Abstract
Expansion of the D-ring of 19-norsteroids with incorporation of the steroid C-18 methyl group into a newly formed six-membered ring provides easy access to the chrysene ring system. By taking advantage of the symmetry of the chrysene ring system and avoiding meso chrysene intermediates, four optically pure 2,8-difunctionalized (C-2 hydroxyl group and C-8 oxo group) hexadecahydrochrysene diastereomers, and their corresponding optically pure enantiomers were prepared from 19-nortestosterone. The eight chrysene stereoisomers are of interest as starting materials for preparing chrysene analogues of physiologically important neurosteroids.
Introduction
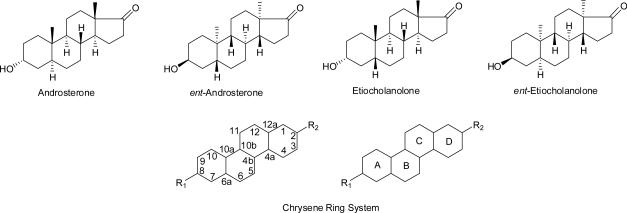
Some endogenous steroids in the androgen and pregnane classes and their analogues are known to be potent modulators of γ-aminobutyric acid type A (GABAA) receptors1. Among the androgens, the non-naturally occurring enantiomers of androsterone and etiocholanolone, ent-androsterone and ent-etiocholanolone, were found to be better modulators of GABAA receptors than their natural enantiomers2,3 (Chart 1).
Chart 1.
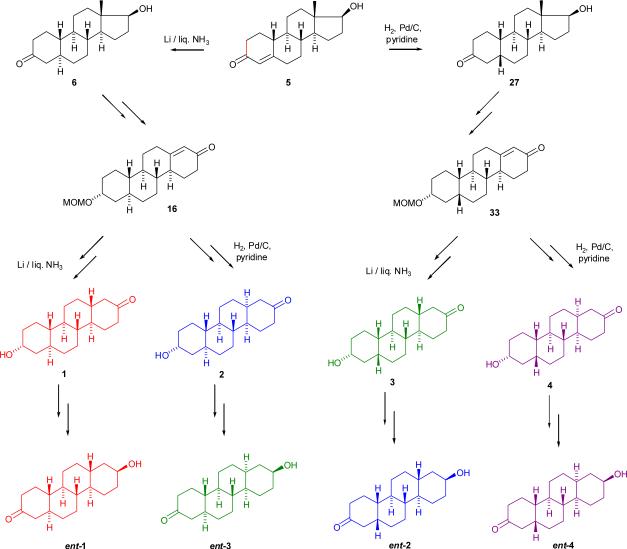
By contrast, the unnatural enantiomers of several other steroids in the pregnane class are not more active than their natural enantiomers.4,5 Currently, the molecular basis for these enantioselectivity differences between the androgen and pregnane classes is not fully understood. One way to gain a further understanding of these previous enantioselectivity findings is to enlarge the steroid studies to include enantioselectivity studies in closely related ring systems. In this regard, the chrysene ring system substituted at the C-2 and C-8 positions is one such option (Chart 1). This is a particularly attractive ring system for an analogue enantioselectivity study because, as described herein, optically pure chrysene diastereomers differing in the configurations of their A,B and C,D ring fusions as well as their optically pure enantiomers can all be readily prepared from optically pure 19-nortestosterone. The synthetic strategy used to prepare diastereomers 1–4 and their corresponding enantiomers ent-1–ent-4 from 19-nortestosterone (5) is outlined in Scheme 1.
Scheme 1.
Stereochemical Plan
For chrysenes 1–4, the appropriate choice of reaction conditions for reduction of the Δ4-double bond of steroid 5 will establish the stereochemistry of the A,B ring fusion. Li/liquid NH3 reduction will lead ultimately to the trans A,B-ring fused chrysenes 1 and 2, whereas Pd/C catalyzed hydrogenation in pyridine will lead ultimately to the cis A,B-ring fused chrysenes 3 and 4. A series of reactions is then used to first convert the 3-oxo groups of steroids 6 and 27 into MOM-protected 3α-hydroxyl groups and then subsequently to expand the steroid five-membered D-ring into the six-membered D-ring of chrysene enones 16 and 33. The double bond of these enones is then either reduced using Li/liquid NH3 to give chrysenes 1 and 3 which have the trans C,D-ring fusion or reduced using Pd/C catalyzed hydrogenation in pyridine to give chrysenes 2 and 4 which have the cis C-D-ring fusion. The corresponding 2,8-diketones formed by oxidation of the hydroxyl groups of chrysenes 1 and 4 have a center of symmetry at the mid-point of the C-4b,C-10b bond and are therefore non-optically active meso structures. Because of this symmetry, we reasoned that chrysenes 1 and 4 could be converted to their corresponding enantiomers ent-1 and ent-4 by interchanging the positions of the hydroxyl (axial for chrysenes 1 and 2, equatorial for chrysenes 3 and 4) and oxo groups of these chrysenes as long as meso structures (e.g., the 2,8-diketones or diaxial-2,8-diols) were avoided. We accomplished this by using protection/deprotection reactions in combination with oxidation/reduction reactions to switch the relative positions of the hydroxyl and oxo groups. The 2,8-diketones derived from chrysenes 2 and 3 do not have a center of symmetry and are not meso structures. Consequently, use of the same strategy to switch the relative positions of the hydroxyl and oxo groups does not lead to their corresponding enantiomers. However, this same chemistry will convert chrysene 2 to ent-3, the enantiomer of chrysene 3, and chrysene 3 to ent-2, the enantiomer of chrysene 2. Thus, we planned to convert chrysenes 1–4 to their corresponding enantiomers by simple procedures that preclude any degree of racemization of the previously established stereocenters at C-4a, C-4b, C-6a, C-10a, C-10b or C-12a.
Results and Discussion
Synthesis of Chrysenes 1 and 2
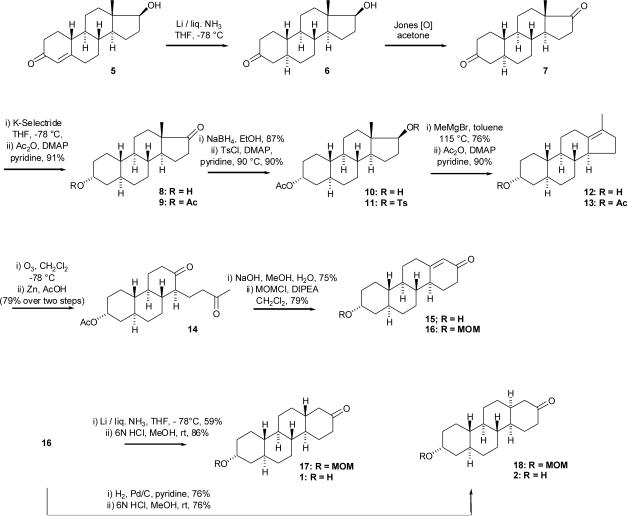
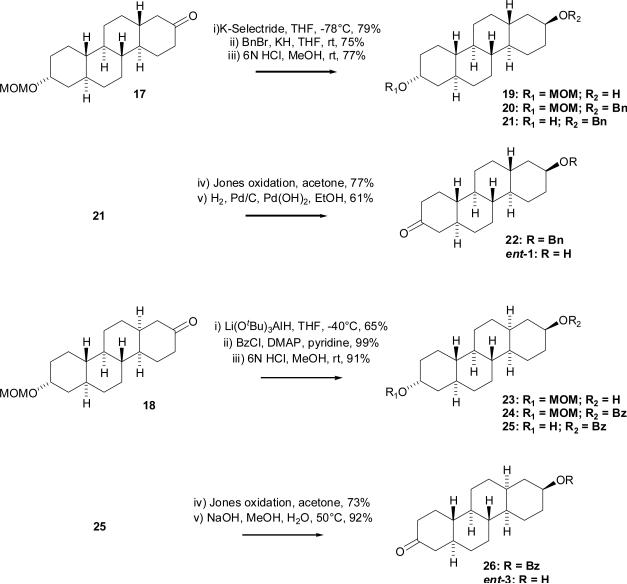
Commercially available 19-nor-testosterone (5) was converted by Li/liquid NH3 reduction followed by Jones oxidation to (5α-estrane-3,20-dione (7) in two steps in 63% overall yield6 (Scheme 2). The 3-oxo group was then selectively reduced by KSelectride in THF at −78 °C to afford the axial-3-alcohol derivative 8 in 55% yield. Treatment of compound 8 with acetic anhydride in pyridine in the presence of DMAP at room temperature gave the axial-3-acetate derivative 9 in 91% yield. NaBH4 reduction of the 17-oxo group in EtOH gave the 17β-alcohol derivative 10 in 87% yield. Compound 10 was then treated with p-toluenesulfonyl chloride in the presence of DMAP in pyridine at 90°C to give the 17β-tosylate 11 in 90% yield. The Kägi-Miescher rearrangement7,8,9 (1,2 shift of the C-18 methyl group) was accomplished by following the modified procedure of Engel et al.10 by refluxing of 17β-tosylate 11 in toluene with methylmagnesium bromide to afford the desired Δ13(17)-ene derivative 12 in 76% yield. The 1H NMR spectrum of compound 12 displayed a singlet for the migrated methyl group at δ 1.61 and the 13C NMR of the compound showed resonances at δ 136.8 and δ 127.8 for the Δ13(17)-double bond. Since the conditions of the Kägi-Miescher rearrangement led to deprotection of the 3-hydroxyl group, compound 12 was treated with acetic anhydride in pyridine in the presence of DMAP at room temperature to afford the 3-acetate derivative 13 in 90% yield. Compound 13 then gave upon ozonolysis in CH2Cl2 at −78 °C and subsequent reduction with Zn/AcOH of the intermediate cyclic ozonide, diketone 14 in 79% yield. Diketone 14 was then cyclized using aqueous NaOH in MeOH to the desired enone 15 in 75% yield. Since enone 15 was sparingly soluble in most solvents (e.g. hexanes, EtOAc), it was treated with MOMCl and N,N-diisopropylethylamine at room temperature overnight to afford the more soluble MOM derivative 16 in 79% yield. Reduction of the double bond of enone 16 with Li/liquid NH3 gave chrysene 17 (79% yield) having the trans C,D-ring fusion, and reduction of this double bond with Pd/C in pyridine gave chrysene 18 (75%) having the cis C,D-ring fusion. Finally, the MOM groups of compounds 17 and 18 were removed using 6 N aqueous HCl in MeOH. Compounds 1 and 2 were prepared in 13 steps from 19-nortestosterone (5) in total yields of 4.5% and 4.6%, respectively.
