Abstract
In this study we determined whether or not 5-hydroxytryptamine (5-HT) has an effect on the pacemaker activities of interstitial cells of Cajal (ICC) from the mouse small intestine. The actions of 5-HT on pacemaker activities were investigated using a whole-cell patch-clamp technique, intracellular Ca2+ ([Ca2+]i) analysis, and RT-PCR in ICC. Exogenously-treated 5-HT showed tonic inward currents on pacemaker currents in ICC under the voltage-clamp mode in a dose-dependent manner. Based on RT-PCR results, we found the existence of 5-HT2B, 3, 4, and 7 receptors in ICC. However, SDZ 205557 (a 5-HT4 receptor antagonist), SB 269970 (a 5-HT7 receptor antagonist), 3-tropanylindole - 3 - carboxylate methiodide (3-TCM; a 5-HT3 antagonist) blocked the 5-HT-induced action on pacemaker activity, but not SB 204741 (a 5-HT2B receptor antagonist). Based on [Ca2+]i analysis, we found that 5-HT increased the intensity of [Ca2+]i. The treatment of PD 98059 or JNK II inhibitor blocked the 5-HT-induced action on pacemaker activity of ICC, but not SB 203580. In summary, these results suggest that 5-HT can modulate pacemaker activity through 5-HT3, 4, and 7 receptors via [Ca2+]i mobilization and regulation of mitogen-activated protein kinases.
Keywords: Interstitial cells of Cajal (ICC), Intestinal motility, Pacemaker currents, 5-hydroxytryptamine (5-HT)
INTRODUCTION
Gastrointestinal (GI) smooth muscle exhibits variable tone with superimposed rhythmic contractions. It was well-known that interstitial cells of Cajal (ICC) are pacemaker cells for GI motility and an active element with unique intracellular timing mechanisms and ionic conductance that generates the pacemaker currents that underlie slow waves. ICC are coupled electrically to each other and to neighboring smooth muscle cells via gap junctions [1]. Slow waves are generated from ICC, actively propagated in ICC networks, and conducted passively to smooth muscle cells [2].
5-hydroxytryptamine (5-HT) serves many diverse physiologic functions, such as the regulation of sleep, appetite, mood, neuroendocrine secretion, sexual behavior, cognition, and GI function [3,4]. In the GI tract, 5-HT has several distinct functions. 5-HT acts as a paracrine factor transducing information from enterochromaffin cells to intrinsic primary afferent neurons and to adjacent cells in the mucosa and submucosa [5]. Furthermore, 5-HT is a neurotransmitter and is increasingly recognized as a survival factor [6,7]. The coordinated movement of food along the GI tract is dependent on 5-HT-mediated regulation of smooth muscle tone, peristalsis, mucosal secretion, and visceral perception [8-10] via an interaction with intrinsic enteric and extrinsic afferent neurons, ICC, smooth muscle cells, and enterocytes [11-13]. The control of GI motility requires the coordinated activity of several cell types, including nerves, smooth muscle cells, and ICC. It is important to understand the role of 5-HT on ICC in the control of GI motility and suggest an understanding of the different mechanisms ICC play in the control of GI motility. With respect to GI disorders, 5-HT has been evaluated clinically to treat conditions, such as irritable bowel syndrome, diarrhea, chronic constipation, functional dyspepsia, and gastroparesis [14].
Many studies have been conducted on the presence of 5-HT receptors in the GI tract and the role of 5-HT receptors on GI motility, but no work has been conducted in pacemaker cells. In this study, we have determined the presence of 5-HT receptors and the effect of 5-HT on ICC.
METHODS
Animal and tissue preparation
Balb/C mice (3~7 days old) of either sex were anesthetized with diethylether and sacrificed by cervical dislocation. All animals were treated ethically according to the guiding principles for the care and use of animals in the field of physiologic sciences approved by the Institutional Animal Use and Care Committee at Chosun University College of Medicine. The small intestine was excised 1 cm below the pyloric ring to the cecum and opened along the mesenteric border. Luminal contents were washed away with Krebs-Ringer bicarbonate solution.
