths in this study and only one case of adverse drug reaction to amoxicillin (urticaria in Vietnam) occurred. Overall compliance with the study drug was over 96%.
Baseline predictors of treatment failure
We identified several baseline characteristics that were predictive of treatment failure up to day 6 (Table 4). Compared with children older than one year, young infants (age 3–5 months) were twice as likely (RR 1.96, 95% CI: 1.09–3.51) to fail treatment. Likewise, children who were enrolled with fast breathing (≥ 50 bpm for children 2–11 months; ≥40 bpm for children 12–59 months) or very fast breathing (≥70 bpm and ≥60, respectively) at enrolment were substantially more likely to fail treatment (RR 6.38; 95% CI: 0.88–46.5 and RR: 12.5; 95% CI: 1.74–89.1, respectively) by six days after initiation of therapy compared with children with normal breathing.
Table 4.
Baseline characteristics associated with treatment failure day 6 among 823** children enrolled in a multicenter study of outpatient treatment of WHO defined severe pneumonia in Bangladesh, Egypt, Ghana and Vietnam (the MASS Study)
| Variable | Exposure | Yes (N = 76) |
No (N = 747) |
Total (N = 823**) |
Relative Risk (95% CI)# |
Adjusted Rela- tive Risk (95% CI)^ |
Cluster Adjusted Relative Risk (95% CI)^ |
|---|---|---|---|---|---|---|---|
| Sex | Female | 24 (7.2%) | 309 (92.8%) | 333 | Reference | Reference | Reference |
| Male | 52 (10.6%) | 438 (89.4%) | 490 | 1.47 (0.93 – 2.34) | 1.43 (0.9 – 2.26) | 1.43 (1.01 – 2.01) | |
| Age Group | 12 – 59 months | 18 (6.1%) | 277 (93.9%) | 295 | Reference | Reference | Reference |
| 6–11 months | 25 (9.8%) | 230 (90.2%) | 255 | 1.61 (0.90 – 2.88) | 1.06 (0.65 – 1.72) | 1.06 (0.76 – 1.46) | |
| 3–5 months | 33 (12.1%) | 240 (87.9%) | 273 | 1.98 (1.14 – 3.43) | 1.96 (1.09 – 3.51) | 1.96 (0.84 – 4.56) | |
| Respiratory Rate Groups* | Normal Breathing | 1 (1.1%) | 91 (98.9%) | 92 | Reference | Reference | Reference |
| Fast Breathing | 24 (7.3%) | 304 (92.7%) | 328 | 6.73 (0.92 – 49.1) | 6.38 (0.88 – 46.5) | 6.38 (0.65 – 63.0) | |
| Very Fast Breathing | 51 (12.7%) | 352 (87.3%) | 403 | 11.6 (1.63 – 83.2) | 12.5 (1.74 – 89.1) | 12.5 (1.19 – 130) | |
| Urine Antibiotic Test Results# | No | 14 (7.4%) | 174 (92.6%) | 188 | Reference | ||
| Yes | 19 (11.2%) | 151 (88.8%) | 170 | 1.50 (0.78 – 2.90) | |||
| Average Respiratory Rate | 59.4 (8.2) | 55.4 (9.6) | 55.8 (9.5) | 4.03***(1.80 – 6.27) | |||
| Audible Wheeze | No | 60 (8.7%) | 626 (91.3%) | 686 | Reference | ||
| Yes | 16 (11.7%) | 121 (88.3%) | 137 | 1.34 (0.79 – 2.25) | |||
| History of Fever | No | 14 (10.3%) | 122 (89.7%) | 136 | Reference | ||
| Yes | 62 (9.0%) | 625 (91.0%) | 687 | 0.88 (0.51 – 1.52) | |||
| Up-to-date Immunization Status | No | 6 (20.0%) | 24 (80.0%) | 30 | Reference | ||
| Yes | 70 (8.8%) | 723 (91.2%) | 793 | 0.44 (0.21 – 0.93) |
For children < 3–11 months: fast breathing ≥50 breaths per minute (bpm) and very fast breathing is > 70; for children 12–59 months fast breathing >=40 bpm and very fast breathing > 60 bpm.
Total excludes subjects loss to follow-up (22) and protocol violations (28) by day 6.