Scheme 2.
Synthesis of Chrysenes 1 and 2
Synthesis of Chrysenes ent-1 and ent-3
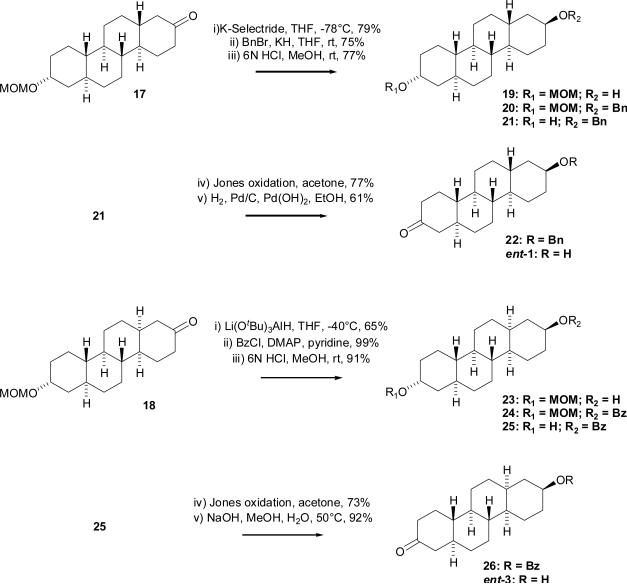
A reaction sequence which utilized protection/deprotection chemistry in combination with oxidation/reduction chemistry was used for the synthesis of compound ent-1 from compound 17 (Scheme 3). The 2-oxo group of compound 17 was selectively reduced by K-Selectride in THF at −78 °C to afford the axial-alcohol 19 in 79% yield. Treatment of compound 19 with BnBr/KH in THF gave the benzyl derivative 20 in 75% yield. Acidic hydrolysis of compound 20 with aqueous HCl in MeOH gave compound 21 which was then subsequently oxidized with Jones reagent to compound 22 (77 % yield in each step). Overnight hydrogenation of chrysene 22 using Pd/C in EtOAc did not lead to the desired product and only starting material was recovered. If EtOH was used instead of EtOAc as solvent, a mixture of desired compound ent-1 and other side products were obtained. A characteristic broad multiplet at δ 3.59 ppm in the 1H NMR spectrum of the major side product indicated that reduction of the oxo group had occurred when EtOH was the solvent. Finally, hydrogenation using a mixture of Pd/C and Pd(OH)2 (4:1) in EtOH afforded the desired compound ent-1 in 61% yield. Chrysene ent-1 was prepared in 17 steps from 19-nortestosterone (5) in a total yield of 0.8%.
Scheme 3.
Synthesis of Chrysenes ent-1 and ent-3
A benzoate ester instead of a benzyl ether protecting group was used for the synthesis of chrysene ent-3 because of the difficulty encountered with removal of the benzyl ether protecting group in the synthesis of chrysene ent-1. Except for the changes in hydroxyl group protection/deprotection reactions, the synthetic strategy used to prepare compounds ent-3 and ent-1 were the same. Thus, the 2-oxo group of compound 18 was selectively reduced by lithium tri(tert-butoxy)aluminum hydride in THF at −40 °C to afford equatorial-alcohol 23 in 65% yield. The hydroxyl group stereochemical assignment was confirmed by 1H-NMR which showed a resonance at δ 3.64 that was a multiplet (W1/2 ~ 36 Hz) as expected for an axial-proton at C-2 in chrysene 23. Treatment of compound 23 with benzoyl chloride in the presence of DMAP gave benzoate ester 24 in 99% yield. Acidic hydrolysis with aqueous HCl in MeOH gave compound 25 (91% yield) and a subsequent Jones oxidation gave intermediate 26 (73% yield). Basic hydrolysis using aqueous NaOH in MeOH at room temperature afforded the desired chrysene ent-3 in 92% yield. Compound ent-3 was prepared in 17 steps from 19-nortestosterone (5) in total yield of 2.7%. The increase in overall yield for the preparation of ent-3 relative to the overall yield obtained for ent-1 is largely due to the improved choice of the benzoate protecting group used for the synthesis of ent-3.
Synthesis of Chrysenes 3 and 4
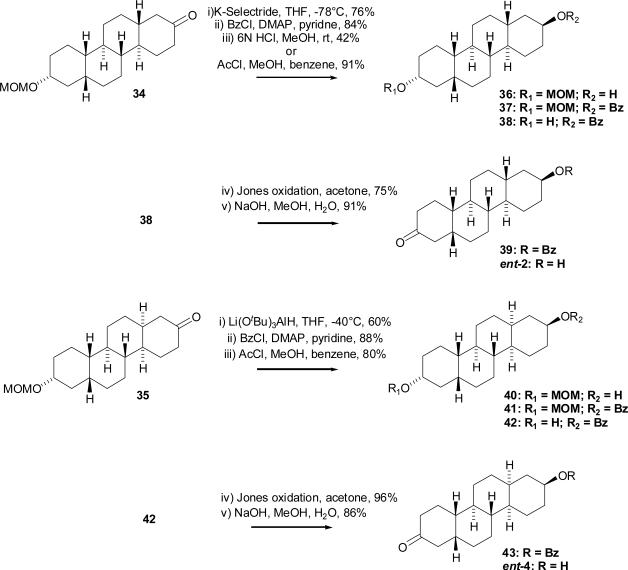
The experience gained in the synthesis of chrysenes 1 and 2 (Scheme 2) motivated us to modify the synthetic sequence to decrease the number of reactions needed to prepare chrysenes 3 and 4 (Scheme 4). Catalytic hydrogenation of 19-nor-testosterone (5) in pyridine using Pd/C gave (5β,17β)-17-hydroxyestrane-3-one (27, 40% yield). Tosylation of the 17-hydroxyl group gave compound 28, and subsequent selective reduction of the 3-oxo group afforded intermediate 29 in yields of 78% and 88%, respectively. Kägi-Miescher rearrangement of compound 29 to the Δ13(17)-ene derivative 30 was carried out in 98% yield. This product's structure was confirmed by its 1H NMR and 13C NMR spectra which showed a resonance for the migrated 18-methyl group as a singlet at δ 1.60 in the 1H NMR spectrum and resonances at δ 136.7 and δ 127.9 for the Δ13(17)-double bond in the 13C NMR spectrum. To avoid potential solubility issues in the subsequent ozonolysis and D-ring closure reactions, compound 30 was first converted to MOM-derivative 31 (99%). Ozonolysis of compound 31 gave compound 32 in 65% yield, and ring closure of this product under basic conditions afforded compound 33 (83% yield). Reduction of the double bond of compound 33 using Li/liquid NH3 gave product 34 (51%) which had the trans C,D ring fusion. Reduction of the double bond of compound 33 by catalytic hydrogenation using Pd/C in pyridine gave product 35 (53%) which had the cis C,D ring fusion. Finally, the MOM groups of compounds 34 and 35 were removed under acidic conditions using aqueous 6 N HCl in MeOH. Compound 3 and compound 4 were each prepared in 9 steps from 19-nortestosterone (5) in total yields of 5.5% and 6%, respectively.
Scheme 4.
Synthesis of Chrysenes 3 and 4
Synthesis of Chrysenesent-2 and ent-4
Protecting groups used during the synthesis of compounds ent-2 and ent-4 were the methoxymethyl and benzoate ester groups (Scheme 5). The 2-oxo group of compound 34 was selectively reduced using K-Selectride to afford derivative 36 in 76% yield. Lithium tri(tert-butoxy)aluminum hydride reduction of compound 35 was used to obtain compound 40 in 60% yield. Treatment of compound 36 as well as compound 40 with benzoyl chloride in the presence of DMAP gave ester 37 in 84% yield and ester 41 in 88% yield, respectively. Overnight acidic hydrolysis of compound 37 with aqueous HCl (Method A) was very slow and afforded only a 42% yield of compound 38 (47% of starting material was recovered). Therefore, acetylchloride in MeOH (Method B) was used to carry out transesterification reactions to obtain compounds 38 and 42 in yields of 91% and 80%, respectively. Jones oxidation of compound 38 (75% yield) followed by basic hydrolysis of oxidation product 39 using aqueous NaOH in MeOH at 50°C (92% yield) afforded chrysene ent-2. Similarly, compound ent-4 was obtained by oxidation of compound 42 (96% yield) and hydrolysis of compound 43 (86% yield). Compound ent-2 was prepared in 13 steps from 19-nortestosterone (5) in a total yield of 3%. Compound ent-4 was prepared in 13 steps from 19-nortestosterone (5) in a total yield of 2.9%.
Scheme 5.
Synthesis of Chrysenes ent-2 and ent-4
Summary
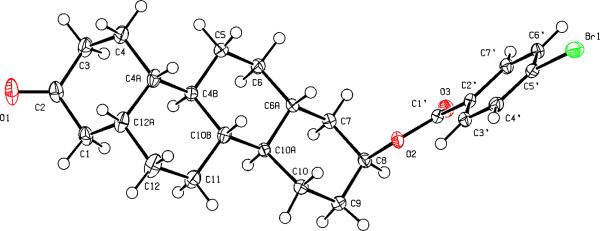
The enantiomeric relationship between chrysenes 1–4 and their corresponding enantiomers ent-1–ent-4 was confirmed by comparison of the physical and spectroscopic properties of the enantiomer pairs. Optical rotations and melting points are summarized in Table 1. Additional comparisons of IR and 1H-NMR spectra for the enantiomer pairs can be made of this data as reported in the Experimental Section. The near zero values for the optical rotations of chrysenes 2 and 4 and their corresponding enantiomers made determinations of their optical purity by optical rotation measurements unreliable. Accordingly, the absolute configuration of compound ent-2 was confirmed by an X-ray structure determination (Figure 1) of the compound's 4-bromobenzoate ester derivative since its optical rotation as well as that of chrysene 2 were both negative.
Table 1.
Values of optical rotation and melting points for chrysenes 1–4 and their corresponding enantiomers.
| nat-Chrysenes | ent-Chrysenes | ||||
|---|---|---|---|---|---|
| 1 | [α]20D −25.65 | 183–184 °C | ent-1 | [α]20D +26.51 | 182–184 °C |
| 2 | [α]20D −0.92 | 158–161 °C | ent-2 | [α]20D −1.88 | 160–162 °C |
| 3 | [α]20D −28.37 | 161–163 °C | ent-3 | [α]20D +29.7 | 160–162 °C |
| 4 | [α]20D −1.67 | 181–183 °C | ent-4 | [α]20D +0.68 | 183–184 °C |
Figure 1.
