Abstract
Caesalpinia sappan (C. sappan) is a medicinal plant used for promoting blood circulation and removing stasis. During a screening procedure on medicinal plants, the ethylacetate extract of the lignum of C. sappan (CLE) showed inhibitory activity on arginase which has recently been reported as a novel therapeutic target for the treatment of cardiovascular diseases such as atherosclerosis. CLE inhibited arginase II activity prepared from kidney lysate in a dose-dependent manner. In HUVECs, inhibition of arginase activity by CLE reciprocally increased NOx production through enhancement of eNOS dimer stability without any significant changes in the protein levels of eNOS and arginase II expression. Furthermore, CLE-dependent arginase inhibition resulted in increase of NO generation and decrease of superoxide production on endothelium of isolated mice aorta. These results indicate that CLE augments NO production on endothelium through inhibition of arginase activity, and may imply their usefulness for the treatment of cardiovascular diseases associated with endothelial dysfunction.
Keywords: Caesalpinia sappan lignum, Arginase, Endothelial nitric oxide synthase, Nitric oxide, Superoxide
INTRODUCTION
Caesalpinia sappan (C. sappan) is a species of flowering tree distributed in Asia, including Korea, China, India, and Vietnam. Its heartwood has been used as a natural reddish dyestuff for years and is also used in oriental folk medicine due to its valuable anti-bacterial, anti-inflammatory, emmenagogue, and analgesic properties [1-3]. The lignum of C. sappan (C. sappan L.) also promotes blood circulation and removal of stasis. Along this same line, the methanolic extract of C. sappan L. was shown to enhance the vasorelaxation response of phenylephrine-preconstricted rat aortic rings in a dose-dependent manner. This has been attributed to increased nitric oxide (NO) biosynthesis, since NG-nitro-L-arginine methyl ester (L-NAME) as a nitric oxide synthase (NOS) inhibitor completely abolishes the vasorelaxant effect [1]. Brazilin and hematoxylin, two purified compounds from C. sappan, show endothelium-dependent vasorelaxation activity that is abolished by treatment with guanylate cyclase inhibitor [1]. However, the molecular target and mechanism underlying the vasorelaxation effect of the extract of C. sappan L. remain to be elucidated.
Nitric oxide (NO) is well established as a major factor in endothelium-dependent vasorelaxation of the vascular system. NO act as a potent vasoprotective molecule by regulating vasoreactivity, platelet activation, leukocyte adhesion, and smooth muscle cell proliferation and migration in vessels. NO is produced from its precursor substrate L-arginine by eNOS in the vasculature and plays critical roles in the regulation of vascular tone and maintenance of vascular integrity. Arginase, which shares L-arginine as a substrate with eNOS, hydrolyzes L-arginine to ornithine and urea as part of the urea cycle. It is well recognized that arginase modulates NOS activity by regulating intracellular L-arginine bioavailability [4-6]. Thus, the balance between arginase and eNOS activities partly regulates vascular endothelial NO production.
Arginase I expression in macrophages, hepatocytes, and vascular smooth muscle cells, is stimulated by lipopolysaccharide (LPS), tumor necrosis factor-α, IL-13, altered oxygen tension, and balloon dilatation of coronary arteries [7-11]. The activation and expression of endothelial arginase II can also be induced by a variety of vascular stimulants, including OxLDL, LPS, TNF-α, IFN-γ, 8-bromo-cGMP, and hypoxia [7,10,12-14]. Arginase activation/upregulation results in arginase/NOS imbalance, as well as decreased NO production, and has been demonstrated to contribute to endothelial dysfunction, in a number of diseases/pathophysiological processes, including aging [4], diabetes [15-17], hypertension [18-20], and atherosclerosis [14,21].
In this study, we investigated whether or not the action of CLE in the vasculature is mediated through inhibition of arginase activity as well as whether or not CLE regulates endothelial NO production in HUVECs and in isolated mice aorta.
METHODS
Materials
Arginase I and II solutions were prepared from liver and kidney lysates of anesthetized C57BL/6 mice. MnTBAP (Mn (III) Tetra (4-benzoic acid) porphyrin chloride) and L-NAME were obtained from Calbiochem. All reagents were purchased from Sigma unless otherwise stated.
