Abstract
Golgi SNAP receptor complex 1 (GS28) has been implicated in vesicular transport between intra-Golgi networks and between endoplasmic reticulum (ER) and Golgi. Additional role(s) of GS28 within cells have not been well characterized. We observed decreased expression of GS28 in rat ischemic hippocampus. In this study, we examined the role of GS28 and its molecular mechanisms in neuronal (SK-N-SH) cell death induced by hydrogen peroxide (H2O2). GS28 siRNA-transfected cells treated with H2O2 showed a significant increase in cytotoxicity under glutathione (GSH)-depleted conditions after pretreatment with buthionine sulfoximine, which corresponded to an increase of intracellular reactive oxygen species (ROS) in the cells. Pretreatment of GS28 siRNA-transfected cells with p38 chemical inhibitor significantly inhibited cytotoxicity; we also observed that p38 was activated in the cells by immunoblot analysis. We confirmed the role of p38 MAPK in cotransfected cells with GS28 siRNA and p38 siRNA in the cell viability assay, flow cytometry, and immunoblot. Involvement of apoptotic or autophagic processes in the cells was not shown in the cell viability, flow cytometry, and immunoblot analyses. However, pretreatment of the cells with necrostatin-1 completely inhibited H2O2-induced cytotoxicity, ROS generation, and p38 activation, indicating that the cell death is necroptotic. Collectively these data imply that H2O2 induces necroptotic cell death in the GS28 siRNA-transfected cells and that the necroptotic signals are mediated by sequential activations in RIP1/p38/ROS. Taken together, these results indicate that GS28 has a protective role in H2O2-induced necroptosis via inhibition of p38 MAPK in GSH-depleted neuronal cells.
Keywords: GS28, Hydrogen peroxide, Glutathione, MAPK, Necroptosis
INTRODUCTION
Under metabolic processes, cells can generate a partially reduced form of oxygen referred to as reactive oxygen species (ROS) [1]. ROS are generated by various enzyme systems, such as the mitochondrial electron transport chain, NADPH oxidase, xanthine oxidase, cytochrome P450, and lipoxygenase [2]. Incomplete reduction of an oxygen molecule during mitochondrial respiration leads to production of a superoxide anion that is enzymatically dismutated to hydrogen peroxide (H2O2), a type of ROS. By Fenton reaction, H2O2 is converted to hydroxyl radicals, which can cause damage to DNA, membrane lipids, and proteins. Mammalian cells maintain intracellular ROS at a low level through antioxidant systems such as superoxide dismutase, vitamins E and C, and glutathione (GSH) [3]. GSH plays a central role in maintaining redox homeostasis [4]. Elevated ROS elicit a variety of oxidative stress responses ranging from cell proliferation to growth arrest to cell death [5,6]. The particular outcome can vary depending on cell type as well as the concentration and duration of ROS exposure.
It is well known that elevated ROS can induce apoptosis or necrosis that occurs usually depending on the level of intracellular ROS and ATP [1]. The existence of necrotic cell death regulated by an intrinsic death program distinct from that of apoptosis was also proposed, termed necroptosis [7]. It was reported that oxidative stress induces necroptosis, which is a caspase-independent nonapoptotic cell death and appears morphologically similar to necrosis [8]. Recent studies demonstrated that ROS induce autophagic cell death, another type of programmed cell death distinct from apoptosis [9,10]. Molecular mechanisms in cell injury have been well demonstrated in ROS-induced apoptosis. Studies have implicated a major role for mitogen-activated protein kinases (MAPKs) in the signaling and have suggested other kinases also play a role, such as phosphatidylinositol 3-kinase (PI3K)/AKT, protein kinase C (PKC), and nuclear factor-κB (NF-κB) [1].
We identified a gene related to oxidative stress, Golgi SNAP receptor complex 1 (GS28), and designed experiments to characterize the roles of this gene in cellular oxidative injury. Decreased expression of the GS28 gene was shown in rat ischemic hippocampus. In this study, we examined the role of GS28 and its molecular mechanisms in neuronal cell death induced by H2O2.