Projection plot showing the absolute configuration of the benzoate derivative of chrysene ent-2.
Conclusions
Because of the symmetry of the chrysene ring system we were able to develop efficient procedures for the preparation of four pairs of 2,8-disubstituted hexadecahyrochrysene enantiomers from 19-nortestosterone. These procedures provide each enantiomer in any pair of chrysene enantiomers in optically pure form. To our knowledge there are no previous reports in the literature of enantiospecific routes to these optically active 2,8-disubstituted hexadecahydrochrysenes. The successful development of the synthetic routes to the reported chrysenes makes these compounds available as starting materials for structure–activity studies of chrysene modulation of GABAA receptors and other ligand-gated ion channels known to be modulated by steroids.
Experimental
General
Solvents were either used as purchased or dried and purified by standard methodology. All extraction solvents were dried with anhydrous Na2SO4. Flash chromatography was performed using silica gel (32–63μm) purchased from Scientific Adsorbents (Atlanta, GA). Melting points were determined on Kofler micro hot stage and are uncorrected. Infrared spectra were recorded as films on a NaCl plate on a Perkin-Elmer Spectrum One FT-IR spectrometer. Optical rotations were measured on a Perkin-Elmer Model 341 Polarimeter in the solvent indicated. NMR spectra were recorded on Varian NMR spectrometer in CDCl3 at ambient temperature operating at 300 MHz (1H) or 75 MHz (13C). Chemical shifts are reported as δ values relative to internal chloroform (δ = 7.27) for 1H and chloroform (δ = 77.23) for 13C. For the1H NMR spectra, only the chemical shifts associated with substituents are listed. The chemical shifts of the remaining steroid or chrysene envelope protons can be found in the spectra reported in the Supportive Information. Reactions were monitored by thin layer chromatography (TLC) with 250 μm precoated silica gel plates (Analtech). Elemental analyses were performed by M-H-W Laboratories (Phoenix, AZ).
Experimental Section
(4aR,4bS,6aS,8R,10aS,10bR,12aR)-8-Hydroxy-hexadecahydro-chrysen-2-one (1)
A solution of compound 17 (110 mg, 0.34 mmol) in MeOH (8 mL) was treated with 6 N HCl (1 mL) at room temperature. After 15 h, it was poured into water-ice and aqueous sat. NaHCO3 was added to pH 8. The precipitated product was filtered off, dried at room temperature, and recrystallized from EtOAc/hexanes to afford desired alcohol 1 (82 mg, 86%): mp 183–184 °C (EtOAc/hexanes); [α]D20 −25.65 (c 0.11 in CHCl3). Found: C, 78.1; H, 10.3. Calc for C18H28O2: C, 78.2; H, 10.2%. IR (νmax/cm−1) 3438, 2946, 2914, 2850, 1702. δH (300 MHz, CDCl3) 4.1 (1H, bs). δC (75 MHz, CDCl3) 212.3, 66.5, 48.8, 47.5, 46.9, 46.8, 46.4, 43.5, 41.5, 40.6, 35.7, 34.2, 33.7, 33.1, 30.5, 30.1, 29.4, 23.5.
(4aS,4bR,6aR,8S,10aR,10bS,12aS)-8-Hydroxy-hexadecahydro-chrysen-2-one (ent-1)
Compound 22 (103 mg, 0.28 mmol) was dissolved in EtOH (50 mL). To this, 10% Pd/Cl (20 mg) and Pd(OH)2 (5 mg) were added. The reaction mixture was hydrogenated (60 psi, H2) for 3.5 h, then additional Pd(OH)2 (5 mg) was added. After 2 h of hydrogenation, the reaction mixture was filtered through a short column of silica gel to remove the catalyst, washing with CH2Cl2. Solvents were removed under vacuo and the residue was purified by column chromatography on silica gel (5% EtOAc in CH2Cl2) to give compound ent-1 (47 mg, 61%) as a white solid: mp 182–184 °C (EtOAc/hexanes), [α]D20 +26.51 (c 0.23 in CHCl3). Found: C, 78.0; H, 10.1. Calc for C18H28O2 C, 78.2; H, 10.2%. IR (νmax/cm−1) 3437, 2914, 2850, 1702. δH (300 MHz, CDCl3) 4.1 (1H, m). δC (75 MHz, CDCl3) 212.3, 66.5, 48.8, 47.4, 46.96, 46.93, 46.45, 43.5, 41.5, 40.7, 35.7, 34.3, 33.8, 33.2, 30.5, 30.1, 29.4, 23.6.
(4aR,4bS,6aS,8R,10aS,10bR,12aS)-8-Hydroxy-hexadecahydro-chrysen-2-one (2)
Compound 2 was prepared in the same manner as compound 1. Starting from compound 18 (221 mg, 0.69 mmol), compound 2 (146 mg, 76%) was obtained as a white solid after column chromatography on silica gel (2% EtOAc in CH2Cl2): mp 158–161 °C (EtOAc/hexanes); [α]D20 −0.92 (c 0.26 in CHCl3). Found: C, 78.3; H, 10.1. Calc. for C18H28O2 C, 78.2; H, 10.2%. IR (νmax/cm−1 3401, 2914, 2857, 1706. δH (300 MHz, CDCl3) 2.58 (1H, t, J = 13.5 Hz), 4.1 (1H, bs). δC (75 MHz, CDCl3) 213.4, 66.4, 47.4, 47.3, 43.0, 40.7, 40.5, 38.1, 37.5, 36.7, 35.6, 33.8, 33.1, 30.8, 29.8, 27.5, 24.1, 23.5.
(4aS,4bR,6aR,8S,10aR,10bS,12aR)-8-Hydroxy-hexadecahydro-chrysen-2-one (ent-2)
A solution of compound 39 (317 mg, 0.83 mmol) in MeOH (50 mL) and water (1 mL) was treated with NaOH (578 mg, 14.4 mmol) at 50 °C. After 1 h, TLC indicated 90% starting material remained. NaOH (400 mg, 10.0 mmol) was added again and the reaction mixture was heated at 50 °C. After 5 h, the solution was poured into water (100 mL) and extracted with CH2Cl2 (2 × 100 mL). The combined extracts were washed with brine and the solvent dried over anhydrous Na2SO4. After solvent evaporation, the oily residue was purified by column chromatography on silica gel (20% EtOAc in hexanes) to afford compound ent-2 (210 mg, 91%) as a white solid: mp 160–162 °C (EtOAc/hexanes); [α]D20 −1.88 (c 0.25 in CHCl3). Found: C, 78.4; H, 10.2. Cacl. for C18H28O2 C, 78.2; H, 10.2%. IR (νmax/cm−1) 3411, 2914, 2857, 2822. δH (300 MHz, CDCl3) 4.09 (1H, m), 2.58 (1H, t, J = 13.8 Hz). δC (75 MHz, CDCl3) 213.1, 66.4, 47.4, 47.2, 42.9, 40.7, 40.4, 38.0, 37.5, 36.6, 35.6, 33.7, 33.1, 30.8, 29.8, 27.4, 24.0, 23.5.
(4aR,4bS,6aR,8R,10aS,10bR,12aR)-8-Hydroxy-hexadecahydro-chrysen-2-one (3)
Compound 3 was prepared in the same manner as compound 1. Starting from compound 34 (156 mg, 0.48 mmol), compound 3 (100 mg, 75%) was obtained as a white solid after column chromatography on silica gel (20% EtOAc in hexanes): mp 161–163 °C (EtOAc/hexanes); [α]D20 −28.37 (c 0.24 in CHCl3). Found: C, 78.5; H, 10.0. Calc. for C18H28O2 calcd. C, 78.2; H, 10.2%. IR (νmax/cm−1) 3409, 2916, 2862, 1714. δH (300 MHz, CDCl3) 3.57 (1H, bm); δC (75 MHz, CDCl3) 212.2, 71.8, 48.8, 47.3, 46.3, 43.4, 41.4, 40.4, 37.3, 36.3, 35.3, 34.2, 31.6, 30.5, 29.9, 29.4, 25.8, 25.3.
(4aS,4bR,6aS,8S,10aR,10bS,12aS)-8-Hydroxy-hexadecahydro-chrysen-2-one (ent-3)
A solution of compound 26 (250 mg, 0.65 mmol) in MeOH (36 mL) and water (1 mL) was treated with NaOH (500 mg, 12.5 mmol) at 50 °C. After 1 h, the solution was poured into water (100 mL) and extracted with CH2Cl2 (2 × 100 mL). The combined extracts were washed with brine and solvent dried over anhydrous Na2SO4. After solvent evaporation, the oily residue was purified by column chromatography on silica gel (20% EtOAc in hexanes) to afford compound ent-3 (168 mg, 92%) as a white solid: mp 160–162 °C (EtOAc/hexanes); [α]D20 +29.7 (c 0.19 in CHCl3). Found: C, 78.3; H, 10.5. Calc. for C18H28O2 calcd. C, 78.2; H, 10.2%. IR (νmax/cm−1) 3410, 2916, 2862, 1714. δH (300 MHz, CDCl3) 3.66 (1H, bm); δC (75 MHz, CDCl3) 212.2, 71.8, 48.8, 47.3, 46.3, 43.5, 41.5, 40.4, 37.4, 36.3, 35.4, 34.2, 31.6, 30.5, 29.9, 29.5, 25.8, 25.3.
(4aR,4bS,6aR,8R,10aS,10bR,12aS)-8-Hydroxy-hexadecahydro-chrysen-2-one (4)
Compound 4 was prepared in the same manner as compound 1. Starting from compound 35 (149 mg, 0.46 mmol), compound 4 (93 mg, 72%) was obtained as a white solid after column chromatography on silica gel (20% EtOAc in hexanes): mp 181–183 °C (EtOAc/hexanes); [α]D20 −1.67 (c 0.21 in CHCl3). Found: C, 78.0; H, 10.1. Calc. for C18H28O2 C, 78.2; H, 10.2%. IR (νmax/cm−1) 3464, 2929, 2907, 2857, 1697. δH (300 MHz, CDCl3) 2.58 (1H, t, J = 14 Hz), 4.1 (1H, bs); δC (75 MHz, CDCl3) 213.2, 71.8, 43.0, 40.4, 40.3, 38.2, 37.9, 37.7, 36.7, 36.3, 35.5, 31.6, 30.8, 29.9, 27.6, 25.8, 25.1, 24.2.