Preparation of ethylacetate extract of C. sappan L. (CLE)
C. sappan L. was purchased at Dong Xuan oriental herbarium market in September 2009 in Hanoi, Vietnam. The plant was botanically identified by professor Pharm Thanh Ki, Hanoi University of Pharmacy. A voucher sample (No. 2009-0038) was deposited at the Herbarium of the Department of Pharmacy of Catholic University. The dried and sliced lignum of C. sappan (1.0 kg) was extracted with ethylacetate (3×3 l) under sonication, and the combined extract was dried under a vacuum to produce a viscous residue (51.6 g). The residue was dissolved in DMSO, and stored at -20℃ until use.
Cell culture
HUVECs were purchased from Cascade biologics and maintained according to the supplier's protocol in Medium 230 containing low-serum growth supplement (LSGS) at 37℃ in 5% CO2.
Arginase activity
Tissue lysates of livers and kidneys were prepared in lysis buffer (50 mM Tris-HCl, pH 7.5, 0.1 mM EDTA and protease inhibitors) by homogenization at 4℃, followed by centrifugation for 20 min at 14,000×g at 4℃. The supernatants were used to assay for arginase activity as previously described [14].
NOx measurement
NO was estimated by Griess reaction based upon the concentration of nitrate/nitrite (NOx) after the conversion of nitrate to nitrite by nitrate reductase using the Nitric oxide assay kit (Calbiochem) [14]. The concentration of NOx in HUVECs was expressed as µmol/mg protein.
Western blot analysis
The treated- and untreated-HUVECs were homogenized in buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Nonidet P-40, 1 mM EDTA, 1 µg/ml of leupeptin, 1 µg/ml of pepstatin, 1 µg/ml of aprotinin, 1 mM phenylmethylsulfonylflouride, 1 mM sodium orthovanadate, and 1 mM NaF) and centrifuged for 30 min at 14,000×g. The protein amount of the supernatant was analyzed by the Bradford method. Proteins (100 µg) were separated in a 10% SDS-PAGE and then transferred to a nitrocellulose membrane (Bio-Rad). The blots were incubated with polyclonal anti-arginase II (Santa Cruz), polyclonal anti-endothelial nitric oxide synthase (BD Bioscience), or monoclonal anti-β-tubulin (BD bioscience) antibodies, followed by the secondary antibody (Amersham). Signals were detected using an enhanced chemiluminescence detection reagent with X-ray film.
Determination of eNOS dimerization
Dimers and monomers of eNOS were separated using low-temperature SDS-PAGE as previously described [22]. Band intensities were analyzed using NIH ImageJ Software.
NO and superoxide generation in endothelium of isolated mice aorta
Mice aortic rings were isolated and incubated overnight at 37℃, 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) containing 2% FBS and antibiotics (1X) in the presence of CLE (20 µg/ml) [5]. The aorta cut longitudinally and pinned to the bottom of a silgard-coated chamber (endothelial layer on top) filled with HEPES buffer (NaCl 120 mM, KH2PO4 2.6 mM, KCl 4 mM, CaCl2 2 mM, MgCl2 0.6 mM, HEPES 25 mM, glucose 14 mM, pH7.4). The chamber was allowed to equilibrate into the heating stage for 30 minutes at 37℃. The chamber allowed for static bath conditions during fluorescence measurements. Tissue background along with DAF-FM (4-Amino-5-methylamino-2',7'-difluorofluorescein) diacetate or DHE (dihydroethidine) fluorescence were measured using an Olympus 10x objective with optimized excitation and emission wavelength (DAF-FM, 470/525 nm; or DHE, 470/580), an intensified camera (Luca 658M-TL), and a custom image acquisition program (Cell software, Olympus). Following initial equilibrium, background fluorescence was recorded and aorta was allowed to return to room temperature for 15 minutes. The aorta were then loaded with 5 µM DAF-FM or 5 µM DHE (molecular Probes) in HEPES buffer for 45 minutes followed by washout of DAF-FM or DHE and a 20 minutes equilibrium period at 37℃. Fluorescence intensity was averaged (5 frames, 2×2 binning) from the entire field of view and recorded by the acquisition program. Changes in DAF-FM or DHE fluorescence were recorded once after washout of DAF-FM or DHE in order to establish baseline changes in intensity and then again following treatment with L-NAME (10-5 mol/l) or MnTBAP (10-5 mol/l). The slopes of changes in fluorescence over time were determined by linear regression in Origin (version 7.5, OrignLab Corp, Northampton, MA) and used for statistical comparison.