Difference in means (95% CI)
In the adjusted model, variables were adjusted for other variables in the table while in the cluster adjusted they were further adjusted for site level clustering using a generalized estimating equation.
Urine antibiotic test results were not available from all children and were not done in Ghana.
A subset of 358 children was tested for antibiotic activity in the urine at baseline as indicator of antibiotic use immediately prior to enrolment. Of these, 170 (47%) were positive and the treatment failure rate was somewhat increased in this group compared with the failure rate among the children whose urine testing was negative (Table 4, 11% vs. 7%, respectively). However, the difference was not statistically significant. Nonetheless, evidence of prior antibiotic use in urine was not uniform across all sites. In Vietnam (45%) and Bangladesh (66%) the rate was far higher than in Egypt (10%). Urine was not tested in Ghana.
DISCUSSION
Our multi-centre observational study demonstrated that clinical treatment failure and adverse event rates among 873 children between 3 and 59 months of age with WHO-defined severe pneumonia who were treated at home with oral amoxicillin did not substantially differ across four geographically dispersed countries despite different respiratory disease ecologies and presenting disease severities. These failure rates are lower than those of previous randomized controlled trials using the same intervention but a different outcome definition (APPIS) (Addo-Yobo et al. 2004)and consistent with a trial using the same outcome measure (NOSHOTS) (Hazir et al. 2008). While clinical treatment failure rates varied between sites from 6.4% to 13.2%, overall these rates were acceptably low, demonstrating that home-based treatment of severe pneumonia can be applied to a wide variety of settings.
Our clinical treatment failure rate of 9.2% by day 6 is considerably lower than reported previously in the amoxicillin arm of the APPIS trial (19%). The lower rate observed here is due, in part, to our use of a less stringent endpoint definition of treatment failure at 48 hours: fever and LCI were considered failure in this study whereas LCI alone was considered failure in the APPIS trial. Our treatment failure rate is, however, comparable to that reported in the home-based treatment arm of the NO-SHOTS trial (7.5%) using very similar methodology and endpoint definitions. The rate of relapse between day 6 and 14 was also very low at 2.7%. These estimates exclude subjects who were lost to follow-up, left against medical advice, withdrew consent or cases of protocol violation. Including these cases modestly increased our estimate of clinical treatment failure and relapse to 14.4% and 4.5%, respectively. This more conservative estimate of clinical failure is comparable to the a priori estimates upon which our sample size calculations were based.
While our study protocol was standardized, the geographic areas and study settings varied considerably. Paediatricians, nurses and community health workers performed home-based management of study subjects. The facility settings were both urban and rural and included tertiary, secondary and primary health centres. We believe that our finding of largely similar treatment failure rates across this range of clinical settings adds to the generalizability of the findings and shows that home-based management of severe pneumonia can be successfully employed in many health care settings.
The primary reasons for clinical treatment failure was the persistence of LCI on day 6 (3.8%) followed by the development of the danger signs abnormally sleepy (1.3%) and central cyanosis (1.3%). It is noteworthy that there were very few instances (<2%) of a child deteriorating to very severe pneumonia (data not shown) or developing serious treatment related adverse events. This underscores the effectiveness of home-based therapy of severe pneumonia, even in settings where less highly trained health workers screened for clinical deterioration, such as in Bangladesh.
Our findings also support the application of home-based treatment of pneumonia with oral therapy in settings where there are very high rates of asthma and wheezing, such as Vietnam. Recent surveys put rates of wheezing and asthma prevalence among schoolchildren in Vietnam at 25%, above global averages (Nga et al. 2003). High relative rates of wheezing among children presenting with severe pneumonia in Vietnam compared with the other sites in this study is consistent with these reports.