Cell culture
The isolated tissue was pinned to the base of a Slygard dish, and the mucosa was removed by sharp dissection. Small strips of intestinal muscle were equilibrated in calcium-free Hank's solution with the following constituents (in mM): KCl, 5.36; NaCl, 125; NaOH, 0.336; Na2HCO3, 0.44; glucose, 10; sucrose, 2.9; and HEPES, 11. The pH was adjusted to 7.4 with Tris for 30 minutes. Cells were dispersed by incubating for 15 minutes at 37℃ in an enzymatic solution containing collagenase (1.3 mg/ml; Wortington Biochemicals, Lakewood, NJ, USA) bovine serum albumin (2 mg/ml; Sigma, St. Louis, MO, USA), trypsin inhibitor (2 mg/ml; Sigma), and ATP (0.27 mg/ml). The cells were finely chopped and placed onto sterile glass coverslips coated with poly-L lysine in 35-mm culture dishes and incubated at 37℃ in a 95% O2 - 5% CO2 incubator in smooth muscle growth medium (SMGM; Cambrex Bio Science, Walkersville, MD, USA) supplemented with 2% antibiotics/antimycotics (Gibco, Grand Island, NY, USA) and murine stem cell factor (SCF, 5 ng/ml; Sigma).
Patch-clamp experiments
The whole cell configuration of the patch-clamp technique was used to record membrane currents (voltage clamp) and membrane potentials (current clamp) from the cultured ICC. Currents or potentials were amplified using an Axopatch 1-D (Axon Instruments, Foster City, CA, USA). Command pulse was applied using an IBM-compatible personal computer and pClamp software (version 9.2; Axon Instruments). The data were filtered at 5 KHz. All experiments were carried out at 30℃.
Solutions
The cells were bathed in a standard solution containing the following (in mM): KCl, 5; NaCl, 135; CaCl2, 2; glucose, 10; MgCl2, 1.2; and HEPES, 10. The standard solution was adjusted to pH 7.4 with Tris. The pipette solution contained the following (in mM): K-aspartate, 120; KCl, 20; MgCl2, 5; K2ATP, 2.7; Na2GTP, 0.1; creatine phosphate disodium, 2.5; EGTA, 0.1; and HEPES, 5. The solution was adjusted to pH 7.4 with Tris.
Cell separation and RT-PCR
The muscle layer isolated from the small intestine was digested by incubating for 20 minutes at 37℃ in an enzymatic solution containing collagenase (1.3 mg/ml; Wortington Biochemicals), bovine serum albumin (2 mg/ml; Sigma), trypsin inhibitor (2 mg/ml; Sigma), and ATP (0.27 mg/ml). The digested muscle was then chopped finely to make a single cell suspension. The large clumps of cells were removed by spinning down and the supernatant containing single cells was transferred to a new tube for the separation.
A Robosep cell separating machine (StemCell Technologies, Inc., Vancouver, BC, Canada) was used for this purpose. The cells were incubated with mouse CD117 PE labeling antibody, magnetic nanoparticles positive selection reagent, and PE selection cocktail according to the protocol stored (manually changed) in the automated machine. After extensive washing with the PBS buffer, we obtained the pure separated ICC. These cells were then used for the molecular studies.
Total RNA from the separated ICC were extracted using the Trizol reagent (Invitrogen, Milan, Italy) and all the steps were followed according to the manufacturer's protocol. The pure form of RNA obtained was then used for PCR amplification. RT-PCR and cDNA amplification were done using SuperScript one-step RT-PCR with Platinum Taq (Invitrogen, Milan, Italy). For amplification, sense and antisense primers were selected according to the mouse sequence, as follows: 5-HT1A (NM_008308), 5'-CTGTTTATCGCCCTGGATGT-3' and 5'-CCACTACCTGGCTGACCA-3' (451 bp); 5-HT1D (NM_008309), 5-TGCTGTCCGTCATCACACTG-3' and 5'-GGATCTGGCCAAAGTTCCAG-3' (197 bp); 5-HT2A (NM_172812), 5'-TGCCGTCTGGATTTACCTGGATGT-3' and 5'-AGTGCTGAGGTCACTCACACACAA-3' (379 bp); 5-HT2B (NM_008311), 5'-CAACTGCCTCCATCATGCATCTCT-3' and 5'-CAGCCAGTGACCCAAAGACCATAA-3' (258 bp); 5-HT2C (NM_008312), 5'-GCTCACTCCTTGTGCACCTA-3' and 5'-CTTAGTCCGCGAATTGAACC-3' (487 bp); 5-HT3 (X72395), 5'-ATCTTCATTGTGCGGCTGGT-3' and 5'-CGCATCTCATCCCGCTTCTC-3' (338 bp); 5-HT4 (NM_ 008313), 5'-TCATGGTGCTGGCCTATTACCGAA-3' and 5'-TGTAGCGCTCATCATCACAGCAGA-3' (384 bp); 5HT6 (NM_021358), 5-GGCATCCTGCTGAGCATGTTCTTT-3' and 5'-AGTCTGAATCTGAGTTGGGTGGCA-3' (334 bp); 5-HT7 (NM_008315), 5'-TGGCATGGCTGAAATGTGACAAGG-3' and 5'-TGCAGTTTGCTTGGAAAGACACCC-3' (360 bp); c-kit, 5'-GCACAGAAGGAGGCACTTATACCT-3' and 5'-TGAGACAGGAGTGGTACACCTTTG-3' (215 bp); and myosin (NM_013607.2), 5'-GAGAAAGGAAACACCAAGGTCAAGC-3' and 5'-AACAAATGAAGCCTCGTTTCCTCTC-3' (233 bp). Polymerase chain reactions (PCRs) with these primer pairs were carried out on cDNA templates derived from mRNA isolated from the separated ICC by performing reverse transcription at 45℃ for 30 min. Cycling parameters consisted of an initial denaturation at 95℃ for 5 min, then 94℃ for 30 sec, 59℃ for 30 sec, 72℃ for 30 sec for 35 cycles, followed by a 10 min final extension at 72℃. Ten microliters of the product was run with molecular weight markers (100 bp ladder) on a 2% agarose gel, then stained with ethidium bromide.