Statistics
All data are represented as mean±S.D. of at least four independent experiments. Unpaired Student's t-test or 1-way ANOVA was used to assess significant differences. A value of p<0.05 was accepted as significant.
RESULTS
CLE inhibited arginase activity
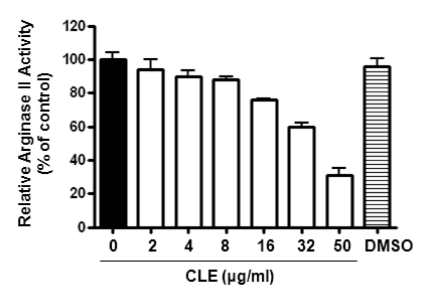
Screening of higher plants to identify a novel arginase inhibitor, found that CLE exhibited significant inhibitory activity. Enzyme solution of arginase II was prepared from kidney lysate of C57BL/6 mice. The predominant expression of the arginase isoforms was previously confirmed by Western blot analysis (data not shown). Treatment with different concentrations of CLE decreased the enzyme activity of arginase in the kidney lysate in a dose-dependent manner (Fig. 1, p<0.01, 1-way ANOVA test). The residual activity of arginase II was 31±10% at 50 µg/ml of CLE, while the calculated IC50 was 36.82 µg/ml to arginase II.
Fig. 1.
CLE inhibits arginase activity in a dose-dependent manner. Arginase II solution was prepared from kidney lysate. Arginase activities were measured in the presence of different concentrations of CLE as described in Methods. Incubation of CLE significantly decreased arginase II activity (n=12 from 4 different experiments; 1-way ANOVA, p<0.01). DMSO (10 µM) was used as a control.
CLE-dependent arginase inhibition resulted in increased NO production in HUVECs
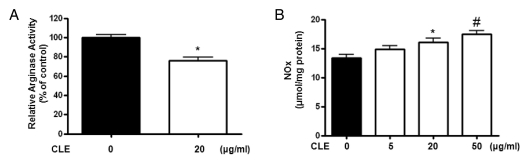
Given recent data suggesting that arginase reciprocally regulates NOx production, we tested whether CLE decreases arginase activity and increases production of NO metabolites (NOx for nitrite and nitrate) in HUVECs. As shown in Fig. 2A, CLE incubation for 18 hours at a concentration of 20 µg/ml significantly decreased arginase activity (* vs. untreated=100±6.0 vs. 76±6.9%, p<0.01). On the other hand, CLE treatment resulted in a dose dependent increase in NOx production (Fig. 2B, * vs. untreated=16.1±1.3 vs. 13.4±1.1 µmol/mg protein, p<0.05). This increase in NOx content reached a maximum level of 130% of the baseline at 50 µg/ml of CLE (Fig. 2B, # vs. untreated=17.5±1.2 vs. 13.4±1.1 µmol/mg protein, p<0.01).
Fig. 2.
CLE-dependent arginase inhibition results in increased NOx production. HUVECs were incubated with 20 µg/ml of CLE for 18 hours. CLE significantly inhibited arginase activity (A, * vs. untreated, p<0.01, n=4) and reciprocally increased NOx production in a dose-dependent manner (B, * vs. untreated, p<0.05; # vs. untreated, p<0.01, n=4).
CLE enhanced eNOS dimerization without altering expression of arginase II and eNOS proteins
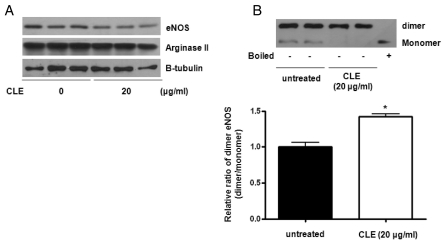
To further test the effect of CLE on the protein levels of arginase II and eNOS, Western blot analysis was performed with CLE-treated HUVECs. As demonstrated in Fig. 3A, CLE had no significant effect on the protein expression levels of arginase II and eNOS, although eNOS expression was slightly decreased. Next, we tested eNOS dimerization in order to elucidate the mechanism associated with increased NOx production by CLE treatment. Interestingly, CLE treatment (6 hours) resulted in an increased eNOS dimer/monomer ratio from 1.00±0.13 to 1.42±0.08 (Fig. 3B, * vs. untreated, p<0.01). Therefore, these data indicate that increased NOx production upon CLE treatment was dependent on the increased bioavailability of L-arginine resulting from arginase inhibition, which itself is associated with eNOS dimerization.
