Abstract
Investigators have administered the opioid receptor antagonist, naloxone, to interrogate the hypothalamic-pituitary-adrenal (HPA) axis response under the assumption that this technique provides a measure of endogenous opioid activity. However it has never been tested whether provocation of the HPA axis with naloxone provides a surrogate marker for direct measurement of endogenous opioid activity using PET imaging as the gold standard. To test this hypothesis, eighteen healthy subjects underwent a PET scan with the mu-opioid receptor (MOR) selective ligand [11C]carfentanil (CFN). The following day ACTH and cortisol responses were assessed using a technique which allows administration of 5 incremental doses of naloxone (0, 25, 50, 100 and 250 µg/kg) in a single session. Relationships between ACTH and cortisol responses and [11C]CFN binding potential (BPND) were examined in 5 brain regions involved in the regulation of the HPA axis and/or regions with high concentrations of MOR. All subjects mounted graded ACTH and cortisol responses to naloxone administrations. There were significant negative relationships between cortisol response to naloxone and [11C]CFN BPND in ventral striatum, putamen and caudate. When sex and smoking were added as covariates to the model, these correlations were strengthened and there was a significant correlation with the hypothalamus. There were no significant correlations between ACTH and any volumes of interest. The opioid receptor antagonist naloxone is not merely a non-specific pharmacologic activator of the HPA axis; it provides information about individual differences in opioid receptor availability.
Keywords: mu opioid receptors, beta-endorphin, naloxone, PET imaging, HPA axis, cortisol
Introduction
Endogenous opioid systems regulate myriad nociceptive and other homeostatic processes. Genetic and environmental factors modulate endogenous opioid systems, and may result in physical and behavioral symptoms as well as chronic neuropsychiatric disorders. For example, mu opioid receptor (MOR) expression is altered in several classes of disorders including substance use disorders, chronic pain and eating disorders (Bencherif et al, 2002; Bencherif et al, 2004; Heinz et al, 2005; Gorelick et al, 2008; Williams et al, 2009).
In the pursuit of understanding the pathophysiologic role of opioid systems in these and other neuropsychiatric illnesses, investigators have administered the opioid receptor antagonist, naloxone, to interrogate the HPA axis. The assumption has been that this challenge provides a measure of endogenous opioid activity (Russell et al, 2008; Adinoff et al, 2005; Wand et al, 1998; Alexander et al, 1995; Torpy et al, 1993). Naloxone administration triggers HPA activity by blocking opioid inhibitory tone directed at hypothalamic regulators of ACTH secretion; ACTH, in turn, stimulates cortisol release. Using naloxone in this manner, studies have presumed to identify differences in opioid activity as a function of alcoholism, family history of alcoholism, gender, neuroticism and genetic variations in the mu opioid receptor (Adinoff et al, 2005; Wand et al, 1998; Wand et al, 1999; Mangold et al, 2000; Wand et al, 2001; Wand et al, 2002; Wand et al, 2006; Oswald et al, 2004; Uhart et al, 2006).
The goal of this study was to examine whether naloxone is merely a non-specific pharmacologic activator of the HPA axis, providing information about ACTH and cortisol secretory capacity, or whether individual differences in hormone responses to naloxone are influenced by individual variations in opioid receptor availability. We suspected the latter explanation because we have previously shown that, within an individual, cortisol response to the biological provocator naloxone do not correlate with cortisol response to a psychological provocator, the Trier Psychosocial Stress Test (TSST) (Oswald et al, 2004). Additionally, we have shown that while men have a greater cortisol response to the TSST than women (Oswald et al, 2004), women have a greater cortisol response to naloxone administration than men (Uhart et al, 2006). Both observations suggest that naloxone activates the HPA axis through mechanisms independent from those activated by mental stress. Thus, the challenge procedure using naloxone may provide more specific information about the status of opioidergic systems than previously realized.
The assumption that studying ACTH and/or cortisol responses to a naloxone challenge provides a surrogate marker for direct measurement of endogenous opioid activity has never been empirically tested. Therefore, we conducted a study to examine the relationship between PET-derived measurements of mu opioid receptor availability and naloxone-induced ACTH and cortisol secretion in healthy subjects. We hypothesized that hormone responses to opioid receptor blockade by naloxone would be correlated with mu opioid receptor availability measured by PET using [11C]carfentanil (CFN).
Methods
Subjects
Healthy male and female subjects (n=18) between 25 and 58 years of age were recruited via advertisement and provided informed consent using an Institutional Review Board approved informed consent document. Subjects underwent a history and physical examination by a physician or nurse practitioner; screening labs were obtained including a pregnancy test in females. Subjects were interviewed by a Masters-level research assistant who utilized the Semi-Structured Assessment of the Genetics of Alcoholism (SSAGA-II) to rule out major DSM IV Axis 1 psychiatric disorders (Bucholz et al, 1994). Assessment results were reviewed and study eligibility determined by author MEM. Subjects were drinking within the NIAAA recommended guidelines (less than 8 drinks/wk for women and 15 drinks/week for men). Individuals were excluded from study participation based any on the following criteria: 1) if they met current or lifetime DSM-IV diagnostic criteria for any major Axis I disorder including alcohol, nicotine and other drug abuse/dependence, 2) if urine drug toxicology was positive at screening or on session days, 3) if they had other ongoing health problems or were taking prescription medication, 4) if screening CBC or liver studies were abnormal, 5) if pregnancy test was positive or if subjects were taking hormonal birth control. Two of the 18 subjects were occasional smokers, and were not nicotine dependent based on the SSAGA and The Fagerstrom Nicotine Dependence Test (Heatherton et al, 1991).