Measurement of the Intracellular Ca2+ concentration
Changes in the intracellular Ca2+ concentration ([Ca2+]i) were monitored using fluo-3/AM, which was initially dissolved in dimethyl sulfoxide and stored at -20℃. The cultured ICC on coverslips (25 mm) were rinsed twice with a bath solution. The coverslips were then incubated in the bath solution containing 1 µM fluo-4 with 5% CO2 at 37℃ for 5 min, rinsed 2 more times with the bath solution, mounted on a perfusion chamber, and scanned every 0.4 second with a Nikon Eclipse TE200 inverted microscope equipped with a Perkin-Elmer Ultraview confocal scanner and a Hamamatsu Orca ER 12-bit CCD camera (×200). Fluorescence was excited at a wavelength of 488 nm, and emitted light was observed at 515 nm. During scanning of the Ca2+ imaging, the temperature of the perfusion chamber containing the cultured ICC was kept at 30℃. The variations of intracellular Ca2+ fluorescence emission intensity were expressed as F1/F0 where F0 is the intensity of the first imaging.
Drugs and chemicals
Drugs used for the experiments, 5-HT, SB 269970, 3-tropanylindole - 3 - carboxylate methiodide, SDZ 205557 hydrochloride, PD 98059, and SB 203580 were purchased from Sigma-Aldrich. JNK inhibitor II was purchased from Calbiochem (San Diego, CA, USA). Appropriate solvents (DMSO or distilled water) were used to dissolve the drugs and stock solutions (10 or 100 mM) were prepared and stored in aliquots at the designated temperatures. All the light-sensitive drugs were protected from light by wrapping the tubes containing drugs with aluminum foil. The required concentrations of the drugs were prepared at the time of the experiments and added to the bath solution. All drugs were applied to the whole cell preparation by superfusion. The final concentration of DMSO in all the drug preparations was <0.5%.
Statistical analysis
Data is expressed as the mean±standard errors. Differences in the data were evaluated by a Student's t-test. A p value <0.05 was taken as a statistically significant difference. The n values reported in the text refer to the number of cells used in the patch-clamp experiments.
RESULTS
Effect of 5-HT on pacemaker potentials generated by ICC
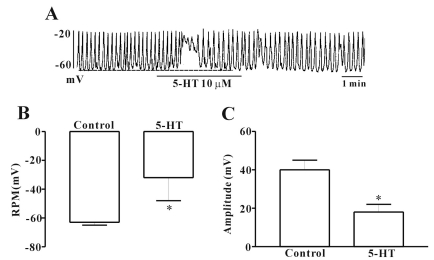
ICC cultured from the murine small intestine were identified with the Kit immunofluroescence. Cells with Kit positive had a distinctive morphology that was easily recognized in cultures. For investigating the effects of 5-HT, we performed electrophysiologic recording from cultured ICC under current clamp mode (I=0), in which spontaneous depolarization (pacemaker potentials) was generated by ICC. The resting membrane potential was -63±2 mV and the amplitude of pacemaker potential was 40±5 mV. In the presence of 5-HT (10 µM), the membrane potentials were depolarized to -32±16 mV, and the amplitude of the pacemaker potentials was decreased to 18±4 mV (n=7; Fig. 1). And the effect of 5-HT on pacemaker potentials recovered about 2 minutes during treatment of drug. So in next experiments, we used 2 minutes treatment times for application of 5-HT on ICC.