History of prior antibiotic use was high in Bangladesh and Vietnam and urine antibacterial activity data showed nearly double the rates of antibiotic use compared to Bangladesh, Egypt and Vietnam (data not shown). Such high rates of antimicrobial use in the community increases the likelihood of community-based antimicrobial resistance, potentially reducing the efficacy of antibiotic therapy for severe pneumonia. Consistent with these findings, rates of in vitro resistance to common antibiotics was reported in more than 70% of pneumococcal organisms isolated in Vietnam (Song et al. 2004). High levels of bacterial resistance to common antibiotics for S. pneumoniae and H. influenzae infections have also been reported in Bangladesh (Saha et al. 2009; Arifeen et al. 2009; Rahman et al. 2008). Despite the presence of these high rates of prior antibiotic use and reports of in vitro antimicrobial resistance, our high rate of treatment success in these settings is comforting. While high rates of antibiotic resistance would potentially increase the failure rate to amoxicillin in a population, prior (effective) antibiotic use may have decreased our observed failure rates. Nonetheless, it is reassuring that clinical failure rates at the Vietnam and Bangladesh sites were only slightly elevated compared to Egypt and Ghana despite the presence of these two factors.
Our findings also demonstrate that high rates of compliance with twice daily dosing schedules for home-based oral amoxicillin therapy for five days can be achieved. While the generalizability of these rates observed in a study setting are somewhat limited, achieving these high rates in this wide array of clinical settings suggests that achieving good compliance with home-based therapy is possible.
Our study had several potential limitations. First, this was a multi-centre observational study which did not have a control arm with which to compare outcomes. We did have the benefit of being able to compare our findings with prior studies conducted with similar protocols, case definitions, case report forms, and follow-up schedules. Second, treatment failure was assessed by study staff and not by an independent observer. This could lead to variability in outcome assessment. However, bias in the determination of study outcomes was minimized by confirmation of each treatment failure by a second member of the study staff. Third, although we attempted to reduce the enrolment of children with recurrent wheeze, some enrolled children likely had viral infection or asthma, which would have resolved without antibiotic therapy. WHO recommends the use of antibiotics in children with chest indrawing and wheeze who do not respond to up to three doses of bronchodilator (World Health Organization 2005b) because the presence of mixed viral and bacterial infections are common in children from both developed and developing countries (Madhi & Klugman 2004; Weber et al. 1998; Ghafoor et al. 1990; Juven et al. 2000; Forgie et al. 1992). Fourth, we could only test 42% of patients’ urine for antibacterial activity which decreased our power to assess the effect that prior antibiotic use had on treatment failure. Finally, our study did not include any area with high HIV prevalence or any sites from Latin America where the population characteristics or disease ecology may differ from the included study sites.
Recently a study from Pakistan compared oral antibiotic versus placebo for non-severe (fast breathing) pneumonia in children 2–59 months of age and found them equivalent (Hazir et al 2011). This obviously has implications for WHO Case management strategy, which assumes that the presentation of cough and or difficult breathing with chest indrawing means that the child has pneumonia and requires an antibiotic. In the Hazir et al. study up to 60% of children had wheeze on examination. In comparison a study from India compared oral antibiotic versus placebo for non-severe (fast breathing) pneumonia and wheezing in children 2–59 months of age and found higher failure rates in the placebo group (Awasthi et al 2008). These data indicate that effectiveness of antibiotics in wheezing children is mixed. We believe the specificity of pneumonia diagnosis is likely low in children who present with wheezing and fast breathing. Cardoso et al (2011) have shown that adding fever to fast breathing in wheezing children more than doubles the specificity of the WHO criteria for syndromic diagnosis of non-severe pneumonia compared to physician diagnosis. Our study enrolled children only when they had chest indrawing and not on fast breathing. The importance and utility of simple signs like lower chest indrawing has been well established and several studies have measured their sensitivities and specificities for radiologic pneumonia (Ayieko 2007; WHO 1991). It is possible that some cases diagnosed as severe pneumonia through WHO algorithm may not need antibiotics. However, WHO guidelines are intended for health workers in developing countries who do not have diagnostic facilities readily available to them, especially in rural areas. In areas where pneumonia is still a major killer of children, it is justifiable to over treat pneumonia with antibiotics.
Our study has several strengths. The large sample size provided good precision to our estimates of treatment failure rates. The diversity of geographic settings, study implementation staff, prevalence of co-morbid conditions such as wheezing, prior antibiotic usage and seasonal variability demonstrates that home-based therapy is applicable to a variety of public health settings. Furthermore, over 95% of children adhered to the treatment, while fewer than 5% of subjects were lost to follow-up. There were no deaths and none had a serious adverse event related to study drug.