General Procedures
Subjects were admitted to the Clinical Research Unit (CRU) the day before the PET scan for a 3-day inpatient stay. Participants were screened for recent alcohol and drug use and pregnancy status at the time of admission and were closely supervised throughout the inpatient stay. No smoking was permitted for the duration of the study. Prior to the CRU admission, subjects underwent magnetic resonance imaging (MRI) to allow anatomical localization and alignment of PET imaging planes within subjects (Meltzer et al, 1990).
PET Procedures
On the morning of the PET scan, subjects were provided a calorie-controlled breakfast. A thermoplastic mask was individually fitted to each subject’s face for immobilization and positioning during imaging. Subjects underwent a PET scan with the mu-opioid selective ligand [11C]carfentanil (CFN) (Lever et al, 1992; Madar et al, 1996).
PET scans were acquired in 3D mode on a GE Advance PET scanner (GE Medical Systems, Milwaukee, WI). Before injection of the radiotracer a transmission scan of 10-min duration was obtained using rotating germanium-68 rods. After intravenous bolus administration of the radiotracer [11C]CFN (19.9±1.2 mCi SA: 17,298 ± 13,907 mCi/µmole), 25 images with variable time intervals (6×30 sec, 5×60 sec, 5×120 sec, 9×480 sec) were acquired during a 90-min period for each subject. The dose of carfentanil injected was less than 0.04 µg/kg body weight and did not produce agonist effects. PET images were reconstructed using the back projection algorithm with a ramp filter using the software provided by the manufacturer that corrects for attenuation, scatter, and dead-time (Kinahan and Rogers, 1989). The radioactivity was corrected for physical decay to the injection time. Each PET frame consisted of a 128×128×35 matrix with voxel size of 2×2×4.25 mm in a spatial resolution of 5.5 and 6.1 mm full-width-at-half-maximum (FWHM) in the radical and tangential directions, respectively, at 10 cm radius from the center of the field-of-view.
VOIs were limited in this study to the hypothalamus, hippocampus, ventral striatum, caudate nucleus and putamen. These regions were selected for analysis because they are involved in regulation of the HPA axis (hypothalamus and hippocampus), contain high concentration of MORs and/or are implicated as playing a role in neuropsychiatric disorders. The VOIs were manually defined on SPGR MRI for putamen, caudate nucleus, and hippocampus using a locally developed VOI defining tool. VOIs for ventral striatum and hypothalamus were customized using a VOI template (Mazziotta et al, 1995; Hammers et al, 2003) available at http://www.loni.ucla.edu. VOIs were transferred to PET space according to the MRI-to-PET co-registration parameters obtained with the SPM2 coregistation module and applied to individual PET frames to obtain time-radioactivity curves (TACs) of VOIs.
The primary dependent variable of interest was [11C]CFN (BPND) (Innis et al, 2007). BPND provides an estimate of the product of the density of available receptors (Bmax' or the receptor density Bmax less those occupied by endogenous transmitters) and the affinity (1/KD). Reference tissue graphical analysis (RTGA)(Logan et al, 1996) was used for [11C]CFN with occipital lobe as the reference region and setting the brain-to-blood clearance rate constant of the reference region (k2R) at 0.104 min−1 (Frost et al, 1990; Endres et al, 2003). Binding potential (BPND) estimates using RTGA are highly correlated with those obtained from the arterial input-based kinetic model (Endres et al, 2003).
Naloxone Cumulative Dosing Procedure
Using our previously published procedure (Mangold et al, 2000), a dose response curve to naloxone was generated in a single session on the day following the PET scan. Following a calorie controlled lunch, participants had an intravenous catheter inserted into a forearm vein at 1200h. Baseline blood samples were obtained −30 min, −15 min and immediately prior to placebo administration. At 1300h (time 0), placebo (0.9% saline) was administered as a bolus. Sequential doses of naloxone (25, 50, 100 and 250 ug/kg) dissolved in 0.9% saline were administered every 30 min thereafter. Blood samples were obtained 15 and 30 min after placebo and each naloxone dose, and then every 30 min through 240 min. Plasma cortisol concentrations were assayed by radioimmunoassay (Diagnostic Products Corporation, Inc.; Los Angeles, CA). Intra-assay and inter-assay coefficients of variance were less than 8%. Plasma ACTH concentrations were measured by the DiaSorin immunoradiometric assay. Intra-assay and inter-assay coefficients of variance were 6% and 9.2%, respectively, for plasma ACTH.