(4aS,4bR,6aS,8S,10aR,10bS,12aR)-8-Hydroxy-hexadecahydro-chrysen-2-one (ent-4)
Compound ent-4 was prepared in the same manner as compound ent-3. Starting from compound 43 (270 mg, 0.71 mmol), compound ent-4 (170 mg, 86%) was obtained as a white solid after column chromatography on silica gel (20% EtOAc in hexanes): mp 183–184 °C (EtOAc/hexanes); [α]D20 +0.68 (c 0.29 in CHCl3). IR (νmax/cm−1) 3466, 2907, 2857, 1697, 1067. δH (300 MHz, CDCl3) 2.57 (1H, t, J = 13.8 Hz), 3.66 (1H, bm); δC (75 MHz, CDCl3) 213.0, 71.8, 43.0, 40.5, 40.4, 38.2, 38.0, 37.8, 36.6, 36.4, 35.5, 31.6, 30.8, 29.9, 27.6, 25.8, 25.1, 24.2. HRMS (FAB) m/z Cacld for C18H29O2 [M]+ 277.2168, found: 277.2170.
(5α)-Estrane-3,17-dione (7)
Compound 7 was prepared in two steps from 19-nortestosterone (5) according to the literature11,12 as a white solid: mp 99–100 °C (EtOAc/hexanes). Found: C, 79.0; H, 9.8. Calc. for C18H26O2 C, 78.8; H, 9.55%. δH (300 MHz, CDCl3) 0.90 (3H, s); δC (75 MHz, CDCl3) 221.0, 211.4, 50.6, 48.7, 48.1, 48.0, 45.9, 43.8, 41.4, 40.7, 36.0, 33.9, 31.7, 30.7, 29.7, 25.6, 21.8, 14.0.
(3α,5α)-3-Hydroxyestran-17-one (8)
Compound 8 was prepared according to the literature13 as a white solid: mp 160–161 °C (EtOAc/CHCl3). Found: C, 78.4; H, 9.9. Calc for C18H28O2 C, 78.2; H, 10.2%. δH (300 MHz, CDCl3) 0.82 (3H, s), 2.34–2.43 (1H, m), 4.03 (1H, bm); δC (75 MHz, CDCl3) 221.6, 66.2, 50.7, 48.3, 47.9, 47.0, 40.8, 40.6, 36.0, 35.9, 33.5, 33.0, 31.6, 29.9, 24.9, 23.7, 21.7, 13.9.
(3α,5α)-3-(Acetyloxy)estran-17-one (9)
4-Dimethylaminopyridine (114 mg, 0.9 mmol) and acetic anhydride (4.2 mL, 44.7 mmol) were added to a solution of steroid 8 (2.6 g, 9.4 mmol) in pyridine (25 mL). After 2 h at room temperature, the reaction mixture was poured into ice-water, stirred for 2 h, and then extracted with EtOAc (3 × 70 mL). Combined extracts were washed with an aqueous solution of KHCO3, brine, and dried over anhydrous Na2SO4. After solvent evaporation, the oily residue was purified by column chromatography on silica gel (15% EtOAc in hexanes) to give compound 9 (2.74 g, 91%) as a white solid: mp 96–98 °C (Et2O/hexanes). Found: C, 75.6; H, 9.3. Calc. for C20H30O3 C, 75.4; H, 9.5%. δH (300 MHz, CDCl3) 0.85 (3H, s), 2.01 (3H, s), 5.02 (1H, bm); δC (75 MHz, CDCl3) 221.4, 170.7, 69.9, 50.8, 48.3, 48.0, 46.7, 40.9, 37.7, 36.9, 35.9, 33.4, 31.7, 30.2, 29.8, 25.0, 24.5, 21.7, 21.6, 13.9.
(3α,5α,17β)-Estrane-3,17,diol 3-acetate (10)
Compound 9 (10 g, 31.4 mmol) was dissolved in EtOH (400 mL) and cooled in an ice-bath. NaBH4 (1.2 g, 31.7 mmol) was added in small portions to a stirred solution. After 1.5 h, the reaction mixture was poured into a solution of brine (500 mL) and AcOH (7 mL) and stirred for 1h. The precipitate was filtered off, dissolved in CH2Cl2 (400 mL), and washed with brine. Solvent was dried over anhydrous Na2SO4 and evaporated. Column chromatography on silica gel (10% EtOAc in CH2Cl2) gave compound 10 (8.7 g, 87%) as a white solid: mp 219–221 °C (CHCl3/hexanes); [α]D20 +28.67 (c 0.75 in CHCl3). Found: C, 75.2; H, 9.9. Calc. for C20H32O3 C, 75.0; H, 10.1%. IR (νmax/cm−1) 3436, 2933, 2951, 2899, 2857, 2840, 1698. δH (300 MHz, CDCl3) 0.74 (3H, s), 2.04 (3H, s), 3.63 (1H, t, J = 8.5 Hz), 5.03 (1H, bm); δC (75 MHz, CDCl3) 170.9, 82.2, 70.2, 50.4, 48.3, 46.8, 43.3, 41.5, 37.8, 37.0, 36.9, 33.6, 30.7 (2 × C), 30.3, 25.4, 24.6, 23.4, 21.7, 11.7.
(3α,5α,17β)-Estrane-3,17,diol 3-acetate, 17-tosylate (11)
A solution of compound 10 (8.7 g, 31.4 mmol), 4-dimethylaminopyridine (330 mg, 2.7 mmol), and p-TsCl (7.5 g, 39.3 mmol) in anhydrous pyridine (75 mL) was heated at 90 °C overnight. Then, 4-dimethylaminopyridine (60 mg, 0.49 mmol) and p-TsCl (1.3 g, 6.82 mmol) were added and the reaction mixture was heated another 24 h. The reaction mixture was poured into ice-water, extracted with CH2Cl2 (2 × 300 mL). The combined extracts were washed with aqueous HCl, aqueous KHCO3, brine, and dried over anhydrous Na2SO4. After solvent evaporation, the oily residue was purified by column chromatography on silica gel (5% EtOAc in CH2Cl2) to give compound 11 (11.6 g, 90%) as a white solid: mp 114–115 °C (CH2Cl2/hexanes); [α]D20 +10.49 (c 0.51 in CHCl3). Found: C, 68.4; H, 8.2; S, 6.8. Calc. for C27H38O5S C, 68.3; H, 8.1; S, 6.8%. IR (νmax/cm−1) 3306, 2919, 2861, 1732, 1598, 1445, 1361, 1176. δH (300 MHz, CDCl3) 0.74 (3H, s), 2.04 (3H, s), 2.44 (1H, s,), 4.27 (1H, m), 5.02 (1H, bm), 7.32 (2H, m), 7.78 (2H, d, J = 8.4 Hz); δC (75 MHz, CDCl3) 170.8, 144.5, 134.6, 129.8, 128.0, 90.3, 70.0, 49.4, 48.1, 46.7, 43.3, 41.1, 37.7, 36.9, 36.3, 34.5, 30.5, 30.2, 27.8, 25.1, 24.5, 23.3, 21.8, 21.7, 11.9.
(3α,5α)-17-methylgon-13(17)-en-3-ol (12)
Compound 11 (550 mg, 1.17 mmol) was dissolved in anhydrous toluene (8 mL) and heated to 100 °C. Methylmagnesium bromide (3.0 M in Et2O, 2 mL, 6 mmol) was added to the stirring hot solution under an N2 atmosphere dropwise as a white precipitate appeared. The reaction mixture was heated for 2 h at 115 °C. The flask was cooled, a few pieces of crushed ice were added, and the pH of the solution was adjusted to pH 2 by dropwise addition of 2 N aqueous H2SO4. The toluene layer was separated and the aqueous layer was extracted with EtOAc (80 mL). The combined extracts were washed with brine, dried over anhydrous Na2SO4 and the solvents evaporated. Column chromatography on silica gel (5% EtOAc in CH2Cl2) gave compound 12 (230 mg, 76%) as a white solid: mp 125–128 °C (CH2Cl2/hexanes); [α]D20 +4.30 (c 0.62 in CHCl3). Found: C, 82.9; H, 11.0. Calc for C18H28O C, 83.0; H, 10.8%. IR (νmax/cm−1) 3350, 2920, 2848, 1595. δH (300 MHz, CDCl3) 1.61 (3H, s), 2.47–2.57 (1H, m), 4.08 (1H, m). δC (75 MHz, CDCl3) 136.8, 127.8, 66.7, 52.9, 51.3, 47.3, 46.4, 40.9, 37.4, 36.0, 33.8, 33.3, 31.5, 29.8, 28.2, 25.6, 23.9, 13.6.
(3α,5α)-3-(Acetyloxy)-17-methylgon-13(17)-ene (13)
Compound 13 was prepared in the same manner as compound 9. Starting from compound 12 (6.9 g, 26.5 mmol), compound 13 (7.23 g, 90%) was obtained as a white solid after column chromatography on silica gel (10 % EtOAc in hexanes): mp 103–104 °C (Et2O/hexanes); [α]D20 +6.41 (c 0.88 in CHCl3). Found: C, 79.5; H, 10.0. Calc. for C20H30O2 C, 79.4; H, 10.0%. IR (νmax/cm−1) 3351, 2917, 2847, 1734, 1597. δH (300 MHz, CDCl3) 1.61 (3H, s), 2.06 (3H, s), 5.0 (1H, bs). δC (75 MHz, CDCl3) 170.9, 136.7, 127.9, 70.2, 53.0, 51.2, 46.9, 46.3, 37.8, 37.4, 36.8, 33.6, 31.4, 30.4, 29.8, 28.2, 25.6, 24.6, 21.7, 13.6.
Acetic acid (1S,4aR,4bS,7R,8aS,10aR)-7-oxo-8-(3-oxo-butyl)-tetradecahydro-phenanthren-2-yl ester (14)
A solution of compound 13 (4 g, 13.2 mmol) in CH2Cl2 (100 mL) was treated with ozone at −78 °C until a blue color persisted (ca. 30 min). Oxygen was passed through the solution for 30 min until the blue color disappeared. AcOH (50 mL) was added and the CH2Cl2 was evaporated under vacuum without heating. Then, AcOH (50 mL) and Zn dust (8.6 g, 130 mmol) were added and the reaction mixture was stirred at room temperature for 1h. Zn dust was filtered off through cotton, washing with CH2Cl2 (100 mL). The Zn dust was stirred with EtOAc (200 mL) for 1 h. The solids were filtered off, the combined solvents were evaporated and the product was purified by a column chromatography on silica gel (15% EtOAc in hexanes) to give compound 14 (3.5 g, 79%) as a white solid: mp 71–73 °C (Et2O/hexanes); [α]D20 +22.92 (c 1.65 in CHCl3). Found: C, 72.0; H, 8.9. Calc. for C20H30O4 C, 71.8; H, 9.0%. IR (νmax/cm−1) 3404, 2917, 2938, 2861, 1732, 1711, 1228, 1249. δH (300 MHz, CDCl3) 2.04 (3H, s), 2.11 (3H, s), 5.04 (1H, m); δC (75 MHz, CDCl3) 212.7, 209.3, 170.8, 69.8, 54.2, 47.9, 46.6, 46.2, 41.8, 41.1, 37.3, 36.2, 33.4, 31.2, 30.7, 30.1, 24.5, 21.6, 19.5.