Fig. 3.
CLE enhances the formation of eNOS dimer without altering expression levels of arginase II and eNOS. Protein levels of arginase II and eNOS were analyzed after incubation with CLE (18 hours, 20 µg/ml). Arginase II and eNOS protein levels were not significantly changed by CLE treatment (A, n=3). CLE incubation (20 µg/ml, 6 hours), however, induced eNOS dimerization, as detected by low-temperature SDS-PAGE and Western blot analysis (B). The dimer to monomer ratio of eNOS was shown in the bar graph from 4 independent experiments (* vs. untreated, p<0.01, n=4). Boiled samples were used as a control.
Arginase inhibition by CLE increased NO production and decreased ROS production in mice aortic endothelium
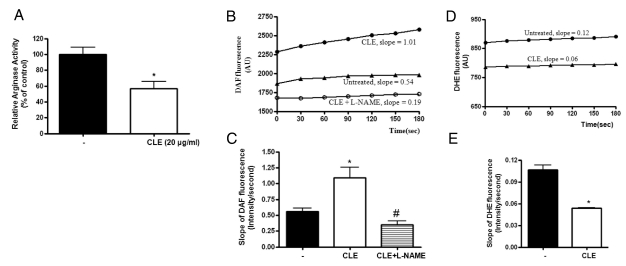
We next investigated whether increased NOx production in HUVECs translates into redox regulation in the endothelium of aortic tissue. Therefore, we measured the intensities of DAF-FM and DHE fluorescence at different time intervals. At first, CLE significantly decreased the arginase activity of isolated mice aorta treated for 16 hours (Fig. 4A, * vs. CLE, 100±9 vs. 57±9%, p<0.01). We next tested whether CLE-dependent arginase inhibition increases in NO production using an NO-sensitive fluorescence dye, DAF-FM. Incubation of aorta with CLE markedly increased the average slope of DAF fluorescence (Fig. 4C, slope of DAF fluorescence; * vs. untreated=1.09±0.16 vs. 0.56±0.05, p<0.01). On the other hand, incubation of L-NAME in the presence of CLE acutely decreased the slope of DAF fluorescence (Fig. 4C, # vs. CLE=0.35±0.06 vs. 1.09±0.16, p<0.01). This is consistent with previous observations using HUVECs. The representative traces of DAF fluorescence in the aortic endothelium were shown in Fig. 4B.
Fig. 4.
Arginase inhibition results in increased NO production and decreased superoxide generation in isolated mice aorta. Incubation of mice aortic rings with CLE (20 µg/ml, 16 hours) resulted in a significant decrease in arginase activity (A, * vs. untreated, p<0.01, n=4). (B) Pretreated aorta were loaded with DAF (5 µM) followed by measurement of fluorescence (endothelial side up). The graph shows representative traces of DAF fluorescence in CLE- and CLE plus L-NAME (10 µM)-treated aorta. (C) The slope of DAF fluorescence was monitored and then determined (* vs. untreated, p<0.01; # vs. CLE, p<0.01; n=4 mice). (D) ROS production in the aortic endothelium was traced at different time points after preloading with DHE (5 µM). (E) The slope of DHE fluorescence was determined based on cumulative data (* vs. untreated, p<0.01; # vs. CLE, p<0.01; n=4 mice).
To determine whether increased NO production upon arginase inhibition contributes to ROS reduction, we measured O2·- generation using the O2·--sensitive dye DHE in the endothelia of CLE-treated aorta. The time-dependent intensity of DHE fluorescence was decreased by incubation with CLE compared to untreated control (Fig. 4E, slope of DHE fluorescence; * vs. untreated, 0.05±0.001 vs. 0.11±0.018, p<0.01). The representative traces of DHE fluorescence are shown in Fig. 4D.