Statistical Plan
Mean ACTH and cortisol time series were plotted to visualize hormone responses. To compare hormone levels at various time points to the placebo level, a linear mixed model with random intercept was constructed. Hormone measurements were log transformed after distribution was checked. An unstructured covariance matrix was used to obtain robust standard errors.
To model the correlation between hormone response and [11C]CFN BPND, we first calculated cortisol and ACTH response area under the curve (AUC). AUC was calculated as the sum of the trapezoids between every pair of consecutive time points subtracting baseline hormone level. Calculated AUC value had units of µg/dl • hour for cortisol and pg/ml • hour for ACTH. The hormone response AUC for the time period from time 0 through time 240 minutes was calculated as the main outcome. We also calculated cortisol response AUCs for each naloxone dose using the area from the time of dose administration to immediately before the next dose was given. A multi-linear model was constructed with BPND as the dependent variable and hormone response AUC as the independent variable for each of the brain regions of interest. Sex can influence naloxone-induced hormone responses and [11C]CFN BPND (Uhart et al, 2006), thus was added as a covariate to the model. Additionally, two subjects were non-nicotine dependent occasional smokers. Therefore the model included sex and smoking status (smoker/non-smoker) as covariates. Although age has previously been reported to modify [11C]CFN BPND (Zubietta et al, 1999), there was not an association between age and[11C]CFN BPND in this data set. We also performed a sensitivity analysis to test models with or without age as a covariate. The significance between [11C]CFN BPND and hormone response remained the same, so age was not included