Fig. 1.
Effects of 5-HT on pacemaker potentials recorded in ICC from mouse small intestine. (A) shows the pacemaker potentials of ICC exposed to 5-HT (10 µM) in the current clamping mode (I=0). 5-HT induced membrane depolarization and inhibited the amplitude and frequency of pacemaker potential in ICC. The dot lines indicate the control resting membrane potentials levels. Responses to 5-HT are summarized in (B) and (C). Bars represent the means±SE. *Asterisks indicate significantly different from controls (p<0.05). RMP, resting membrane potentials.
Effect of 5-HT on pacemaker currents generated by ICC
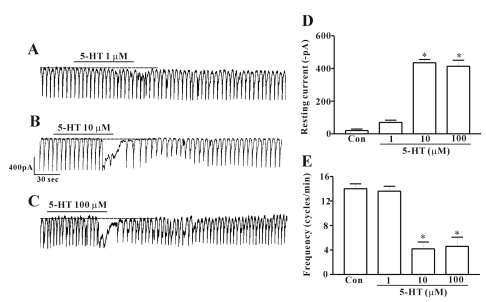
Under a voltage clamp at a holding potential of -70 mV, ICC generated pacemaker currents. When ICC were treated with 5-HT, inward currents occurred. At a concentration of 1 µM, the effect of 5-HT on the pacemaker current was not significant compared with control (n=7; Fig. 2A). However, when the concentration of 5-HT was increased to 10 µM, a significant effect was noted (Fig. 2B). At a concentration of 10 µM, the resting current was -463±67 pA and the corresponding frequency was 9±0.6 cycles/min (n=10; Figs. 2D and E). Also, when ICC were treated with 100 µM 5-HT, the effect to the 10 µM concentration was similar (n=8; Fig. 2C). The value obtained after treatment with 5-HT was significantly different from the control values and 5-HT at a concentration of 10 µM showed the maximum effect. Thus, further experiments were carried out at this concentration. And we found 5-HT also showed a desensitization effect on the pacemaker current (data not shown) and after treatment with 5-HT, it took 15 minutes washing time for showing the same effect on the pacemaker current by retreatment of 5-HT. So after treatment with the drug in this experiment, the cells were washed for 15 minutes to washout all the drug completely before treating with drugs again.
Fig. 2.
Effects of 5-HT on pacemaker currents recorded in cultured ICC from mouse small intestine. (A), (B) and (C) show pacemaker currents of ICC exposed to 5-HT (1, 10, or 100 µM) at a holding potential of -70 mV. Vertical solid line represents amplitude of pacemaker current and horizontal solid line represents duration of recording(s) pacemaker currents. The dot lines indicate the control resting current levels. (D) and (E) summarize the effects of 5-HT on pacemaker currents in ICC. Bars represent the means±SE. *Asterisks indicate significantly different from controls (p<0.05). Con, control.
Identification of receptor subtypes of 5-HT in cultured ICC
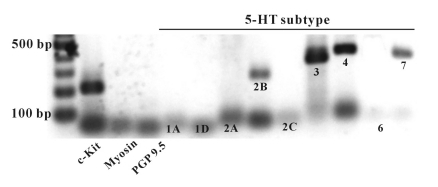
In this study, the receptor subtypes of 5-HT were identified by the RT-PCR method. To determine the presence of 5-HT receptor subtypes, RT-PCR with isolated c-kit-positive cells was performed using 5-HT receptor subtype gene-specific primers. In ICC, RT-PCR detected transcripts for 5-HT2B, 3, 4, and 7 (Fig. 3), but the specific primers (5-HT1A, 1D, 2A, 2C, and 6) did not produce cDNA fragments of appropriate size (Fig. 3).
Fig. 3.
Agarose gels of the RT-PCR products of 5-HT receptor subtypes using separated ICC. Amplified cDNA prepared from pure ICC separated by magnetic cell separation and visualized in 2% gel. c-Kit - ICC marker, myosin - smooth muscle cells marker, PGP9.5 - neuronal cell marker.