DISCUSSION
Based on the idea that arginase appears to contribute to the pathobiology of a number of diseases in which NO is dysregulated by limiting the bioavailability of L-arginine substrate, we here show that CLE, the ethylacetate extract of C. sappan L., inhibits arginases activity and reciprocally increases NO production while reducing ROS generation through enhanced stability of eNOS dimer.
The extract of C. sappan L. shows biological activities such as activation of blood circulation, and elimination of stasis. It has also been reported that the immunocompetence of lymphocytes and macrophages is significantly suppressed by the ethanolic and water extracts of C. sappan L. and that anti-complementary activity is exhibited by the methanolic extract [3]. Furthermore, the ethanolic extract of C. sappan L. inhibits proliferation of T and B lymphocytes [23]. Together with its immunosuppressive effect, the methanolic extract of C. sappan L. relaxes phenylephrine-preconstricted rat thoracic aorta, a condition associated with endothelium-dependent NO production and its signaling event such as cGMP formation [1].
CLE-dependent inhibition of arginase activity contributed to an increase in NO production in both HUVECs and the endothelium of isolated mice aorta (Fig. 2 and 4). These results are consistent with previous observations that arginase inhibition accentuates NO release in rat aortic endothelium [4], bovine pulmonary endothelial cells [7], and a porcine coronary artery model [24]. CLE mediated its increase in NO production through enhanced eNOS coupling. Under normal physiological conditions, NO synthase produces the potent vasodilator NO by catalyzing L-arginine to L-citrulline. This normal function of endothelial NOS (eNOS) requires dimerization of the enzyme, the substrate L-arginine, and the essential cofactor (6R)-5,6,7,8-tetrahydro-L-biopterin (BH4) [25]. However, the enzyme arginase uses L-arginine as a substrate and reciprocally regulates NOS by substrate depletion. There is increasing evidence that upregulation of arginase can decrease L-arginine concentrations and has been shown to promote uncoupling of eNOS, which process contributes to the pathophysiology of vascular dysfunction [4,21]. With together, several mechanisms could explain eNOS uncoupling under pathophysiological conditions, including: 1) substrate (L-arginine) depletion, 2) cofactor (BH4) depletion, 3) loss of dimerization, and 4) altered eNOS phosphorylation. During eNOS uncoupling, electrons flow from the reductase domain in the heme to molecular oxygen rather than L-arginine, resulting in production of O2·- instead of NO. As shown in Fig. 2 and Fig. 3, the availability of substrate as well as the local eNOS microdomain concentration of L-arginine by CLE-dependent arginase inhibition, rather than the expression level and abundance of eNOS enzyme, were critical to increased NO production and decreased ROS production. ROS generation is enhanced in blood vessel under various pathophysiological conditions. Superoxide is a free radical that rapidly reacts NO, thereby decreasing NO bioavailbiltiy through peoxynitrite (ONOO-) production. Finally raised peroxynitrite can give a detrimental effect to vascular cell function and viability.
Regarding the physiological roles of arginase in reciprocal NO regulation, arginase isoforms are important in regulating the synthesis of polyamines and proline [26,27], and arginase inhibition blocks HUVECs proliferation, which is an emerging phenomenon associated with angiogenesis [28]. Furthermore, reciprocal regulation of NOS by arginase has been demonstrated in cells and organs in which NO is an important signaling molecule, including the endothelium, cardiac myocytes, penis, airway, skin, and inflammatory cells [4-6,10,16,29-31]. It was demonstrated that arginase II activity is upregulated in atherosclerosis-prone mice and is associated with impaired endothelial NO production, endothelial dysfunction, vascular stiffness, and ultimately, aortic plaque development. Conversely, inhibition of endothelial arginase or deletion of the arginase II gene enhances NO production, restores endothelial function and aortic compliance, and reduces plaque burden. Therefore, arginase II represents a novel target for the prevention and treatment of atherosclerotic vascular disease [21].
Therefore, CLE inhibited the enzyme activity of arginase II in a dose-dependent manner, and this was associated with a reciprocal increase in NO production through eNOS dimerization in HUVECs. Furthermore, CLE inhibited arginase activity, increased NO production, and decreased ROS production in isolated mice aorta. Therefore, CLE may useful for the treatment of diseases associated with impaired NO production.