in the final model. Across VOIs, the obtained p values of correlations between hormone AUC and BPND were adjusted using the adaptive step-up Bonferroni method for multiple comparisons (Hochberg and Benjamini, 1990). An adjusted p-value less than 0.05 was considered statistically significant. All the analyses were carried out using SAS 9.2.
Results
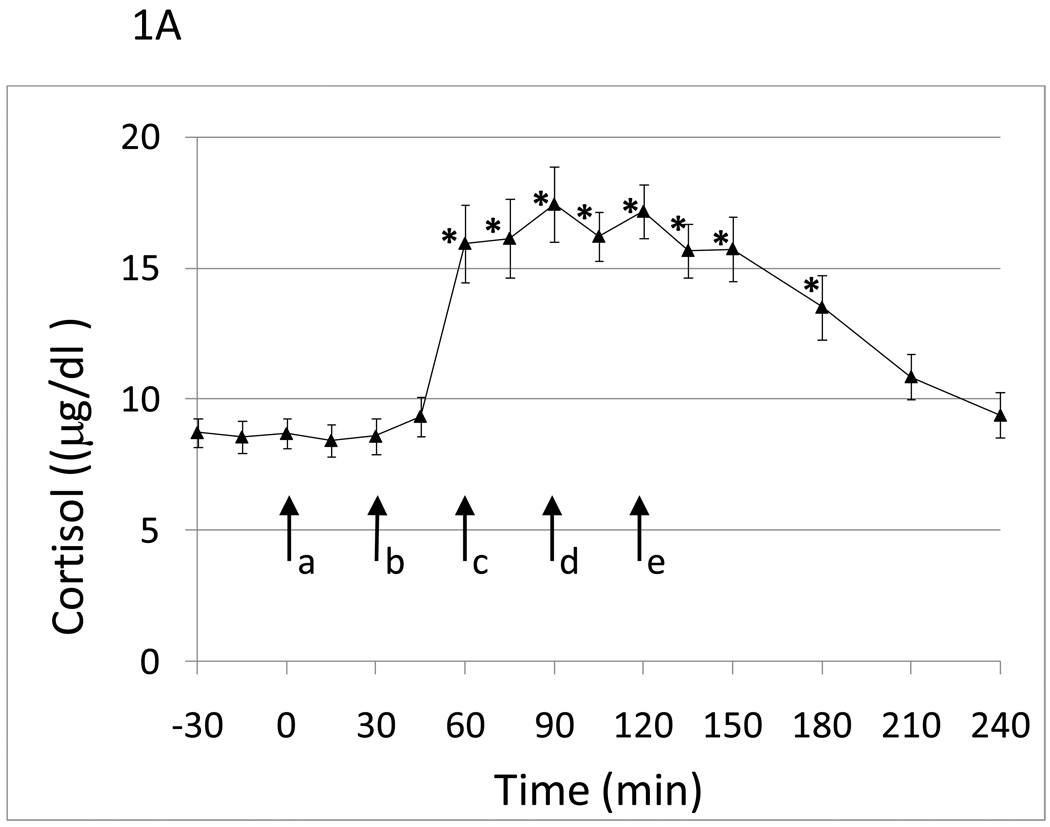
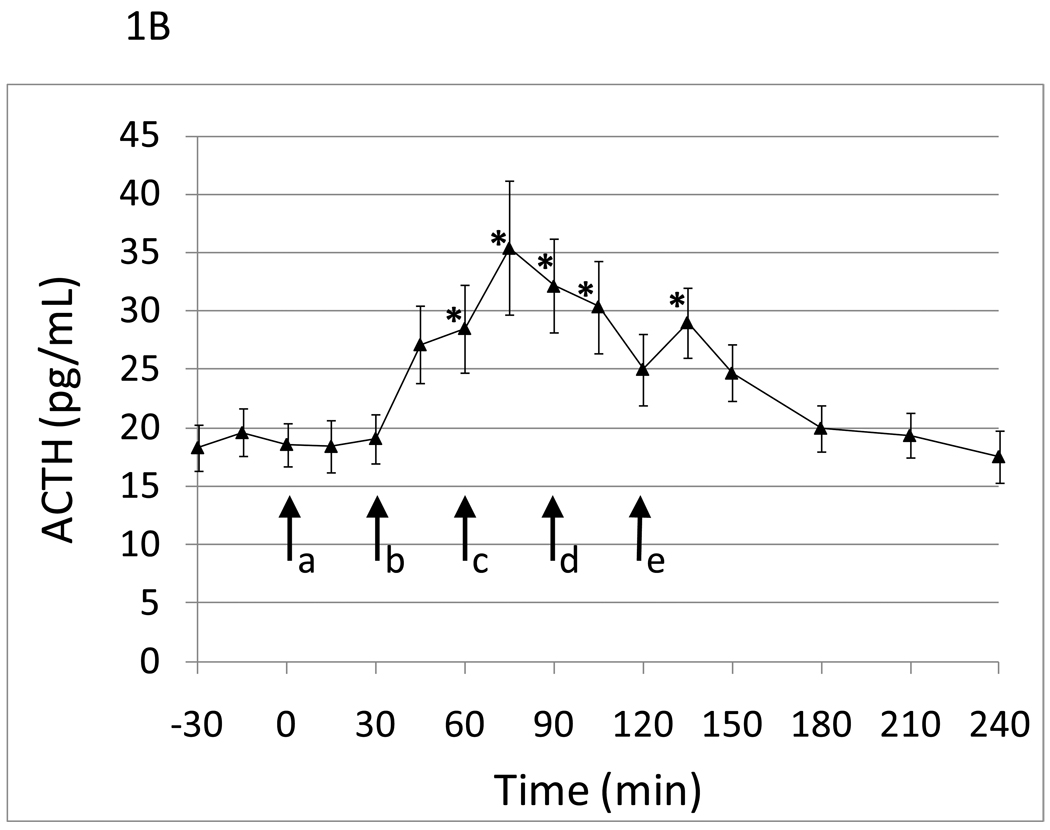
Eighteen healthy white (n=11) and black subjects of both sexes (males, n=11) were studied with an average age of 47.1 (SD 8.8). Within-session cumulative naloxone dose administration induced statistically significant graded cortisol and ACTH responses (Figure 1A and B). Cortisol and ACTH responses peaked following the 50µg/kg dose of naloxone. There was an approximate 2 fold increase in cortisol and 1.75 fold increase in ACTH levels in response to naloxone administration. There was greater variability in the ACTH response compared to the cortisol response to naloxone.
Figure 1.
Hormone responses to five graded doses of naloxone as a function of time. a, placebo; b, 25 µg/kg naloxone; c, 50 µg/kg naloxone; d, 100 µg/kg naloxone; e., 250 µg/kg naloxone. Data points are mean (SEM). A) Cortisol. *time points significantly different from mean of placebo time point with p<.05. adjusting for sex and smoking status. B) ACTH. *time points significantly different from mean of placebo time point with p<.05. adjusting for sex and smoking status.
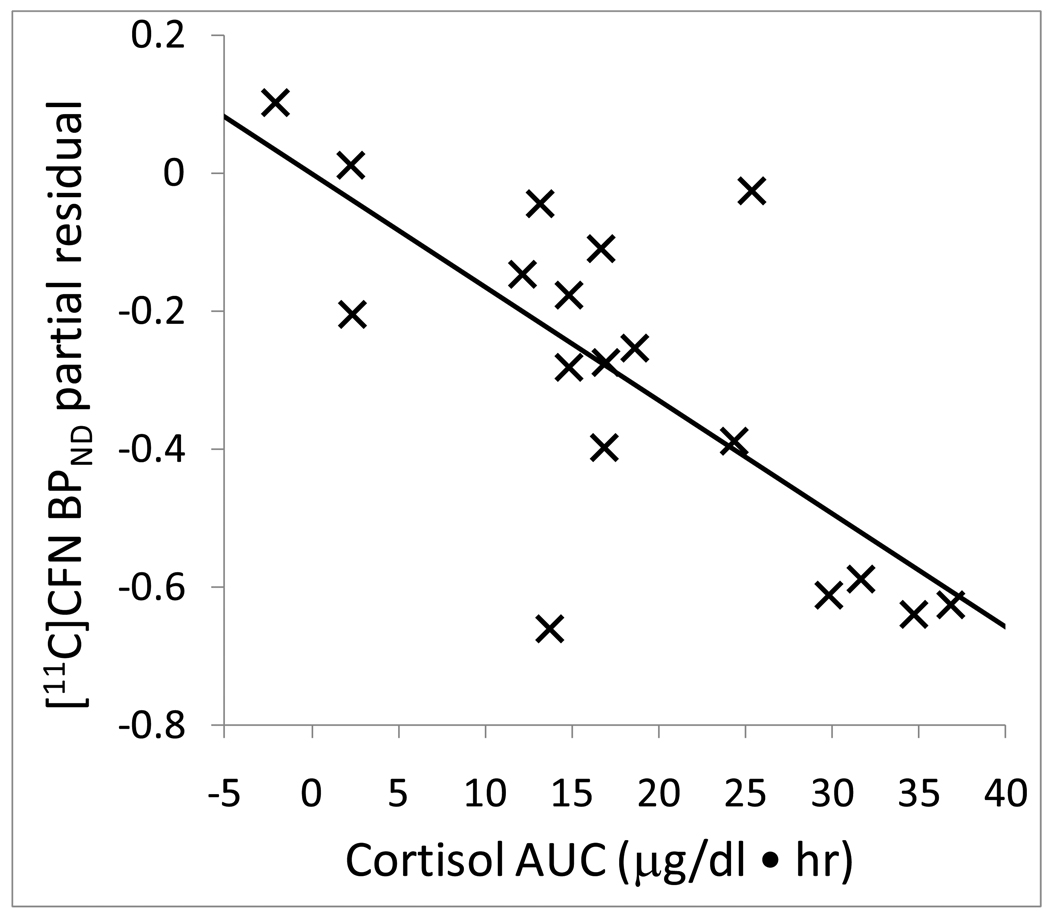
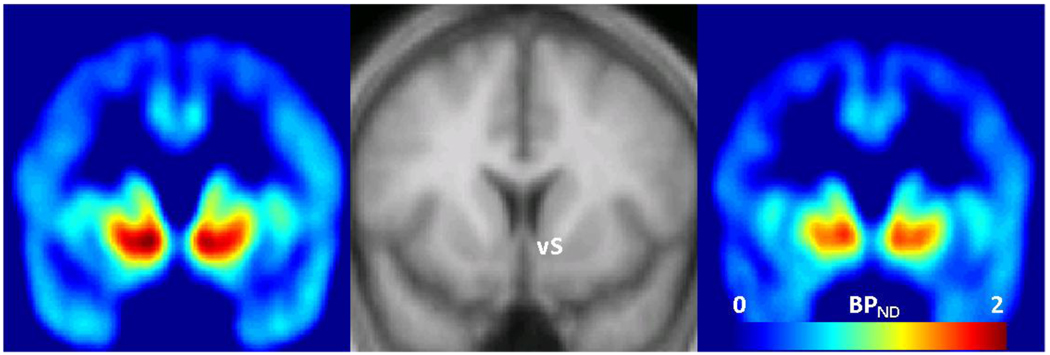
Mean [11C]CFN BPND across VOIs is shown in Table 1. The highest [11C]CFN BPND was measured in the ventral striatum and the lowest level was measured in the hippocampus. With or without adjusting for covariates there were no significant correlations between baseline cortisol or