METHODS
Materials
The SK-N-SH cell line (HTB-11) was purchased from ATCC (Rockville, MD). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were from Thermo Scientific (Waltham, MA). Lipofectamine™ 2000 and Opti-MEM were from Invitrogen Corporation (Carlsbad, CA). Hydrogen peroxide (H2O2), buthionine sulfoximine (BSO), deferoxamine mesylate (DFO), bafilomycin A1, dimethylsulfoxide (DMSO), phosphate-buffered saline (PBS), and 2',7'-dichlorodihydrofluorescein diacetate (H2DCF-DA) were from Sigma (St Louis, MO). The GS28 antibody was from BD Transduction Laboratories (San Jose, CA). The β-actin antibody and secondary mouse and rabbit antibodies were from Sigma. The Bax antibody was from Delta Biolabs (Gilroy, CA). Microtubule-associated protein 1 light chain 3 (LC3), caspase-3, PARP, p-p38, and beclin-1 antibodies were from Cell Signaling Technology (Danvers, MA). The GAPDH antibody and necrostatin-1 (Nec-1) were from Santa Cruz Biotechnology (Santa Cruz, CA). SB202190, SP600125, wortmannin, and tempol were from Calbiochem (San Diego, CA), and zVAD-fmk was from Tocris (Ellisville, MO). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was from Duchefa (Haarlem, Netherlands). The PVDF membrane was from GE Healthcare (Buckinghamshire, England). ECL Western Blotting Detection Reagent was from Pierce (Rockford, IL). Immobilon™ Western was from Millipore (Billerica, MA). Bradford reagent was from Bio-Rad (Hercules, CA). The ApoScan Annexin V-FITC apoptosis detection kit was from BioBud (Seoul, Korea).
Cell culture, siRNA transfection, and cell viability assay
Human neuroblastoma SK-N-SH cells were cultured in DMEM supplemented with 10% heat-inactivated FBS at 37℃ in 5% CO2/95% air. Cells were transfected using Lipofectamine™ 2000 according to the manufacturer's instructions. The day before transfection, cells were seeded at a concentration of 4.5×105 cells per well in a 6 well plate. Cells were transfected with small interfering RNA (siRNA) targeted for each gene. After 2 days, the cells were seeded into 96-well plates at 1.2×104 cells per well in medium containing with 200 µM BSO, a specific inhibitor of glutathione (GSH) synthesis. After overnight culture, the cells were treated with various concentrations of H2O2 with 200 µM BSO for 24 h. Cell viability was analyzed by MTT reduction assay. The cell viability assay also was performed following pretreatment with MAPK inhibitors or chemicals 1 h before addition of H2O2. The inhibitors or chemicals used were SB202190 (P38 inhibitor), SP600125 (JNK/SAPK inhibitor), wortmannin (PI3K inhibitor), zVAD-fmk (pan-specific caspase inhibitor), Nec-1 (selective RIP1 inhibitor), DFO (iron chelator), and tempol (ROS scavenger). Duplex siRNAs for GS28 and Atg5 were 5'-GCAUUGCUAUGGCAACAAA-3' and 5'-GGAAUAUCCUGCAGAAGAA-3', respectively, and were synthesized by Ambion (Austin, TX). MAPK14 siRNA was from Dharmacon (Lafayette, CO). Negative control siRNA was purchased from Ambion. Efficiency in silencing gene expression was determined three days after transfection by immunoblots. Cell viabilities were expressed as a percentage of that observed in untreated cells. Effect on cell viability by the silencing siRNAs was compared with that in cells transfected with a non-silencing siRNA control.