ACKNOWLEDGEMENTS
This work was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2010-0027720, 2010-0001289 and 2010-0023741), and the Korea Research Foundation Grand founded by the Korea government (MEST) (Regional Research Universities Program/Medical & Bio-materials Research Center).
ABBREVIATIONS
- CLE
the ethylacetate extract of the lignum of C. sappan
- NO
nitric oxide
- BH4
5,6,7,8-tetrahydro-L-biopterine
- L-NAME
NG-nitro-L-arginine methyl ester
- NOS
nitric oxide synthase
- DHE
dihydroethidine
- DAF-FM
4-Amino-5-methylamino-2',7'-difluorofluorescein
References
- 1.Xie YW, Ming DS, Xu HX, Dong H, But PP. Vasorelaxing effects of Caesalpinia sappan involvement of endogenous nitric oxide. Life Sci. 2000;67:1913–1918. doi: 10.1016/s0024-3205(00)00772-4. [DOI] [PubMed] [Google Scholar]
- 2.Baek NI, Jeon SG, Ahn EM, Hahn JT, Bahn JH, Jang JS, Cho SW, Park JK, Choi SY. Anticonvulsant compounds from the wood of Caesalpinia sappan L. Arch Pharm Res. 2000;23:344–348. doi: 10.1007/BF02975445. [DOI] [PubMed] [Google Scholar]
- 3.Oh SR, Kim DS, Lee IS, Jung KY, Lee JJ, Lee HK. Anticomplementary activity of constituents from the heartwood of Caesalpinia sappan. Planta Med. 1998;64:456–458. doi: 10.1055/s-2006-957481. [DOI] [PubMed] [Google Scholar]
- 4.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 5.White AR, Ryoo S, Li D, Champion HC, Steppan J, Wang D, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension. 2006;47:245–251. doi: 10.1161/01.HYP.0000198543.34502.d7. [DOI] [PubMed] [Google Scholar]
- 6.Steppan J, Ryoo S, Schuleri KH, Gregg C, Hasan RK, White AR, Bugaj LJ, Khan M, Santhanam L, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE. Arginase modulates myocardial contractility by a nitric oxide synthase 1-dependent mechanism. Proc Natl Acad Sci USA. 2006;103:4759–4764. doi: 10.1073/pnas.0506589103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chicoine LG, Paffett ML, Young TL, Nelin LD. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L60–L68. doi: 10.1152/ajplung.00194.2003. [DOI] [PubMed] [Google Scholar]
- 8.Klasen S, Hammermann R, Fuhrmann M, Lindemann D, Beck KF, Pfeilschifter J, Racké K. Glucocorticoids inhibit lipopolysaccharide-induced up-regulation of arginase in rat alveolar macrophages. Br J Pharmacol. 2001;132:1349–1357. doi: 10.1038/sj.bjp.0703951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis CA, Reichner JS, Henry WL, Jr, Mastrofrancesco B, Gotoh T, Mori M, Albina JE. Distinct arginase isoforms expressed in primary and transformed macrophages: regulation by oxygen tension. Am J Physiol. 1998;274:R775–R782. doi: 10.1152/ajpregu.1998.274.3.R775. [DOI] [PubMed] [Google Scholar]
- 10.Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM., Jr Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med. 2004;170:148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]
- 11.Modolell M, Corraliza IM, Link F, Soler G, Eichmann K. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur J Immunol. 1995;25:1101–1104. doi: 10.1002/eji.1830250436. [DOI] [PubMed] [Google Scholar]
- 12.Nelin LD, Wang X, Zhao Q, Chicoine LG, Young TL, Hatch DM, English BK, Liu Y. MKP-1 switches arginine metabolism from nitric oxide synthase to arginase following endotoxin challenge. Am J Physiol Cell Physiol. 2007;293:C632–C640. doi: 10.1152/ajpcell.00137.2006. [DOI] [PubMed] [Google Scholar]
- 13.Que LG, Kantrow SP, Jenkinson CP, Piantadosi CA, Huang YC. Induction of arginase isoforms in the lung during hyperoxia. Am J Physiol. 1998;275:L96–L102. doi: 10.1152/ajplung.1998.275.1.L96. [DOI] [PubMed] [Google Scholar]
- 14.Ryoo S, Lemmon CA, Soucy KG, Gupta G, White AR, Nyhan D, Shoukas A, Romer LH, Berkowitz DE. Oxidized low-density lipoprotein-dependent endothelial arginase II activation contributes to impaired nitric oxide signaling. Circ Res. 2006;99:951–960. doi: 10.1161/01.RES.0000247034.24662.b4. [DOI] [PubMed] [Google Scholar]