ACTH levels (mean of −30, −15 and 0 time points) and [11C]CFN BPND in any volume of interest. Without adjusting for sex and smoking, there were significant negative correlations between area under the cortisol response curve and [11C]CFN BPND in the ventral striatum, putamen and caudate. When sex and smoking were added as covariates to the model, these relationships were strengthened, and there was also a significant correlation with the hypothalamus (Table 2). As an example of these relationships, the partial residual plot shows the association between [11C]CFN BPND in the ventral striatum and cortisol responses to naloxone after adjusting for sex and smoking status (Figure 2). Figure 3 shows brain images of higher mean [11C]CFN BPND in the ventral striatum for the subjects in the lowest cortisol response tertile (left panel) compared to subjects in the highest cortisol response tertile (right panel).
Table 1.
Mean and standard deviation of [11C]CFN BPND
| Region | [11C]CFN BPND (N=18) | |
|---|---|---|
| Mean | Std dev | |
| Hypothalamus | 1.216 | 0.459 |
| Hippocampus | 0.130 | 0.083 |
| Ventral Striatum | 1.405 | 0.272 |
| Caudate | 1.121 | 0.212 |
| Putamen | 1.002 | 0.170 |
Table 2.
Correlation of cortisol AUC (0 minute to 240 minute) response to naloxone and [11C]CFN BPND adjusted by sex and smoking. The adaptive step-up Bonferroni method was used to adjust P values for multiple comparisons.
| Region | Partial correlation coefficient |
Adjusted P |
|---|---|---|
| Hypothalamus | −0.557 | 0.024 |
| Hippocampus | −0.442 | 0.080 |
| Ventral Striatum | −0.619 | 0.010 |
| Caudate | −0.648 | 0.006 |
| Putamen | −0.599 | 0.014 |
Figure 2.
Partial residual plot of [11C]CFN BPND in ventral striatum (vS) with cortisol response AUC (0–240 min) adjusted for sex and smoking. Statistics are displayed in Table 2.
Figure 3.