We must be cautious in treating these children as they do need to be monitored for clinical deterioration through follow-up visits. In addition, as most children can be managed at home after being identified at the health facility, it is necessary to explore the effectiveness of identification and treatment of children with chest indrawing in the community. However, our findings have important policy and programmatic implications for treatment of pneumonia with chest indrawing in outpatient settings, principally for Integrated Management of Childhood Illness (IMCI) guidelines. A short course of oral amoxicillin will reduce hospital referrals and admissions. It also has the potential of reducing health care costs, hospital acquired infections, and could improve access to health care and reduce inequity for needy populations.
Acknowledgments
Narendra K. Arora, Professor of Paediatrics, Executive Director, INCLEN Trust, New Delhi, India. We are grateful to INCLEN Inc and JHU for their technical support. We acknowledge the participation and cooperation of the Ministry of Health (MOH) of Ismailia governorate Egypt, and its director Dr. Mahmoud El-Sharkawy and Ismailia IMCI Program Coordinator Dr. Mohamed Nasr. Special thanks to the residents of the Primary Health Care Units in Ismailia governorate and the units personnel. We are in debt to all the families that participated in the study for their kind cooperation. The study was funded by the Department of Child and Adolescent Health and Development, WHO (Bangladesh and Vietnam), the Center of Global Health and Development, Boston University (Ghana), and through USAID grants and INCLEN-Inc/JHU (Egypt). Matthew Fox was funded by a grant from the National Institute of Allergy And Infectious Diseases.
Footnotes
Disclaimer
The content of this publication does not necessarily reflect the views or policies of NIAID, WHO or USAID nor does mention of trade names, commercial projects, or organizations imply endorsement by the US Government. The content is solely the responsibility of the authors and does not represent the official views of the National Institute of Allergy and Infectious Diseases, USAID, or WHO.
References
- Addo-Yobo E, Chisaka N, Hassan M, et al. Oral amoxicillin versus injectable penicillin for severe pneumonia in children aged 3 to 59 months: a randomised multicentre equivalency study. Lancet. 2004;364:1141–1148. doi: 10.1016/S0140-6736(04)17100-6. [DOI] [PubMed] [Google Scholar]
- Arifeen SE, Saha SK, Rahman S, et al. Invasive pneumococcal disease among children in rural Bangladesh: results from a population-based surveillance. Clin.Infect.Dis. 2009;48(Suppl 2):S103–S113. doi: 10.1086/596543. [DOI] [PubMed] [Google Scholar]
- Awasthi S, Agarwal G, Kabra SK, et al. Does 3-day course of oral amoxycillin benefit children of non-severe pneumonia with wheeze: a multicentric randomised controlled trial. PLoS One. 2008;3:e1991. doi: 10.1371/journal.pone.0001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayieko P, English M. Case management of childhood pneumonia in developing countries. Pediatr Infect Dis J. 2007;26:432–440. doi: 10.1097/01.inf.0000260107.79355.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- Cardoso MR, Nascimento-Carvalho CM, Ferrero F, et al. Adding fever to WHO criteria for diagnosing pneumonia enhances the ability to identify pneumonia cases among wheezing children. Arch Dis Child. 2011;96:58–61. doi: 10.1136/adc.2010.189894. [DOI] [PubMed] [Google Scholar]
- Craig JC, Williams GJ, Jones M, et al. The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: prospective cohort study of 15 781 febrile illnesses. BMJ. 2010;340:c1594. doi: 10.1136/bmj.c1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran WG. Sampling techniques. 3rd ed. New York: John Wiley & Sons; 1977. [Google Scholar]
- Forgie IM, Campbell H, Lloyd-Evans N, et al. Etiology of acute lower respiratory tract infections in children in a rural community in The Gambia. Pediatr Infect Dis J. 1992;11:466–473. doi: 10.1097/00006454-199206000-00009. [DOI] [PubMed] [Google Scholar]