(4aS,4bR,6aS,8R,10aS,10bR)-8-Hydroxy-4,4a,4b,5,6,6a,7,8,9,10,10a,10b,11,12-tetradecahydro-3H-chrysen-2-one (15)
A solution of NaOH (10% w/v in MeOH/water (9:1), 30 mL) was added to a solution of compound 14 (3.5 g, 10.5 mmol) in MeOH (100 mL) and the mixture was stirred for 1 h at 40 °C. A few pieces if crushed ice were added and the pH was adjusted to pH 1 by adding aqueous 1 N HCl. Solids were filtered off, washed several times with water and dried overnight at room temperature to give 15 (2.16 g, 75%) as a yellow solid, which was then crystallized from MeOH/CHCl3 to afford white crystals. The aqueous phase was extracted with CH2Cl2 (2 × 100 mL), the organic extract was washed with brine, dried over anhydrous Na2SO4, and the solvent evaporated to afford additional, but slightly impure, oily product 15 (570 mg, 19%). Crystalline product 15 had: mp 250–251 °C (MeOH/CHCl3), [α]D20 −14.73 (c 0.27 in CHCl3). Found: C, 78.6; H, 9.6. Calc. for C18H26O2 C, 78.8; H, 9.55%. IR (νmax/cm−1) 3403, 2928, 2856, 1648, 1611. δH (300 MHz, CDCl3) 4.12 (1H, m), 5.83 (1H, s); δC (75 MHz, CDCl3) 200.3, 167.2, 124.3, 66.5, 48.9, 47.2, 46.4, 43.1, 40.5, 36.7, 35.8, 35.5, 33.6, 33.1, 30.5, 30.0, 26.4, 23.7.
(4aS,4bR,6aS,8R,10aS,10bR)-8-Methoxymethoxy-4,4a,4b,5,6,6a,7,8,9,10,10a,10b,11,12-tetradecahydro-3H-chrysen-2-one (16)
N,N-Diisopropylethylamine (0.52 mL, 2.9 mmol) was added to a solution of compound 15 (410 mg, 1.49 mmol) in dry CH2Cl2 (40 mL). The solution was cooled to 0 °C, MOMCl (0.22 mL, 2.8 mmol) was added and the mixture was stirred under Ar at room temperature. After 30 h, CH2Cl2 (50 mL) was added and the organic layer was washed with the aqueous HCl (5%), aqueous NaHCO3, and brine. Solvent was dried over anhydrous Na2SO4 and evaporated. Column chromatography on silica gel(10 % EtOAc in hexanes) gave compound 16 (380 mg, 79%) as a white solid: mp 64–65 °C (EtOAc/hexanes); [α]D20 −19.46 (c 0.35 in CHCl3). Found: C, 75.15; H, 9.5. Calc. for C20H30O3 C, 75.4; H, 9.5%. IR (νmax/cm−1) 3332, 2915, 2858, 1673, 1621, 1045. δH (300 MHz, CDCl3) 3.35 (3H, s), 3.86 (1H, bm), 4.64 (2H, s), 5.79 (1H, s). δC (75 MHz, CDCl3) 200.2, 167.1, 124.2, 94.7, 71.4, 55.3, 48.9, 46.9, 46.3, 43.0, 38.3, 36.6, 36.0, 35.7, 33.6, 30.4 (2 × C), 29.9, 26.3, 24.2.
(4aR,4bS,6aS,8R,10aS,10bR,12aR)-8-Methoxymethoxy-hexahydro-chrysen-2-one (17)
Anhydrous NH3 (10 mL) was condensed with a gas condenser into a three-neck flask containing Li metal (18 mg, 2.6 mmol) at −78 °C. Then, anhydrous THF (12 mL) was added and the resulting blue solution was stirred for 0.5 h. A solution of compound 16 (168 mg, 0.53 mmol) in dry THF (6 mL) was added dropwise to the vigorously stirred solution. After 3 h of stirring, the reaction color was discharged by careful addition of solid NH4Cl in portions and left overnight while the NH3 evaporated. The reaction was then acidified with aqueous 1 N HCl up to pH <7 and the product was extracted with EtOAc (2 × 50 mL). The combined organic phases were washed with sat. aqueous NaHCO3, brine, dried with anhydrous Na2SO4, and the solvent evaporated. Purification by prep-TLC (4 plates, 40% EtOAc/hexanes) afforded ketone 17 (110 mg, 59%) as a white solid: mp 103–105 °C (hexanes), [α]D20 ∔32.09 (c 0.21 in CHCl3). Found: C, 75.1; H, 10.2. Calc. for C20H32O3 C, 75.0; H, 10.0%. IR (νmax/cm−) 3368, 2952, 2910, 2848, 2836, 1721, 1042. δH (300 MHz, CDCl3) 3.37 (3H, s), 3.87 (1H, bm), 4.66 (2H, s); δC (75 MHz, CDCl3) 211.9, 94.7, 71.4, 55.2, 48.7, 47.2, 46.8, 46.8, 46.3, 43.4, 41.4, 38.4, 36.1, 34.2, 33.7, 30.43, 30.40, 29.9, 29.3, 24.1.
(4aR,4bS,6aS,8R,10aS,10bR,12aS)-8-Methoxymethoxy-50 hexadecahydro-chrysen-2-one (18)
A solution of compound 16 (200 mg, 0.63 mmol) in pyridine (25 ml) was hydrogenated in the presence of Pd/C (5%, 30 mg) at 60 psi. After 12 h, the catalyst was filtered through a short column of silica gel, washing with CH2Cl2. The solvent was removed in vacuo to yield a white solid. Column chromatography on silica gel (2% EtOAc in CH2Cl2) gave compound 18 (153 mg, 76%) as a white solid: mp 94–96 °C (hexanes), [α]D20 −6.66 (c 0.195 in CHCl3). Found: C, 75.1; H, 10.0. Calc. for C20H32O3 C, 75.0; H, 10.1%. IR (νmax/cm−1) 2909, 2856, 2823, 1710, 1034. δH (300 MHz, CDCl3) 2.58 (1H, t, J = 13.5 Hz), 3.37 (3H, s), 3.87 (1H, bs), 4.66 (2H, s); δC (75 MHz, CDCl) 213.3, 94.8, 71.5, 55.3, 47.3, 47.3, 43.0, 40.5, 38.6, 38.1, 37.5, 36.7, 36.3, 33.8, 30.9, 30.5, 29.8, 27.5, 24.1, 24.1.
(2S,4aR,4bS,6aS,8R,10aS,10bR,12aR)-8-Methoxymethoxy octadecahydro-chrysen-2-ol (19)
Compound 19 was prepared in the same manner as compound 8. Starting from compound 17 (538 mg, 1.67 mmol), compound 19 (429 mg, 79%) was obtained as a white solid after column chromatography on silica gel (10% EtOAc in hexanes): mp 141–142 °C (EtOAc/hexanes);[α]D20 −5.59 (c 0.22 in CHCl3). Found: C, 74.2; H, 10.7. Calc. for C20H34O3 C, 74.5; H, 10.6%. IR (νmax/cm−1) 3293, 2915, 2848, 1050. δH (300 MHz, CDCl3) 3.37 (3H, s), 3.88 (1H, bm), 4.09 (1H, m), 4.67 (2H, s); δC (75 MHz, CDCl3) 94.8, 71.7, 66.7, 55.3, 47.7, 47.6, 47.3 (2 × C), 40.8, 38.6, 36.4, 35.8, 34.1, 34.0, 33.2, 30.6, 29.6 (2 × C), 24.2, 23.6.
(2S,4aR,4bS,6aS,8R,10aS,10bR,12aR)-2-Benzyloxy-8-methoxymethoxy-octadecahydro-chrysene (20)
KH (106 mg, 30% suspension in mineral oil, 0.82 mmol) was added to a solution of compound 19 (75 mg, 0.23 mmol) in dry THF (12 mL). After 60 min reflux under an N2 atmosphere, the mixture was allowed to attain room temperature. Then, BnBr (0.1 mL, 0.82 mmol) was added and the resulting mixture was stirred at room temperature. After 3 h, the reaction mixture was carefully quenched with MeOH (2 mL), extracted with CH2Cl2 (50 mL). The organic phase was washed with brine and dried over anhydrous Na2SO4. Column chromatography on silica gel (2% EtOAc in CH2Cl2) gave compound 20 (72 mg, 75%) as a white solid: mp 95–97 °C (hexanes), [α]D20 −2.85 (c 0.31 in CHCl3). Found: C, 78.9; H, 9.45. Calc. for C27H40O3 C, 78.6; H, 9.7%. IR (νmax/cm−1) 3368, 2903, 2847, 1605, 1496, 1099, 1049. δH (300 MHz, CDCl3) 3.38 (3H, s), 3.69 (1H, bm), 3.88 (1H, m), 4.5 (2H, d, J = 3.3 Hz), 4.67 (2H, s), 7.28–7.36 (5H, m); δH (75 MHz, CDCl3) 139.7, 128.5 (2 × C), 127.5 (2 × C), 127.4, 94.8, 73.3, 71.7, 69.8, 55.4, 47.8, 47.6, 47.3, 47.3, 38.6, 38.2, 36.4, 36.3, 34.1, 31.2, 30.6, 29.9, 29.6 (2 × C), 24.2, 24.1.
(2S,4aR,4bS,6aS,8R,10aS,10bR,12aR)-8-Benzyloxyoctahydro-chrysen-2-ol (21)
Compound 21 was prepared in the same manner as compound 1. Starting from compound 20 (450 mg, 1.09 mmol), compound 21 (310 mg, 77%) was obtained as a white solid after column chromatography on silica gel (10% EtOAc in hexanes): mp 156–158 °C (EtOAc/hexanes); [α]D20 −1.42 (c 0.15 in CHCl3). Found: C, 81.4; H, 9.8. Calc. for C25H36O2 C, 81.5; H, 9.85%. IR (νmax/cm−1) 3271, 2916, 2850, 1595, 1094, 730, 694. δH (300 MHz, CDCl3) 3.62 (1H, t, J = 2.7 Hz), 4.01 (1H, t, J = 2.7 Hz), 4.43 (2H, dd, J1 = 12 Hz, J2 = 15.3 Hz, ), 4.5 (2H, d, J = 3.3 Hz), 7.19–7.3 (5H, m); δC (75 MHz, CDCl3) 139.7, 128.5 (2 × C), 127.6 (2 × C), 127.4, 73.3, 69.8, 66.7, 47.8, 47.7, 47.3, 47.2, 40.8, 38.2, 36.3, 35.8, 34.1, 34.0, 33.2, 29.9, 29.64, 29.67, 27.1, 23.6.