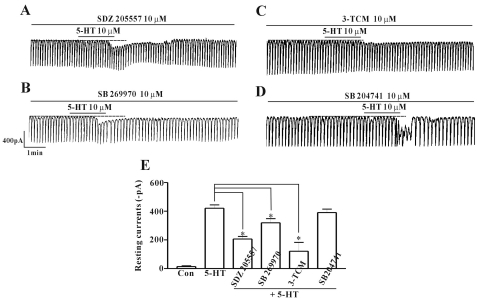
To verify the receptor subtypes of 5-HT, various 5-HT receptor antagonists were pre-treated, then co-treated with 5-HT. SDZ 205557 (a 5-HT4 receptor antagonist), SB 269970 (a 5-HT7 receptor antagonist), 3-tropanylindole - 3 - carboxylate methiodide (3-TCM; a 5-HT3 antagonist), and SB 204741 (a 5-HT2B receptor antagonist) were all used to pre-treat cultured ICC at a concentration of 10 µM for 10 min and then co-treated with 5-HT. After pre-treatment with SDZ 205557, the inward current produced by 5-HT was partially blocked (Fig. 4A). The resting current produced in the presence of SDZ 205557 was -206±18 pA (n=6; Fig. 4E). SB 269970 also partially blocked the inward current produced by 5-HT with an inward current of - 319±29 pA (n=5; Figs. 4B and E). The 5-HT-induced inward current was also blocked by 3-TCM (n=5; Figs. 4C and E). As a commercial antagonist specific for 5-HT3, 4, and 7 receptors at the same time was not available, the mixture of each antagonists was pre-treated and the action of 5-HT was observed. This completely blocked the effect of 5-HT action (data not shown). Next, SB 204741 was used to check if there was any role on the 5-HT-induced inward current. The pre-treated ICC with SB 204741 did not block the effect of 5-HT (n=5; Figs. 4D and E).
Fig. 4.
Effects of 5-HT receptor subtype antagonists on 5-HT-induced responses on pacemaker currents in cultured ICC of the mouse small intestine. (A) Pacemaker currents of ICC exposed to 5-HT (10 µM) in the presence of 5-HT4 receptor antagonist (SDZ 205557; 10 µM). (B) Pacemaker currents of ICC exposed to 5-HT (10 µM) in the presence of 5-HT7 receptor antagonist (SB 269970; 10 µM). (C) Pacemaker currents of ICC exposed to 5-HT (10 µM) in the presence of 5-HT3 receptor antagonist 3-TCM (10 µM). (D) Pacemaker currents of ICC exposed to the selective 5-HT2B receptor antagonist SB 204741 (10 µM). Responses to 5-HT in presence of different receptor antagonists are summarized in (E). Bars represent the mean values±S.E. The dotted lines indicate the zero current levels. *Asterisks indicate significantly different from the control (p<0.05). Con, control.
Excitation of [Ca2+]i oscillation by 5-HT
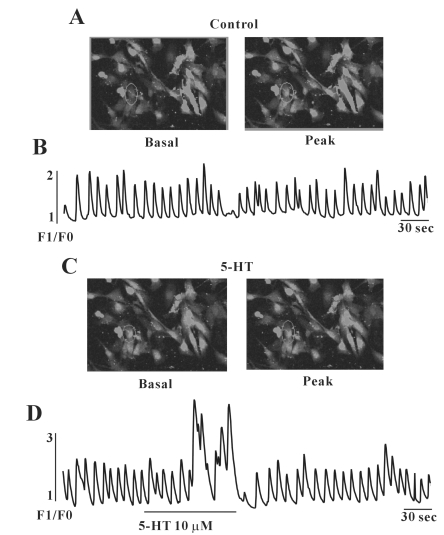
Because many reports suggested that [Ca2+]i oscillations in ICC are considered to be the primary mechanism for the pacemaker activity in gastrointestinal activity, we examined the effect of 5-HT on [Ca2+]i oscillations in ICC. In this study, we measured spontaneous [Ca2+]i oscillations of ICC which are connected with cell clusters. Spontaneous [Ca2+]i oscillations were observed in many ICC which were loaded with 1 µM fluo-3. Figs. 5A and B show images of basal (F0) and peak point (F1) of Ca2+ oscillations. Furthermore, in the presence of 5-HT (10 µM), the [Ca2+]i concentration in ICC was increased, i.e., excited (Figs. 5C and D).
Fig. 5.
Effects of 5-HT on [Ca2+]i oscillation in cultured ICC from mouse small intestine. (A) shows the basal and peak point of ICC image and (B) Ca2+ oscillation in the normal condition. (C) shows the basal and peak point of ICC image and (D) Ca2+ oscillation in the presence of 5-HT (10 µM). The interval of representative frame was 1 second and the exposure time of each frame was 500 ms.