Coronal view images of [11C]CFN BPND, averaged across the 6 subjects in the lowest tertile of cortisol responses (left) compared to the 6 subjects in the highest tertile of cortisol responses (right). A standard MRI to which BPND images were spatially normalized is displayed in the middle panel to indicate image locations around the anterior-posterior center of ventral striatum (vS). Colored legend depicts [11C]CFN BPND from 0 (light blue) to 2.0 (red).
We then examined the relationship of [11C]CFN BPND and area under the cortisol response curve following each of the naloxone doses to evaluate whether the entire cumulative dosing procedure was required to identify significant correlations with brain regions of interest or whether significant correlations could be identified with fewer naloxone doses. While some statistically significant results were observed for AUCs of individual doses, results were not consistent across regions, and the associations were weaker than those obtained using the entire AUC curve. As shown in Table 3, there was considerable variability in the amount of naloxone required to obtain peak cortisol response across study participants.
Table 3.
The number of subjects reaching peak cortisol response at each naloxone dose. Timepoints for cortisol measurements are shown in parenthesis after each dose.
| Peak cortisol | Number of Subjects |
|---|---|
| placebo (30 min) | 1 |
| Dose 25µg (45, 60 min) | 4 |
| Dose 50µg (75, 90 min) | 8 |
| Dose 100µg (105, 120 min) | 2 |
| Dose 250µg (135, 150) | 3 |
No significant correlations were found for ACTH AUC and [11C]CFN BPND in any VOI.
Discussion
The primary regulators of ACTH secretion, and thus cortisol, are the CRF neurons in the paraventricular nucleus of the hypothalamus, and these neurons are under opioid inhibitory tone. This study observed that naloxone-induced cortisol secretion correlated with [11C]CFN BPND measured in hypothalamus as well as with several mesostriatal brain regions of healthy subjects. Higher [11C]CFN BPND was associated with lower cortisol responses to opioid receptor blockade. Higher [11C]CFN BPND indicates either decreased endogenous beta-endorphin occupancy or increased mu opioid receptor number/affinity compared to lower [11C]CFN BPND; the scanning technique cannot distinguish among these possibilities. However, the negative correlation makes the most sense if subjects with higher binding potential in this study have lower endogenous beta-endorphin occupancy and thus place less inhibitory tone on the HPA axis. Under this assumption there would be lower levels of cortisol induced by opioid blockade in subjects with high MOR BP (e.g., low opioid inhibitory tone) compared to subjects with low MOR BP (e.g., high opioid inhibitory tone).
We identified significant correlations between [11C]CFN BPND and naloxone-induced cortisol responses not only in the hypothalamus but also with ventral and dorsal striatum. The correlation of cortisol with [11C]CFN BPND in the ventral striatum is particularly important since opioids modulate dopaminergic transmission in the nucleus accumbens, and thus influence reinforcement/reward to both internal and external stimuli (Wise, 2008). This system is crucial in understanding the neurobiology of alcohol dependence and other drug dependencies (Barson et al, 2010). It may also be dysfunctional in eating disorders, gambling and other “impulse” disorders (Berridge et al, 2010).
We have previously shown that cortisol response following mental stress as well as amphetamine administration correlates with mesolimbic dopamine release and positive hedonic responses to amphetamine (Wand et al, 2007; Oswald et al, 2005). Preclinical studies have shown that glucocorticoids can amplify the dopaminergic signal in the nucleus accumbens (Marinelli and Piazza, 2002). The interaction of cortisol and the endogenous opioid system observed in this study may point to a mechanism whereby cortisol can alter mesolimbic dopamine release through individual differences in opioid activity induced by genetic and/or environmental determinants (Wand, 2008).
When adjusted for sex and smoking status, there was a trend for a correlation of naloxone-induced cortisol AUC and [11C]CFN BPND in the hippocampus. This structure is an important region modulating cortisol dynamics though positive and negative feedback loops (Herman et al, 2005). Recent studies have also shown involvement of the opioidergic systems in both the dorsal and ventral hippocampus in modulating anxiety-like behaviors and stress (Solati et al, 2010; Zarrindast et al, 2008).
It is not clear why ACTH AUC was not associated with [11C]CFN BPND since it is ACTH that stimulates cortisol release. One possible explanation is that cortisol negative feedback truncates the ACTH response to naloxone, and therefore a full ACTH response cannot be realized. This explanation is supported by the more rapid return of ACTH than cortisol to baseline level s (see Figure 1). Another possibility relates to the short half-life of ACTH in plasma. There may not have been frequent enough blood samplings to capture the true area under the ACTH curve. Failure of naloxone-induced cortisol to predict [11C]CFNBPND in all brain regions indicates that the technique is useful for only a subset of brain regions. Future studies should better define the brain regions where this technique will be informative and also the mechanism that allows its predictive ability. It should also be mentioned that the naloxone challenge may only be useful for characterizing mu opioid receptor availability when it is executed as a cumulative dosing procedure (Mangold et al, 2000) since cortisol responses to individual doses of naloxone did not consistently correlate with [11C]CFNBPND across brain regions. This may be the result of the marked individual differences in the amount of naloxone needed to obtain peak cortisol response. Last, the most robust correlations were observed when adjusting for sex and smoking, two factors with known effects on the HPA axis.