(4aS,4bR,6aR,8S,10aR,10bS,12aS)-8-Benzyloxyhexadecahydro-chrysen-2-one (22)
Jones reagent was added dropwise to a solution of compound 21 (320 mg, 0.87 mmol) in acetone (20 mL) at 0 °C until an orange color persisted. The course of the reaction was checked by TLC. Then, 2-propanol was added dropwise until the reaction mixture turned green. After 30 min, the reaction mixture was poured into water-ice. The product was extracted with EtOAc (2 × 50 mL) and the water phase was partially evaporated (1/3) and extracted with EtOAc (50 mL). The combined extracts were washed with brine, dried over anhydrous Na2SO4, and the solvent was evaporated. Column chromatography on silica gel (10% EtOAc in hexanes) gave compound 22 (247 mg, 77%) as a white solid: mp 109–110 °C (Et2 O); [α]D20 +17.88 (c 0.34 in CHCl3). Found: C, 82.0; H, 9.3. Calc. for C25H34O2 C, 81.9; H, 9.35%. IR (νmax/cm−1) 3407, 2948, 2913, 2847, 1715, 1096, 1068. δH (300 MHz, CDCl3) 3.60 (1H, bm), 4.41 (2H, dd, J = 12 Hz, J = 15.9Hz), 7.17–7.26 (5H, m); δC (75 MHz, CDCl3) 212.3, 139.6, 128.5 (2 × C), 127.5 (2 × C), 127.4, 73.1, 69.8, 48.8, 47.5, 46.94, 46.97, 46.5, 43.5, 41.5, 38.1, 36.2, 34.3, 33.9, 30.5, 30.1, 29.9, 29.4, 24.1.
(2S,4aR,4bS,6aS,8R,10aS,10bR,12aS)-8-Methoxymethoxyoctahexahydro-chrysen-2-ol (23)
Lithium tri(tert-butoxy)aluminum hydride (1M solution in THF, 4.8 mL) was added dropwise to a cooled solution (−40 °C) of compound 18 (860 mg, 2.68 mmol) in anhydrous THF (200 mL) under an N2 atmosphere. After 2 h at −40 °C, aqueous 1 N HCl (50 mL) was carefully added and the mixture was allowed to attain room temperature. The product was extracted with CH2Cl2 (2 × 150 mL), combined extracts were washed with sat. aqueous NaHCO3, brine, dried over anhydrous Na2SO4. After solvent evaporation, the residue was purified by column chromatography on silica gel (10% EtOAc in hexanes) to afford compound 23 (570 mg, 65%) as a white solid: mp 118–119 °C (EtOAc/hexanes), [α]D20 −4.0 (c 0.15 in CHCl3); IR (νmax/cm−1) 3306, 2915, 2864, 1049. δH (300 MHz, CDCl3) 3.37 (3H, s), 3.64 (1H, bm), 3.87 (1H, bs), 4.66 (2H, s). δC (75 MHz, CDCl3) 94.8, 72.1, 71.6, 55.4, 47.7, 47.5, 40.6, 38.6, 37.6, 36.5, 36.4, 35.5, 34.0, 31.8, 30.6, 30.0, 29.7, 25.8, 24.8, 24.2. HRMS (FAB) m/z Calcd for C20H34O3 [M]+ 332.2508, found: 332.2495.
Benzoicacid (2S,4aR,4bS,6aS,8R,10aS,10bR,12aS)-8-35 methoxymethoxy-octadecahydro-chrysen-2-yl ester (24)
A solution of compound 23 (520 mg, 1.61 mmol) and 4-dimethylaminopyridine (10 mg, 0.01 mmol) in dry pyridine (45 mL) was cooled in an ice-bath. Then, BzCl (0.9 mL, 8.06 mmol) was added dropwise while stirring. After 15 h at room temperature, the reaction mixture was poured into water and extracted with CH2Cl2 (2 × 150 mL). The combined extracts were washed with aqueous HCl, sat. aqueous NaHCO3, brine, and dried over anhydrous Na2SO4. After solvent evaporation, the oily residue was purified by column chromatography on silica gel (10% EtOAc in hexanes) to afford compound 24 (683 mg, 99%)as a white solid: [α]D20 −15.85 (c 0.2 in CHCl3); IR (νmax/cm−1) 2915, 2867, 1715, 1275, 1039. δH (300 MHz, CDCl3) 3.38 (3H, s), 3.88 (1H, bs), 4.67 (2H, s), 5.01 (1H, bm), 7.41ȁ37.46 (2H, m), 7.52–7.57 (1H, m), 8.03–8.06 (2H, m); δC (75 MHz, CDCl3) 166.3, 132.8, 131.1, 129.7 (2 × C), 128.4 (2 × C), 94.8, 75.22, 71.6, 55.3, 47.6, 47.5, 40.6, 38.7, 37.6, 36.3, 35.4, 34.0, 32.5, 31.6, 30.6, 29.7, 26.2, 25.7, 24.7, 24.2. HRMS (FAB) m/z Calcd for C27H38O4Na [M + Na]+ 449.2668, found: 449.2664.
Benzoicacid (2S,4aR,4bS,6aS,8R,10aS,10bR,12aS)-8-55 Hydroxy-octadecahydro-chrysen-2-yl ester (25)
Compound 25 was prepared in the similar fashion as compound 1, wherein MeOH (60 mL) and 6 N HCl (4 mL) were used to carry out the hydrolysis. Starting from compound 24 (550 mg, 1.28 mmol), compound 25 (450 mg, 91%) was obtained as a white solid after column chromatography on silica gel (10% EtOAc in hexanes): mp 164–166 °C (EtOAc/hexanes); [α]D20 −17.81 (c 0.16 in CHCl3). Found: C, 78.6; H, 8.8. Calc. for C25H34O3 C, 78.5; H, 9.0%. IR (νmax/cm−1) 3364, 2913, 2865, 1715,1275, 772. δH (300 MHz, CDCl3) 4.1 (1H, bs), 5.01 (1H, (1H, bm), 7.41–7.46 (2H, m), 7.52–7.58 (1H, m), 8.04–8.07 (2H, m); δC (75 MHz, CDCl3) 166.3, 132.8, 131.1, 129.7 (2 × C), 128.4 (2 × C), 75.2, 66.6, 47.61, 47.58, 40.8, 40.6, 37.6, 35.7, 35.4, 33.9, 33.2, 32.5, 31.6, 29.7, 26.2, 25.6, 24.7, 23.6.
Benzoic acid (4aS,4bR,6aS,8S,10aR,10bS,12aS)-8-Oxo-70 octadecahydro-chrysen-2-yl ester (26)
Compound 26 was prepared in the same manner as compound 22. Starting from compound 25 (400 mg, 1.04 mmol), compound 26 (289 mg, 73%) was obtained as a solidified foam after column chromatography on silica gel (10% EtOAc in hexanes): [α]D20 +1.3 (c 0.16 in CHCl3); IR (νmax/cm−1 ) 2914, 2866, 1714, 1274. δH (300 MHz, CDCl3) 5.02 (1H, bm), 7.41–7.47 (2H, m), 7.53–7.59 (1H, m), 8.04–8.07 (2H, m). δC (75 MHz, CDCl3) 212.0, 166.3, 132.9, 131.1, 129.7 (2 × C), 128.5 (2 × C), 74.9, 48.8, 47.3, 46.3, 43.4, 41.5, 40.4, 37.4, 35.3, 34.2, 32.4, 31.5, 30.5, 29.5, 26.2, 25.7, 25.3. HRMS (EI) m/z Calcd for C25H32O3 [M]+ 380.2351, found: 380.2355.
(5β,17β)-17-Hydroxyestran-3-one (27)
Compound 27 was prepared in the same manner as compound 18. Starting from compound 5 (6 g, 21.8 mmol), pure 5β-isomer 27 (2.39 g, 40 %) and a mixture of 5β/5α-isomers (95:5, 1.98 g, 33%) were obtained after column chromatography on silica gel (15% EtOAc in hexanes): mp 105–106 °C (EtOAc, hexanes). δH (300 MHz, CDCl3) 0.77 (3H, s), 2.57 (1H, t, J = 13.9 Hz), 3.66 (1H, t, J = 8.4 Hz); δC (75 MHz, CDCl3) 213.2, 82.1, 50.2, 43.4, 43.1, 41.7, 39.9, 38.6, 38.5, 36.8, 36.6, 30.68, 30.72, 27.9, 25.7, 25.2, 23.4, 11.3. HRMS (FAB) m/z Calcd for C18H29O2 [M + H]+ 277.2168, found: 277.2168.
(5β,17β)-17-hydroxyestran-3-one 17-tosylate (28)
Compound 28 was prepared in the same manner as compound 11. Starting from compound 27 (5.8 g, 20.1 mmol), compound 28 (7.03 g, 78 %) was obtained after column chromatography on silica gel (10% EtOAc in hexanes): mp 130–132 °C (EtOAc/hexanes); [α]D20 +7.49 (c 0.25 in CHCl3). Found: C, 69.6; H, 8.25; S, 7.3. Calc. for C25H34O4S C, 69.4; H, 8.4; S, 7.4%. IR (νmax/cm−1) 3400, 2920, 2871, 1708, 1357, 1175. δH (300 MHz, CDCl3) 0.83 (3H, s), 2.45 (3H, s), 2.53 (1H, t, J = 14 Hz), 4.28 (1H, t, J = 9 Hz), 7.31–7.35 (2H, m), 7.77–7.79 (2H, m); δC (75 MHz, CDCl3) 212.8, 144.6, 134.4, 129.8 (2 × C), 128.0 (2 × C), 90.0, 49.2, 43.4, 43.0, 41.3, 39.8, 38.4, 38.3, 36.5, 36.3, 30.6, 27.87, 27.82, 25.3, 25.0, 23.3, 21.8, 11.9.