Involvement of mitogen-activated protein kinases (MAPKs) in the 5-HT-induced depolarization of pacemaker currents
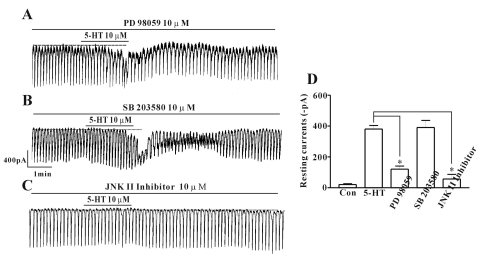
Because many reports have suggested 5-HT activates MAPKs in many cell types, we determined whether or not MAPKs are involved in the 5-HT-induced effects using PD 98059 (a p42/44 MAPK inhibitor), SB 203580 (a p38 MAPK inhibitor), or a c-jun NH2-terminal kinase (JNK) II inhibitor. In the presence of PD 98059 (10 µM), 5-HT still activated an inward current, of which amplitude is, however, smaller than that activated by 5-HT treatment alone. Thus, p42/44 has a role in the5-HT-induced inward current, as PD 98059 partially blocked the effect (n=6; Figs. 6A and D). In the presence of SB 203580 (10 µM), there was no change in the effect of 5-HT on the pacemaker current (n=5; Figs. 6B and D), but the JNK II inhibitor blocked the effect of 5-HT in the pacemaker current (n=8; Figs. 6C and D).
Fig. 6.
Effects of various MAPK inhibitors on 5-HT induced responses on pacemaker currents in cultured ICC of the mouse small intestine. (A) Pacemaker currents of ICC exposed to 5-HT (10 µM) in the presence of PD 98059, a p42/44 MAPK inhibitor (10 µM). (B) Pacemaker currents of ICC exposed to 5-HT (10 µM) in the presence of SB 203580, a p38 MAPK inhibitor (10 µM). (C) Pacemaker currents of ICC exposed to 5-HT (10 µM) in the presence of JNK II inhibitor (10 µM). Responses to 5-HT in presence of different MAPK inhibitors are summarized in (D). Bars represent mean values±S.E. The dotted lines indicate the zero current levels. *Asterisks indicate significantly different from control (p<0.05). Con, control.
DISCUSSION
In the present study, it was found that 5-HT depolarizes the membrane potential and produced a tonic inward pacemaker current in ICC. The effect produced by 5-HT was mediated by the 5-HT3, 5-HT4, and 5-HT7 receptors through MAPK.
To date, many experiments have been carried out and the 5-HT receptors in ICC [13,15,16] have been shown, but the physiologic action on ICC and which receptor types for functional action are involved have not been determined. Of the 7 classes of 5-HT receptors, 5-HT1, 5-HT2, 5-HT3, 5-HT4, and 5-HT7 are known to be expressed in the GI tract [17]. The distribution in GI tissues is heterogeneous; some are distributed on nerve terminals and others are distributed on smooth muscle [18]. The main objective of the present study was to determine the action of 5-HT on the spontaneous pacemaker current generated by ICC and the receptor subtypes involved. In this study, 5-HT produced the tonic inward current. Together, 5-HT also depolarized the membrane potential of ICC. Thus, the 5-HT regulation on GI motility can be by modulation of pacemaker activity in ICC.
The next goal of this study was to determine which receptor subtypes of 5-HT were involved in the effect produced by 5-HT. Molecular studies were conducted to determine which 5-HT receptors genes were expressed in ICC. From the molecular studies, 5-HT2B, 3, 4, and 7 were shown to be expressed in ICC. In the whole-cell patch-clamp study, ICC showing spontaneous inward current was treated with potent 5-HT3, 4, and 7 receptor antagonists, which could block the effect of 5-HT. Each blocker alone did not completely block the effect of 5-HT, suggesting the possibility that 5-HT has action on pacemaker activity in ICC through proper action with these receptor subtypes. In a previous study, 5-HT3 receptor immunoreactivity was expressed on neurons of the myenteric and submucosal plexuses, ICC, and fibers in the circular and longitudinal muscle layers, submucosa and mucosa [16], and 5-HT3 receptor activation was associated with increased electrically-evoked contractions of guinea pig and mouse stomach corpus and fundus circular smooth muscle [19,20]. Interestingly, the 5-HT2B receptor was reported to be present in ICC, which regulates the proliferation of ICC in mouse jejunum [13]. Thus, 5-HT2B, 3, 4, and 7 expressed in ICC and 5-HT3, 4, and 7 have the role for regulating the functional electrical activity of ICC, but not 5-HT2B.