In summary, cortisol responses obtained using our cumulative naloxone challenge procedure were associated with [11C]CFN BPND in healthy subjects in several important brain regions involved in alcohol and drug reward, substance use disorders and other psychiatric and impulse control disorders. Future studies will examine for additional utility of the technique by performing similar correlations in populations with neuropsychiatric disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, Williams MJ. Dissection of hypothalamic-pituitary-adrenal pathology in one-month abstinent alcohol-dependent men: Response to CRH and Naloxone. Alcohol: Clin. Exp. Res. 2005;29:528–537. doi: 10.1097/01.ALC.0000158939.25531.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SL, Irvine CH. The effect of naloxone administration oon the secretion of corticotrophin-releasing hormone, arginine vasopressin, and adrenocorticotropin in unperturbed horses. Endocrinology. 1995;136(11):5139–5147. doi: 10.1210/endo.136.11.7588252. [DOI] [PubMed] [Google Scholar]
- Barson JR, Carr AJ, Soun JE, Sobhani NC, Rada P, Leibowitz SF, Hoebel BG. Opioids in the hypothalamic paraventricular nucleus stimulate ethanol intake. Alcohol: Clin. Exp. Res. 2010;34(2):214–222. doi: 10.1111/j.1530-0277.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencherif B, Wand GS, McCaul ME, Kim YK, Ilgin N, Dannals RF, Frost JJ. Mu-opoid receptor binding measured by [11C]carfentanil positron emission tomography is related to craving and mood in alcohol dependence. Boil Psychiatry. 2004;55:255–262. doi: 10.1016/j.biopsych.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Bencherif B, Fuchs PN, Sheth R, Dannals RF, Campbell JN, Frost JJ. Pain activation of human supraspinal opioid pathways as demonstrated by [11C]-carfentanil and positron emission tomography (PET) Pain. 2002;99(3):589–598. doi: 10.1016/S0304-3959(02)00266-X. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, Difeliceantonio AG. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010 doi: 10.1016/j.brainres.2010.04.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Xu M, Chavkin C. Repeated swim stress induces kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2. Neuroreport. 2008 Sep 17;19(14):1417–1422. doi: 10.1097/WNR.0b013e32830dd655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J. Stud. Alchohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Endres CJ, Bencherif B, Hilton J, Madar I, Frost JJ. Quantification of brain mu-opioid receptors with [11C]carfentanil: reference-tissue methods. Nucl. Med. Biol. 2003;30:177–186. doi: 10.1016/s0969-8051(02)00411-0. [DOI] [PubMed] [Google Scholar]
- Frost JJ, Mayberg HS, Sadzot B, Dannals RF, Lever JR, Ravert HT, Wilson AA, Wagner HN, Jr, Links JM. Comparison of [11C]diprenorphine and [11C]carfentanil binding to opiate receptors in humans by positron emission tomography. J. Cereb. Blood Flow Metab. 1990;10:484–492. doi: 10.1038/jcbfm.1990.90. [DOI] [PubMed] [Google Scholar]
- Frost JJ, Wagner HN, Jr, Dannals RF, Ravert HT, Links JM, Wilson AA, Burns HD, Wong DF, McPherson RW, Rosenbaum AE. Imaging opiate receptors in the human brain by positron tomography. J. Comput. Assist. Tomogr. 1985;9:231–236. doi: 10.1097/00004728-198503000-00001. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino ML, Dannals RF, Frost JJ. Brain mu-opioid receptor binding: relationship to relapse to cocaine use after monitored abstinence. Psychopharmacology (Berl) 2008;200(4):475–486. doi: 10.1007/s00213-008-1225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, Mitchell TN, Brooks DJ, Duncan JS. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum. Brain Mapp. 2003;19:224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br. J. Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Reimold M, Wrase J, Hermann D, Croissant B, Mundle G, Dohmen BM, Braus DF, Schumann G, Machulla HJ, Bares R, Mann K. Correlation of stable elevations in striatal mu-opioid receptor availability in detoxified alcoholic patients with alcohol craving: a positron emission tomography study using carbon 11-labeled carfentanil. Arch. Gen. Psychi. 2005;62(1):57–64. doi: 10.1001/archpsyc.62.1.57. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y. More Powerful Procedures for Multiple Significance Testing,”. Statistics in Medicine. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J. Cereb. Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Kinahan PE, Rogers JG. Analytic 3D image reconstruction using all detected events. Nuc. Sci., IEEE Transactions on. 1989;36:964–968. [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010 Feb 16;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J. Cereb. Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Mangold D, Wand G. Cortisol and ACTH responses to naloxone in subjects with high and low neuroticism. Biol. Psychi. 2006;60:850–855. doi: 10.1016/j.biopsych.2006.03.049. [DOI] [PubMed] [Google Scholar]