(3α,5α,17α)-estrane-3,17-diol 17-tosylate (29)
Compound 29 was prepared in the same manner as compound 23. Starting from compound 28 (6.94 g, 16.1 mmol), compound 29 (6.19 g, 88%) was obtained as a white solid after column chromatography on silica gel (20% EtOAc in hexanes): mp 174–176 °C (EtOAc/hexanes); [α]D20 +7.89 (c 0.38 in CHCl3). Found: C, 69.6; H, 8.25; S, 7.3. Calc. for C25H36O4S C, 69.4; H, 8.4; S, 7.4%. IR (νmax/cm−1) 3418, 2923, 2861, 1644, 1356, 1175. δH (300 MHz, CDCl3) 0.77 (3H, s), 2.44 (3H, s), 3.60 (1H, bm), 4.23 (1H, t, J = 8 Hz), 7.33 (2H, d, J = 8.4 Hz), 7.78 (2H, d, J = 8.7 Hz); δC (75 MHz, CDCl3) 144.5, 134.3, 129.8 (2 × C), 127.9 (2 × C), 90.3, 71.7, 49.2, 43.3, 41.6, 39.9, 384, 36.4, 36.3, 35.6, 31.4, 29.7, 27.8, 26.1, 25.7, 25.0, 23.3, 21.8, 11.9.
(3α,5β-17-methylgon-13(17)-en-3-ol (30)
Compound 30 was prepared in the same manner as compound 12. Starting from compound 29 (2.9 g, 6.7 mmol), compound 30 (1.71 g, 98%) was obtained as a white solid after column chromatography on silica gel (20% EtOAc in hexanes): mp 130–131 °C (EtOAc/hexanes); [α]D20 −7.05 (c 0.105 in CHCl3). Found: C, 83.0; H, 10.7. Calc. for C18H28O C, 83.0; H, 10.8%. IR (νmax/cm−1) 3368, 2923, 2861, 1638, 1068, 1034. δH (300 MHz, CDCl3) 1.60 (3H, s), 2.48–2.55 (1H, m), 3.64 (1H, bm); δC (75 MHz, CDCl3) 136.7, 127.9, 72.0, 52.8, 51.6, 40.2, 37.4, 36.8, 36.5, 35.8, 31.6, 30.1, 30.1, 28.2, 26.6, 26.1, 25.7, 13.6.
(3α,5β)-3-Methoxymethoxy-17-methylgon-13(17)-ene (31)
Compound 31 was prepared in the same manner as compound 16. Starting from compound 30 (5.28 g, 20.2 mmol), compound 31 (6.11 g, 99%) was obtained as an oily material after column chromatography on silica gel (5% EtOAc in hexanes). Compound 31 had: [α]D20 +1.35 (c 0.17 in CHCl3); IR (νmax/cm−1) 2926, 2865, 1454, 1444, 1043. δH (300 MHz, CDCl3) 1.60 (3H, s), 2.48–2.55 (1H, m), 3.38 (3H, s), 3.56 (1H, bm), 4.70 (2H, s); δC (75 MHz, CDCl3) 136.7, 127.9, 94.7, 77.01, 55.3, 52.8, 51.6, 40.4, 37.4, 36.8, 35.8, 33.6, 31.7, 30.1, 28.2, 27.3, 26.5, 26.2, 25.7, 13.6. HRMS (FAB) m/z Calcd for C20H32O2Na [M + Na]+ 327.2300, found: 327.2297.
(1S,4aR,4bS,7R,8aR,10aR)-7-Methoxymethoxy-1-(3-oxobutyl)-dodecahydro-phenanthren-2-one (32)
Compound 32 was prepared in the same manner as compound 14. Starting from compound 31 (6.11 g, 20.06 mmol), compound 32 (4.43 g, 65%) was obtained as an oily material after column chromatography on silica gel (10% EtOAc in hexanes): [α]D20 +22.6 (c 0.29 in CHCl3); IR (νmax/cm−1) 2935, 2869, 1711, 1041. δH (300 MHz, CDCl3) 2.10 (3H, s), 3.36 (3H, s), 3.56 (1H, bm), 4.68 (2H, s); δC (75 MHz, CDCl3) 212.7, 209.2, 94.7, 76.6, 55.3, 54.1, 48.4, 42.0, 41.1, 40.3, 36.9, 35.0, 33.4, 31.6, 31.0, 30.0, 27.1, 26.7, 26.0, 19.5. HRMS (FAB) m/z Calcd for C20H33O4 [M + H]+ 337.2379, found: 337.2376.
(4aS,4bR,6aR,8R,10aS,10bR)-8-Methoxymethoxy-4,4a,4b,5,6,6a,7,8,9,10,10a,11,12-tetradecahydro-3H-chrysen-2-one (33)
Compound 32 was prepared in the same manner as compound 15. Starting from compound 32 (4.37 g, 12.9 mmol), compound 33 (3.45 g, 83%) was obtained as an low melting solid after column chromatography on silica gel (20% EtOAc in hexanes): mp <65 °C (hexanes); [α]D20 −13.77 (c 0.39 in CHCl3). Found: C, 75.5; H, 9.4. Calc. for C20H30O3 C, 75.4; H, 9.5%. IR (νmax/cm−1) 3382, 2931, 2865, 1671, 1040. δH (300 MHz, CDCl3) 3.36 (3H, s), 3.56 (1H, bm), 4.68 (2H, s), 5.81 (1H, bs); δC (75 MHz, CDCl3) 200.1, 166.9, 124.3, 94.7, 76.7, 55.3, 49.3, 42.9, 40.3, 36.8, 36.6, 35.7, 35.1, 33.5, 31.5, 30.2, 27.2, 26.4, 25.8, 25.6.
(4aR,4bS,6aR,8R,10aS,10bR,12aR)-8-Methoxymethoxyhexadecahydro-chrysen-2-one (34)
Compound 34 was prepared in the same manner as compound 17. Starting from compound 33 (500 mg, 1.57 mmol), compound 34 (260 mg, 51%) and starting material 33 (140 mg, 28%) were obtained as white solids after column chromatography on silica gel (20% EtOAc in hexanes). Compound 34 had: mp 83–84 °C (hexanes); [α]D20 −19.9 (c 0.31 in CHCl3). Found: C, 75.2; H, 10.0. Calc. for C20H32O3 C, 75.0; H, 10.1%. IR (νmax/cm−1) 2931, 2910, 2862, 1717, 1035. δH (300 MHz, CDCl3) 3.36 (3H, s), 3.54 (1H, bm), 4.68 (2H, s); δC (75 MHz, CDCl3) 212.1, 94.7, 76.8, 55.3, 48.8, 47.3, 46.3, 43.4, 41.4, 40.6, 37.3, 35.4, 34.2, 33.5, 31.6, 30.5, 29.4, 27.1, 25.8, 25.3
(4aR,4bS,6aR,8R,10aS,10bR,12aS)-8-Methoxymethoxy-hexadecahydro-chrysen-2-one (35)
Compound 35 was prepared in the same manner as compound 18. Starting from compound 33 (400 mg, 1.25 mmol), cis-isomer 35 (214 mg, 53%), trans-isomer 34 (99 mg, 24%) and a mixture of 34 and 35 (1:1, 52 mg, 13%) were obtained after column chromatography on silica gel (10% EtOAc in hexanes): mp 102–103 °C (ether/hexanes); [α]D20 +6.06 (c 0.21 in CHCl3). Found: C, 75.1; H, 9.9. Calc. for C20H32O3 C, 75.0; H, 10.1%. IR (νmax/cm−1) 2925, 2869, 2849, 1712, 1039. δH (300 MHz, CDCl3) 2.58 (1H, t, J = 13.8 Hz), 3.37 (3H, s), 3.56 (1H, bm), 4.69 (2H, s); δC (75 MHz, CDCl3) 213.1, 94.7, 76.8, 55.3, 43.0, 40.6, 40.3, 38.1, 37.9, 37.7, 36.6, 35.4, 33.5, 31.6, 30.8, 27.6, 27.1, 25.8, 25.0, 24.2.
(2S,4aR,4bS,6aR,8R,10aS,10bR,12aR)-8-Methoxymethoxyoctadecahydro-chrysen-2-ol (36)
Compound 36 was prepared in the same manner as compound 8. Starting from compound 34 (860 mg, 2.68 mmol), compound 36 (660 mg, 76%) was obtained as a white solid after chromatography on silica gel (15% EtOAc in hexanes): mp 150–151 °C (Et2O); [α]D20 +4.7 (c 0.27 in CHCl3). Found: C, 74.6; H, 10.8. Calc. for C20H34O3 C, 74.5; H, 10.6%. IR (νmax/cm−1) 3275, 2914, 2866, 2845. δH (300 MHz, CDCl3) 3.37 (3H, s), 3.54 (1H, bm), 4.07 (1H, bs), 4.69 (2H, s); δC (75 MHz, CDCl3) 94.7, 76.8, 66.6, 55.3, 47.7, 47.6, 40.8 (2 x C), 37.5, 35.8, 35.5, 33.9, 33.6, 33.2, 31.8, 29.7, 27.1, 25.8, 24.7, 23.6.
Benzoic acid (2S,4aR,4bS,6aR,8R,10aS,10bR,12aR)-8-methoxymethoxy-octadecahydro-chrysen-2-yl ester (37)
Compound 37 was prepared in the same manner as compound 24. Starting from compound 36 (610 mg, 1.89 mmol), compound 37 (680 mg, 84%) was obtained as a white solid after column chromatography on silica gel (10% EtOAc in hexanes): mp 130–131 °C (Et2O/hexanes); [α]D20 +5.52 (c 0.39 in CHCl3). Found: C, 76.25; H, 9.1. Calc. for C27H38O4 C, 76.0; H, 9.0%. IR (νmax/cm−1) 2930, 2865, 1715, 1450, 1275, 1040, 714. δH (300 MHz, CDCl3) 3.38 (3H, s), 3.56 (1H, bm), 4.70 (2H, s), 5.31 (1H, m), 7.42–7.47 (2H, m), 7.53–7.59 (1H, m), 8.04–8.08 (2H, m); δC (75 MHz, CDCl3) 166.1, 132.8, 1631.3, 129.7 (2 × C), 128.5 (2 × C), 94.7, 77.0, 70.7, 55.3, 47.6, 47.2, 40.8, 37.9, 37.5, 36.9, 35.5, 33.8, 33.6, 31.8, 30.5, 29.6, 27.1, 25.8, 24.7, 24.5.