Many reports have suggested pacemaker activity in ICC depends on [Ca2+]i release [21]. It is well-known that inositol 1,4,5-triphosphate receptor plays an important role for generating spontaneous electrical activity in pacemaker cells [22] and periodic Ca2+ release from [Ca2+]i stores produces [Ca2+]i oscillations in ICC from mouse ileum [23], and these actions in ICC are considered to be the primary pacemaker activity in the gut. Furthermore in our previous reports, we showed spontaneous [Ca2+]i oscillations in ICC and found that carbachol and PGE2 regulated the [Ca2+]i oscillations [24,25], suggesting that [Ca2+]i in ICC is the important element regulating pacemaker activity. In the studies conducted on 5-HT, the intracellular calcium was increased and returned back to normal conditions showing a similar effect as electrophysiologic studies. This result means that the generation of tonic inward currents by 5-HT is due to the modulation of [Ca2+]i in ICC.
The MAPKs signaling pathway plays an important role in the mediation of cellular responses, including visceral smooth muscle contraction. Three principal MAPKs are expressed in various tissues (p42/44, JNK, and p38 MAPK) [26]. Reports are there demonstrating activation of MAP kinase by 5-HT7 receptors in hippocampal neurons [27] and 5-HT induces cyclooxygenase (COX)-2 by acting on MAPK activation in vascular smooth muscle cells [28]. Therefore, 5-HT can regulate the MAPK system. In the present study, an inhibitor of p42/44 and the JNK II inhibitor inhibited the effect of 5-HT suggesting that p42/44 and JNK MAPK may be involved in the modulation of pacemaker currents by 5-HT, but a p38 MAPK inhibitor did not inhibit the action of 5-HT. However, the action on MAPK by 5-HT commonly relate with pathologic conditions in various tissues. In pathologic conditions, 5-HT stimulates the production of the COX enzyme by acting MAPK and then prostanoids exert functions in most cells. In our previous study, we showed prostaglandin E2 and F2α have actions on pacemaker activity in ICC [24,29]. Whether or not the effect of 5-HT on ICC is by COX induction was not proven in the current study, thus further studies are needed.
In summary, the present results indicate that 5-HT directly alters pacemaker currents through 5-HT3, 4, and 7 receptors via [Ca2+]i mobilization and MAPKs regulation, thus suggesting a possibility that the function of GI motility induced by 5-HT may involve the regulation of ICC by 5-HT. However, additional studies are required for elucidation of the signaling pathway involved in the action of 5-HT in ICC.
ACKNOWLEDGEMENTS
This work was supported by Korea Research Foundation Grant funded by Korea Government (311-2008-2-E0040) and Chosun University.
ABBREVIATIONS
- 5-HT
5-hydroxytryptamine
- GI
gastrointestinal
- ICC
interstitial cells of Cajal
- [Ca2+]i
intracellular Ca2+ concentration
- MAPKs
mitogen-activated protein kinases
- JNK
c-jun NH2-terminal kinase
- COX
cyclooxygenase
References
- 1.Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995;269:C1577–C1585. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- 2.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 3.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 4.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 5.Zhu JX, Zhu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001;530:431–442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiorica-Howells E, Maroteaux L, Gershon MD. Serotonin and the 5-HT(2B) receptor in the development of enteric neurons. J Neurosci. 2000;20:294–305. doi: 10.1523/JNEUROSCI.20-01-00294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nebigil CG, Etienne N, Messaddeq N, Maroteaux L. Serotonin is a novel survival factor of cardiomyocytes: mitochondria as a target of 5-HT2B receptor signaling. FASEB J. 2003;17:1373–1375. doi: 10.1096/fj.02-1122fje. [DOI] [PubMed] [Google Scholar]
- 8.Baker DE. Rationale for using serotonergic agents to treat irritable bowel syndrome. Am J Health Syst Pharm. 2005;62:700–711. doi: 10.1093/ajhp/62.7.700. [DOI] [PubMed] [Google Scholar]
- 9.Hansen MB, Skadhauge E. Signal transduction pathways for serotonin as an intestinal secretagogue. Comp Biochem Physiol A Physiol. 1997;118:283–290. doi: 10.1016/s0300-9629(97)00085-6. [DOI] [PubMed] [Google Scholar]