Detection of intracellular ROS
Detection of intracellular ROS was performed as described previously [1]. To visualize intracellular ROS generation at the end of treatments, cells were incubated for 10 min with 5 µM H2DCF-DA. The cells were washed with PBS, and DCF fluorescence intensity was monitored using a fluorescence microscope (IX71, Olympus, Tokyo, Japan). To determine the intracellular ROS levels, the fluorescence intensities emitted at 515~545 nm from the suspended cells incubated with 5 µM H2DCF-DA were measured using a flow cytometer (FACSCalibur, Becton Dickinson, San Jose, CA) and analyzed using CellQuest software (Becton Dickinson). The fluorescent intensity in the cells transfected with silencing siRNAs was compared to that in the cells transfected with control siRNA.
Flow cytometric analysis of apoptosis
Determination of apoptosis was performed using an ApoScan Annexin V-FITC apoptosis detection kit according to the manufacturer's protocol as described previously [11]. Cells transfected with siRNA were treated with H2O2 for 2 h, and the cells were collected and washed twice in PBS. The cells were incubated for 15 min at room temperature with annexin V-FITC, and propidium iodide (PI) was added. The fluorescence intensities from the cells were measured with the FL1 channel for detecting FITC (518 nm) and with the FL2 channel for detecting PI (620 nm) using a flow cytometer (FACSCalibur) and analyzed using CellQuest software (Becton Dickinson).
Immunoblot analysis
On the third day of transfection, cells were treated with H2O2. After incubation of indicated times, cells were harvested and lysed with RIPA buffer. After centrifugation of lysates, supernatants were mixed with an equal volume of 2× SDS-PAGE loading buffer. Protein concentrations were quantified using Bradford solution. Equal amounts of the cell lysates were loaded and separated by SDS-PAGE, and proteins were transferred to PVDF membranes. The membranes were incubated with primary antibodies; next, they were incubated with secondary antibodies conjugated to horseradish peroxidase. Immunoreactive protein signals were visualized using ECL™ Western Blotting Detection Reagents or Immobilon™ Western. β-actin or GAPDH were used as internal controls in the immunoblot analysis. The conversion of LC3 was expressed in the ratio of LC3-II to LC3-I determined by densitometry of the immunoblots. Western blots also were performed following pretreatment with DFO, tempol, or Nec-1 1 h before the addition of H2O2.
Statistical analysis
Analysis and data graphing were done with Prism 4.0 (GraphPad Software, San Diego, CA). Data are expressed as means±SEM of at least three independent experiments. Statistical analysis was performed by two-way ANOVA for multiple group comparisons followed by Bonferroni post-tests. A value of p<0.05 was considered statistically significant.
RESULTS
GS28 on H2O2-induced neuronal cytotoxicity in GSH-depleted conditions
Libraries of cDNA prepared from ischemia/reperfusion (I/R)-treated and -untreated hippocampal tissues were used to isolate genes related to oxidative injury by a differential screening method as described previously [12]. One of the identified genes showing decreased expression in the I/R-treated hippocampus was GS28 (data not shown). In this study, we examined the role of GS28 and its molecular mechanisms in neuronal (SK-N-SH) cell death induced by H2O2 as an oxidative stress. In contrast to a decrease in ischemic hippocampal tissues, the increase of GS28 gene expression in the neuronal cells treated with H2O2 was demonstrated in immunoblot analysis (Fig. 1A), which suggests that GS28 plays roles in oxidative injury. To examine the molecular mechanisms in this condition, we introduced GS28 siRNA into the cells to silence gene expression. GS28 siRNA was efficient in silencing gene expression compared to that of control siRNA three days after transfection as seen by immunoblot (Fig. 1B). Without pretreatment with BSO, the change of cytotoxicity in the GS28 siRNA-transfected cells treated with H2O2 was not shown in a cell viability assay (Fig. 1C). After overnight pretreatment with BSO, GS28 siRNA-transfected cells treated with H2O2 showed a significant, dose-dependent increase in cytotoxicity compared to that of cells transfected with a control non-silencing siRNA (Fig. 1D). The following experiments were performed under GSH-depleted conditions with BSO pretreatment. In the assays, H2O2 was applied to cells at concentrations ranging from 0 to 400 µM. The GSH level in cells treated with 200 µM BSO was negligible compared to that of non-treated cells (data not shown). These data suggest that GS28 plays protective roles against H2O2-induced cytotoxicity under GSH-depleted conditions.