- Mangold D, McCaul M, Ali M, Wand GS. Generating a dose response curve to naloxone in a single session. Biol. Psychi. 2000;48:310–314. doi: 10.1016/s0006-3223(00)00885-4. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur. J. Neurosci. 2002;16(3):387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography. Park II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J. Cereb. Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The international Consortium for Brain Mapping (ICBM) Neuroimage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Bryan RN, Holcomb HH, Kimball AW, Mayberg HS, Sadzot B, Leal JP, Wagner HN, Jr, Frost JJ. Anatomical localization for PET using MR imaging. J. Comput. Assist. Tomogr. 1990;14:418–426. doi: 10.1097/00004728-199005000-00019. [DOI] [PubMed] [Google Scholar]
- Oswald L, Wand G. Opioids and Alcohol. Physiol Behav. 2004;81(2):339–358. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Mathena JR, Wand GS. Comparison of HPA axis hormonal responses to naloxone vs psychologically-induced stress. Psychoneuroendocrinol. 2004;29(3):371–388. doi: 10.1016/s0306-4530(03)00048-9. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, Brasic J, Wand GS. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychophar. 2005;30:821–832. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- Russell JA, Douglas AJ, Brunton PJ. Reduced hypothalamo-pituitary-adrenal axis stress responses in late pregnancy: central opioid inhibition and noradrenergic mechanisms. Ann. N.Y. Acad. Sci. 2008;1148:428–438. doi: 10.1196/annals.1410.032. [DOI] [PubMed] [Google Scholar]
- Schluger JH, Ho A, Borg PL, Porter M, Maniar S, Gunduz M, Perret G, King A, Kreek MJ. Nalmefene causes greater HPA axis activation than naloxone in normal volunteers: implications for the treatment of alcoholism. Alcohol Clin Exp Res. 1998;22(7):1430–1436. doi: 10.1111/j.1530-0277.1998.tb03931.x. [DOI] [PubMed] [Google Scholar]
- Solati J, Zarrindast MR, Salari AA. Dorsal hippocampal opioidergic system modulates anxiety-like behaviors in adult male Wistar rats. Psychiatry Clin Neurosci. 2010 Oct 28; doi: 10.1111/j.1440-1819.2010.02143.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Torpy DJ, Grice JE, Hockings GI, Walters MM, Crosbie GV, Jackson RV. Alprazolam blocks the naloxone-stimulated hypothalamo-pituitary-adrenal axis in man. J. Clin. Endocrinol. Metab. 1993;76(2):388–391. doi: 10.1210/jcem.76.2.8381800. [DOI] [PubMed] [Google Scholar]
- Uhart M, Chong R, Oswald L, Ping L, Wand GS. Gender differences in hypothalamic-pituitary-adrenal (HPa) axis reactivity. Psychoneuroendocrinol. 2006;31(5):642–652. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Wand GS. The influence of stress on the transition from drug use to addiction. National Institutes of Health’s Alcohol Health Res. 2008;31(2):119–136. [PMC free article] [PubMed] [Google Scholar]
- Wand GS, Oswald LM, McCaul ME, Wong DF, Johnson E, Zhou Y, Kuwabara H, Kumar A. Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology. 2007;32(11):2310–2320. doi: 10.1038/sj.npp.1301373. [DOI] [PubMed] [Google Scholar]
- Wand GS, Mangold D, Ali M. Adrenocorticotropin response to naloxone in sons of alcohol dependent men. J. Clin. Endocrinol. Metab. 1999;84:64–68. doi: 10.1210/jcem.84.1.5373. [DOI] [PubMed] [Google Scholar]
- Wand GS, Mangold D, El Diery S, McCaul M, Hoover D. Family history of alcoholism and hypothalamic opioidergic activity. Arch. Gen. Psychi. 1998;55:1114–1119. doi: 10.1001/archpsyc.55.12.1114. [DOI] [PubMed] [Google Scholar]
- Wand GS, McCaul M, Gotjen D, Reynolds J, Lee S. Confirmation that offspring from families with alcohol dependent individuals have greater HPA axis activation-induced by naloxone compared to offspring without a family history of alcohol dependence. Alcohol: Clin. Exp. Res. 2001;25:1134–1139. [PubMed] [Google Scholar]
- Wand GS, McCaul M, Gotjen D, Reynolds J, Lee S. The mu opioid receptor gene polymorphism A(118)G alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacol. 2002;26:106–114. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Williams TM, Davies SJ, Taylor LG, Daglish MR, Hammers A, Brooks DJ, Nutt DJ, Lingford-Hughes A. A Brain opioid receptor binding in early abstinence from alcohol dependence and relationship to craving: an [11C]diprenorphine PET study. Eur. Neuropsychopharmacol. 2009;19:740–748. doi: 10.1016/j.euroneuro.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox. Res. 2008;14(2–3):169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast MR, Babapoor-Farrokhran S, Babapoor-Farrokhran S, Rezayof A. Involvement of opioidergic system of the ventral hippocampus, the nucleus accumbens or the central amygdala in anxiety-related behavior. Life Sci. 2008 Jun 6;82(23–24):1175–1181. doi: 10.1016/j.lfs.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Dannals RF, Frost JJ. Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am. J Psychi. 1999;156(6):842–848. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]