- 10.Jin JG, Foxx-Orenstein AE, Grider JR. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther. 1999;288:93–97. [PubMed] [Google Scholar]
- 11.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Read NW, Gwee KA. The importance of 5-hydroxytryptamine receptors in the gut. Pharmacol Ther. 1994;62:159–173. doi: 10.1016/0163-7258(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 13.Wouters MM, Gibbons SJ, Roeder JL, Distad M, Ou Y, Strege PR, Szurszewski JH, Farrugia G. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology. 2007;133:897–906. doi: 10.1053/j.gastro.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Beattie DT, Smith JA. Serotonin pharmacology in the gastrointestinal tract: a review. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:181–203. doi: 10.1007/s00210-008-0276-9. [DOI] [PubMed] [Google Scholar]
- 15.Epperson A, Hatton WJ, Callaghan B, Doherty P, Walker RL, Sanders KM, Ward SM, Horowitz B. Molecular markers expressed in cultured and freshly isolated interstitial cells of Cajal. Am J Physiol Cell Physiol. 2000;279:C529–C539. doi: 10.1152/ajpcell.2000.279.2.C529. [DOI] [PubMed] [Google Scholar]
- 16.Glatzle J, Sternini C, Robin C, Zittel TT, Wong H, Reeve JR, Jr, Raybould HE. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology. 2002;123:217–226. doi: 10.1053/gast.2002.34245. [DOI] [PubMed] [Google Scholar]
- 17.De Ponti F. Pharmacology of serotonin: what a clinician should know. Gut. 2004;53:1520–1535. doi: 10.1136/gut.2003.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gershon MD. Review article: serotonin receptors and transporters -- roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther. 2004;20(Suppl 7):3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 19.Buchheit KH, Buhl T. Stimulant effects of 5-hydroxytryptamine on guinea pig stomach preparations in vitro. Eur J Pharmacol. 1994;262:91–97. doi: 10.1016/0014-2999(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 20.Xue L, Camilleri M, Locke GR, 3rd, Schuurkes JA, Meulemans A, Coulie BJ, Szurszewski JH, Farrugia G. Serotonergic modulation of murine fundic tone. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1180–G1186. doi: 10.1152/ajpgi.00224.2006. [DOI] [PubMed] [Google Scholar]
- 21.Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525:355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, Mikoshiba K. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J Physiol. 2000;525:105–111. doi: 10.1111/j.1469-7793.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aoyama M, Yamada A, Wang J, Ohya S, Furuzono S, Goto T, Hotta S, Ito Y, Matsubara T, Shimokata K, Chen SR, Imaizumi Y, Nakayama S. Requirement of ryanodine receptors for pacemaker Ca2+ activity in ICC and HEK293 cells. J Cell Sci. 2004;117:2813–2825. doi: 10.1242/jcs.01136. [DOI] [PubMed] [Google Scholar]
- 24.Choi S, Yeum CH, Chang IY, You HJ, Park JS, Jeong HS, So I, Kim KW, Jun JY. Activating of ATP-dependent K+ channels comprised of K(ir) 6.2 and SUR 2B by PGE2 through EP2 receptor in cultured interstitial cells of Cajal from murine small intestine. Cell Physiol Biochem. 2006;18:187–198. doi: 10.1159/000097516. [DOI] [PubMed] [Google Scholar]
- 25.So KY, Kim SH, Sohn HM, Choi SJ, Parajuli SP, Choi S, Yeum CH, Yoon PJ, Jun JY. Carbachol regulates pacemaker activities in cultured interstitial cells of Cajal from the mouse small intestine. Mol Cells. 2009;27:525–531. doi: 10.1007/s10059-009-0076-1. [DOI] [PubMed] [Google Scholar]
- 26.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 27.Errico M, Crozier RA, Plummer MR, Cowen DS. 5-HT(7) receptors activate the mitogen activated protein kinase extracellular signal related kinase in cultured rat hippocampal neurons. Neuroscience. 2001;102:361–367. doi: 10.1016/s0306-4522(00)00460-7. [DOI] [PubMed] [Google Scholar]
- 28.Machida T, Ohta M, Onoguchi A, Iizuka K, Sakai M, Minami M, Hirafuji M. 5-Hydroxytryptamine induces cyclooxygenase-2 in rat vascular smooth muscle cells: mechanisms involving Src, PKC and MAPK activation. Eur J Pharmacol. 2011;656:19–26. doi: 10.1016/j.ejphar.2010.12.044. [DOI] [PubMed] [Google Scholar]
- 29.Park CG, Kim YD, Kim MY, Koh JW, Jun JY, Yeum CH, So I, Choi S. Effects of prostaglandin F2α on small intestinal interstitial cells of Cajal. World J Gastroenterol. 2011;17:1143–1151. doi: 10.3748/wjg.v17.i9.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]