- 15.Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008;102:95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ, Champion HC. Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem Biophys Res Commun. 2001;283:923–927. doi: 10.1006/bbrc.2001.4874. [DOI] [PubMed] [Google Scholar]
- 17.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demougeot C, Prigent-Tessier A, Marie C, Berthelot A. Arginase inhibition reduces endothelial dysfunction and blood pressure rising in spontaneously hypertensive rats. J Hypertens. 2005;23:971–978. doi: 10.1097/01.hjh.0000166837.78559.93. [DOI] [PubMed] [Google Scholar]
- 19.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J. 2004;18:1746–1748. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- 20.Johnson FK, Johnson RA, Peyton KJ, Durante W. Arginase inhibition restores arteriolar endothelial function in Dahl rats with salt-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1057–R1062. doi: 10.1152/ajpregu.00758.2004. [DOI] [PubMed] [Google Scholar]
- 21.Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, Lim HK, Sohi J, Santhanam L, Soucy K, Tuday E, Baraban E, Ilies M, Gerstenblith G, Nyhan D, Shoukas A, Christianson DW, Alp NJ, Champion HC, Huso D, Berkowitz DE. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res. 2008;102:923–932. doi: 10.1161/CIRCRESAHA.107.169573. [DOI] [PubMed] [Google Scholar]
- 22.Woo A, Min B, Ryoo S. Piceatannol-3'-O-beta-D-glucopyranoside as an active component of rhubarb activates endothelial nitric oxide synthase through inhibition of arginase activity. Exp Mol Med. 2010;42:524–532. doi: 10.3858/emm.2010.42.7.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye M, Xie WD, Lei F, Meng Z, Zhao YN, Su H, Du LJ. Brazilein, an important immunosuppressive component from Caesalpinia sappan L. Int Immunopharmacol. 2006;6:426–432. doi: 10.1016/j.intimp.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C, Hein TW, Wang W, Chang CI, Kuo L. Constitutive expression of arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function. FASEB J. 2001;15:1264–1266. doi: 10.1096/fj.00-0681fje. [DOI] [PubMed] [Google Scholar]
- 25.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Meininger CJ, Hawker JR, Jr, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM, Jr, Wu G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab. 2001;280:E75–E82. doi: 10.1152/ajpendo.2001.280.1.E75. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Meininger CJ, Kelly KA, Hawker JR, Jr, Morris SM, Jr, Wu G. Activities of arginase I and II are limiting for endothelial cell proliferation. Am J Physiol Regul Integr Comp Physiol. 2002;282:R64–R69. doi: 10.1152/ajpregu.2002.282.1.R64. [DOI] [PubMed] [Google Scholar]
- 28.Faffe DS, Flynt L, Mellema M, Whitehead TR, Bourgeois K, Panettieri RA, Jr, Silverman ES, Shore SA. Oncostatin M causes VEGF release from human airway smooth muscle: synergy with IL-1beta. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1040–L1048. doi: 10.1152/ajplung.00333.2004. [DOI] [PubMed] [Google Scholar]
- 29.Bivalacqua TJ, Liu T, Musicki B, Champion HC, Burnett AL. Endothelial nitric oxide synthase keeps erection regulatory function balance in the penis. Eur Urol. 2007;51:1732–1740. doi: 10.1016/j.eururo.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT, Gladwin MT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109:3088–3098. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Gao X, Potter BJ, Cao JM, Zhang C. Anti-LOX-1 rescues endothelial function in coronary arterioles in atherosclerotic ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:871–877. doi: 10.1161/01.ATV.0000259358.31234.37. [DOI] [PubMed] [Google Scholar]