- Ghafoor A, Nomani NK, Ishaq Z, et al. Diagnoses of acute lower respiratory tract infections in children in Rawalpindi and Islamabad, Pakistan. Reviews of Infectious Diseases. 1990;12(Suppl-14) doi: 10.1093/clinids/12.supplement_8.s907. [DOI] [PubMed] [Google Scholar]
- Hazir T, Fox LM, Nisar YB, et al. Ambulatory short-course high-dose oral amoxicillin for treatment of severe pneumonia in children: a randomised equivalency trial. Lancet. 2008;371:49–56. doi: 10.1016/S0140-6736(08)60071-9. [DOI] [PubMed] [Google Scholar]
- Hazir T, Nisar YB, Abbasi S, et al. Comparison of oral amoxicillin with placebo for the treatment of world health organization defined non-severe pneumonia in children aged 2–59 months: a multicenter, double-blind, randomized, placebo-controlled trial in Pakistan. Clinical Infectious Diseases. 2011;52:293–300. doi: 10.1093/cid/ciq142. [DOI] [PubMed] [Google Scholar]
- Juven T, Mertsola J, Waris M, et al. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr.Infect.Dis.J. 2000;19:293–298. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- Liu YC, Huang WK, Huang TS, Kunin CM. Detection of antimicrobial activity in urine for epidemiologic studies of antibiotic use. J.Clin.Epidemiol. 1999;52:539–545. doi: 10.1016/s0895-4356(99)00027-x. [DOI] [PubMed] [Google Scholar]
- Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat.Med. 2004;10:811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nga NN, Chai SK, Bihn TT, et al. ISAAC-based asthma and atopic symptoms among Ha Noi school children. Pediatr.Allergy Immunol. 2003;14:272–279. doi: 10.1034/j.1399-3038.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Pepin J, Demers AM, Mberyo-Yaah F, et al. Acute lower respiratory infections among children hospitalized in Bangui, Central African Republic: toward a new case-management algorithm. Trans.R.Soc.Trop.Med.Hyg. 2001;95:410–417. doi: 10.1016/s0035-9203(01)90199-3. [DOI] [PubMed] [Google Scholar]
- Qazi SA, Rehman GN, Khan MA. Standard management of acute respiratory infections in a children's hospital in Pakistan: impact on antibiotic use and case fatality. Bulletin of the World Health Organization. 1996;74:501–507. [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Hossain S, Baqui AH, et al. Haemophilus influenzae type-b and non-b-type invasive diseases in urban children (<5years) of Bangladesh: implications for therapy and vaccination. J.Infect. 2008;56:191–196. doi: 10.1016/j.jinf.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Saha SK, Naheed A, el Arifeen S, et al. Surveillance for invasive Streptococcus pneumoniae disease among hospitalized children in Bangladesh: antimicrobial susceptibility and serotype distribution. Clin.Infect.Dis. 2009;48(Suppl 2):S75–S81. doi: 10.1086/596544. [DOI] [PubMed] [Google Scholar]
- Singhi S, Jain V, Gupta G. Pediatric emergencies at a tertiary care hospital in India. J.Trop.Pediatr. 2003;49:207–211. doi: 10.1093/tropej/49.4.207. [DOI] [PubMed] [Google Scholar]
- Song JH, Jung SI, Ko KS, et al. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study) Antimicrob.Agents Chemother. 2004;48:2101–2107. doi: 10.1128/AAC.48.6.2101-2107.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MW, Mulholland EK, Greenwood BM. Respiratory syncytial virus infection in tropical and developing countries. Tropical Medicine & International Health. 1998;3:268–280. doi: 10.1046/j.1365-3156.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Geneva: World Health Organization; 1991. Technical bases for the WHO recommendations on management of pneumonia in children at first level health facilities. [WHO/ARI/91.20] [Google Scholar]
- World Health Organization. Geneva: World Health Organization; 2005a. Pocket book of hospital care for children: guidelines for the management of common illnesses with limited resources. [Google Scholar]
- World Health Organization. Child and Adolescent Health. Geneva: World Health Organization; 2005b. Technical updates of the guidelines on the Integrated Management of Childhood Illness (IMCI): evidence and recommendations for further adaptations. in press. [Google Scholar]
- Zeitz PS, Harrison LH, Lopez M, Cornale G. Community health worker competency in managing acute respiratory infections of childhood in Bolivia. Bull Pan Am Health Organ. 1993;27:109–119. [PubMed] [Google Scholar]