Benzoic acid (2S,4aR,4bS,6aR,8R,10aS,10bR,12aR)-8-hydroxy-octadecahydro-chrysen-2-yl ester (38)
Method A: Compound 38 was prepared in the same manner as compound 1. Starting from compound 37 (600 mg, 1.4 mmol), compound 38 (228 mg, 42%) and starting material 37 (286 mg, 47%) were obtained after column chromatography on silica gel (20% EtOAc in hexanes). Method B: To a solution of compound 37 (286 mg, 0.67 mmol) in MeOH (18 mL) and benzene (6 mL) was added dropwise AcCl (0.8 mL, 11.2 mmol) at 0 °C and the reaction mixture was allowed to stir overnight at room temperature. Then, a few pieces of crushed ice were added and the pH was adjusted with sat. aqueous NaHCO3 to pH 6. The product was extracted with CH2Cl2 (2 × 80 mL) and the combined extracts were washed with brine and dried over anhydrous Na2SO4. After solvent evaporation, the residue was purified by a column chromatography (20% EtOAc/hexanes) to afford compound 38 (233 mg, 91%): mp 196–198 °C (EtOAc); [α]D20 −0.92 (c 0.24 in CHCl3). Found: C, 78.3; H, 9.0. Calc. for C25H34O3 C, 78.5; H, 9.0%. IR (νmax/cm−1) 3377, 2922, 2862, 1714, 1450, 1275, 713. δH (300 MHz, CDCl3) 3.65 (1H, m), 5.32 (1H, m), 7.42–7.48 (2H, m), 7.53–7.59 (1H, m), 8.04–8.07 (2H, m); δC (75 MHz, CDCl3) 166.1, 132.8, 131.4, 129.7 (2 × C), 128.5 (2 × C), 72.0, 70.7, 47.7, 47.3, 40.7, 37.9, 37.6, 36.9, 36.5, 35.5, 33.8, 31.8, 30.5, 30.1, 29.7, 25.8, 24.8, 24.6.
Benzoic acid (4 aS,4bR,6aR,8S,10aR,10bS,12aR)-8-Oxooctadecahydro-chrysen-2-yl ester (39)
Compound 39 was prepared in the same manner as compound 22. Starting from compound 38 (447 mg, 1.16 mmol), compound 39 (335 mg, 75%) was obtained as a white solid after column chromatography on silica gel (15% EtOAc in hexanes): mp 115–117 °C (EtOAc); [α]D +1.84 (c 0.39 in CHCl3). Found: C, 78.8; H, 8.6. Calc. for C25H32O3 C, 78.9; H, 8.5%. IR (νmax/cm−1) 2916, 2862, 1712, 1274. δH (300 MHz, CDCl3) δ 2.60 (1H, t, J = 13.8 Hz), 5.33 (1H, m), 7.43–7.49 (2H, m), 7.54–7.59 (1H, m), 8.05–8.09 (2H, m); δC (75 MHz, CDCl3) 212.9, 166.1, 132.9, 131.3, 129.7 (2 × C), 128.5 (2 × C), 70.5, 47.3, 47.3, 43.0, 40.5, 38.1, 37.9, 37.5, 36.9, 36.7, 33.7, 30.9, 30.5, 29.8, 27.6, 24.5, 24.1.
(2S,4aR,4bS,6aR,8R,10aS,10bR,12aS)-8-Methoxymethoxyoctadecahydro-chrysen-2-ol (40)
Compound 40 was prepared in the same manner as compound 23. Starting from compound 35 (264 mg, 0.823 mmol), compound 40 (160 mg, 60%) and starting material 35 (42 mg, 16%) were obtained as white solids after column chromatography on silica gel (15% EtOAc in hexanes). Compound 40 had: mp 151–152 °C (Et2O); [α]D20 +6.75 (c 0.24 in CHCl3). Found: C, 74.4; H, 10.6. Calc. for C20H34O3 C, 74.5; H, 10.6%. IR (νmax/cm−1) 3272, 2934, 2911, 2869, 2845, 1058. δH (300 MHz, CDCl3) 3.37 (3H, s), 3.52–3.65 (2H, m), 4.68 (2H, s); δC (75 MHz, CDCl3) 94.7, 76.8, 71.9, 55.3, 40.6, 40.4, 38.0 (2 × C), 36.4, 35.6, 35.5, 33.6, 31.8, 31.7, 29.9, 27.1, 25.9, 25.8, 24.9 (2 × C).
Benzoic acid (2S,4aR,4bS,6aR,8R,10aS,10bR,12aS)-8-Methoxymethoxy-octadecahydro-chrysen-2-yl ester (41)
Compound 41 was prepared in the same manner as compound 24. Starting from compound 40 (480 mg, 1.48 mmol), compound 41 (560 mg, 88%) was obtained as a white solid after column chromatography on silica gel (5 % EtOAc in hexanes): mp 126–128 °C (EtOAc/hexanes); [α]D20 −8.71 (c 0.28 in CHCl3). Found: C, 76.1; H, 8.8. Calc. for C27H38O4 C, 76.0; H, 9.0%. IR (νmax/cm−1) 2932, 2869, 1719, 1450, 1273, 1044, 712. δH (300 MHz, CDCl3) 3.38 (3H, s), 3.55 (1H, m), 4.70 (2H, s), 5.00 (1H, m), 7.40–7.46 (2H, m), 7.52–7.57 (1H, m), 8.03–8.07 (2H, m); δC (75 MHz, CDCl3) 166.3, 132.8, 131.2, 129.7 (2 × C), 128.4 (2 × C), 94.8, 76.8, 75.1, 55.3, 40.7, 40.5, 38.10, 38.07, 35.6, 35.5, 33.6, 32.5, 31.8, 31.6, 27.2, 26.2, 25.9, 25.7, 24.95, 24.91.
Benzoic acid (2S,4aR,4bS,6aR,8R,10aS,10bR,12aS)-8-Hydroxy-octadecahydro-chrysen-2-yl ester (42)
Compound 42 was prepared in the same manner as compound 38 by Method B. Starting from compound 41 (530 mg, 1.24 mmol), compound 42 (380 mg, 80%) was obtained as a white solid after column chromatography on silica gel (25% EtOAc in hexanes): mp 173–174 °C (EtOAc); [α]D20 −15.15 (c 0.2 in CHCl3). Found: C, 78.7; H, 8.9. Calc. for C25H34O3 C, 78.5; H, 9.0%. IR (νmax/cm−1) 3292, 2917, 2867, 1714, 1451, 1275, 711. δH (300 MHz, CDCl3) 3.65 (1H, m), 5.00 (1H, m), 7.40–7.46 (2H, m), 7.52–7.57 (1H, m), 8.04–8.07 (2H, m); δC (75 MHz, CDCl3) 166.3, 132.8, 131.2, 129.7 (2 × C), 128.4 (2 × C), 75.2, 71.9, 40.5, 38.1 (2 × C), 36.5, 35.6, 35.5, 32.5, 31.7, 31.6, 30.0, 26.2, 25.9, 25.7, 24.98, 24.92.
Benzoic acid (4 aS,4bR,6aS,8S,10aR,10bS,12aR)-8-Oxooctadecahydro-chrysen-2-yl ester (43)
Compound 43 was prepared in the same manner as compound 22. Starting from compound 42 (357 mg, 0.93 mmol), compound 43 (342 mg, 96%) was obtained as a white solid after column chromatography on silica gel (15% EtOAc in hexanes): mp 116–117 °C (hexanes), [α]D20 −16.3 (c 0.24 in CHCl3). Found: C, 79.0; H, 8.4. Calc. for C25H32O3 C, 78.9; H, 8.5%. IR (νmax/cm−1) 2916, 2868, 1711, 1274. δH (300 MHz, CDCl3) 2.60 (1H, t, J = 13.8 Hz), 5.02 (1H, bm), 7.41–7.46 (2H, m), 7.52–7.58 (1H, m), 8.04–8.08 (2H, m); δC (75 MHz, CDCl3) 212.8, 166.2, 132.9, 131.1, 129.7 (2 × C), 128.4 (2 × C), 74.9, 43.0, 40.5, 40.4, 38.2, 38.0, 37.8, 36.6, 35.4, 32.4, 31.5, 30.8, 27.6, 26.2, 25.7, 25.1, 24.3.
Supplementary Material
Acknowledgements
The authors thank Dr. Achintya K. Bandyopadhyaya for his preliminary studies on methods for expansion of the steroid D-ring. This work was supported by NIH Grant GM47969 to DFC. The x-ray structure determinations were made possible by NSF Shared Instrument Grant No. CHE-042097.
Footnotes
Electronic Supplementary Information (ESI) available: 1H NMR and 13C NMR spectra. The cif file for the structure shown in Figure 1. The X-ray crystal structure has also been deposited at The Cambridge Crystallographic Data Centre (CCDC 814522.) See DOI: 10.1039/b000000x/
References
- (1).Belelli D, Lambert JJ. Nat. Rev. Neurosci. 2005;6:565. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- (2).Katona BW, Krishnan K, Cai ZY, Manion BD, Benz A, Taylor A, Evers AS, Zorumski CF, Mennerick S, Covey DF. Eur. J. Med. Chem. 2008;43:107. doi: 10.1016/j.ejmech.2007.02.017. [DOI] [PubMed] [Google Scholar]
- (3).Li P, Bracamontes J, Katona BW, Covey DF, Steinbach JH, Akk G. Mol. Pharmacol. 2007;71:1582. doi: 10.1124/mol.106.033407. [DOI] [PubMed] [Google Scholar]
- (4).Covey DF, Nathan D, Kalkbrenner M, Nilsson KR, Hu Y, Zorumski CF, Evers AS. J. Pharmacol. Exp. Ther. 2000;293:1009. [PubMed] [Google Scholar]
- (5).Wittmer LL, Hu Y, Kalkbrenner M, Evers AS, Zorumski CHF, Covey DF. Mol. Pharmacol. 1996;50:1581. [PubMed] [Google Scholar]
- (6).Bowers A, Ringold HJ, Denot E. J. Amer. Chem. Soc. 1958;80:6115. [Google Scholar]
- (7).Parker KA, Johnson WS. J. Amer. Chem. Soc. 1974;96:2556. [Google Scholar]
- (8).Madaeva OS. Zh. Obshch. Khim. 1955;25:1427. [Google Scholar]
- (9).Rodewald WJ, Morzycki JW. Polish J. Chem. 1978;52:2361. [Google Scholar]
- (10).Engel CHR, Lachance P, Capitaine J, Zee J, Mukhetjee D, Mérand Y. Org. Chem. 1983;48:1954. [Google Scholar]
- (11).Menberu D, Van Phuoc N, Onan KD, P., Le Quesne W. J. Org. Chem. 1992;57:2100. [Google Scholar]
- (12).Farnsworth WE. Steroids. 1966;8:825. doi: 10.1016/0039-128x(66)91012-9. [DOI] [PubMed] [Google Scholar]
- (13).Hu Y, Zorumski CHF, Covey DF. J. Med. Chem. 1993;36:3956. doi: 10.1021/jm00076a025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.