Fig. 1.
Increase of H2O2-induced cytotoxicity by silencing gene expression of the GS28 in glutathione (GSH)-depleted neuronal cells. (A) After treatment of neuroblastoma SK-N-SH cells with 400 µM H2O2 for indicated times, expression of GS28 in the cell lysates was examined by immunoblot analysis using GS28 or GAPDH antibody. (B) siRNAs were transfected into cells by lipofectamine, and efficiency of silencing GS28 expression was examined with immunoblot 3 days after transfection. The transfected cells were treated with various concentrations of H2O2 for 24 h in the absence (C) or presence (D) of 200 µM buthionine sulfoximine (BSO). Cell viability was determined by an MTT reduction assay. The data represent the mean±SEM of 3 independent experiments. *p<0.05 versus the values in control siRNA-transfected cells (two-way ANOVA, Bonferroni posttests).
H2O2-induced ROS responsible for cytotoxicity
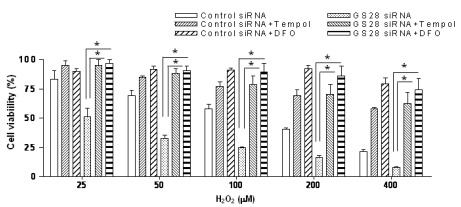
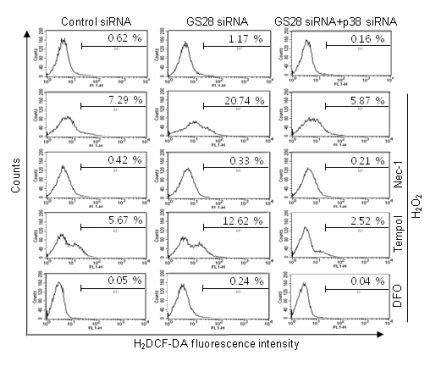
It was well-demonstrated that intracellular H2O2 can be converted to hydroxyl radicals via Fenton reaction. We examined whether or not H2O2 addition correlates with the generation of ROS in the GS28 siRNA-transfected cells. Pretreatment of GS28 siRNA-transfected cells with tempol or DFO significantly inhibited the cytotoxicity of H2O2 in cell viability assays (Fig. 2). We measured the intracellular ROS accumulation by staining cells with H2DCF-DA. A significant increase of DCF fluorescence in flow cytometry analysis was shown in GS28 siRNA-transfected cells treated with H2O2 for 3 h (Fig. 4). The increase of fluorescence in the cells was inhibited by pretreatment with iron chelator (DFO) and ROS scavenger (tempol). These data suggest that ROS generated by the H2O2 are responsible for the cytotoxicity in GS28 siRNA-transfected cells, and GS28 is involved in regulation of intracellular ROS levels.
Fig. 2.
Contribution of H2O2-induced reactive oxygen species (ROS) to cytotoxicity in GS28 siRNA-transfected cells. Contribution of H2O2-induced ROS to cytotoxicity in GS28 siRNA-transfected cells was examined. siRNA-transfected cells were treated with H2O2 for 24 h in the presence of BSO without or with pretreatment with 1 mM tempol and 50 µM deferoxamine (DFO). Cell viability was determined by an MTT reduction assay. The data represent the mean±SEM of 3 independent experiments. *p<0.05 versus the values in GS28 siRNA-transfected cells without pretreatment.
Fig. 4.
Determination of reactive oxygen species (ROS) generated by H2O2 in GS28 siRNA-transfected cells. siRNA-transfected cells were treated with 200 µM H2O2 for 3 h in the presence of BSO without or with pretreatment with 40 µM necrostatin-1 (Nec-1), 1 mM tempol and 50 µM deferoxamine (DFO). The cells were incubated with 5 µM H2DCF-DA, and fluorescence intensities were measured in flow cytometry, as described in Materials and Methods.
Non-apoptotic cell death by H2O2 via p38 MAPK activation
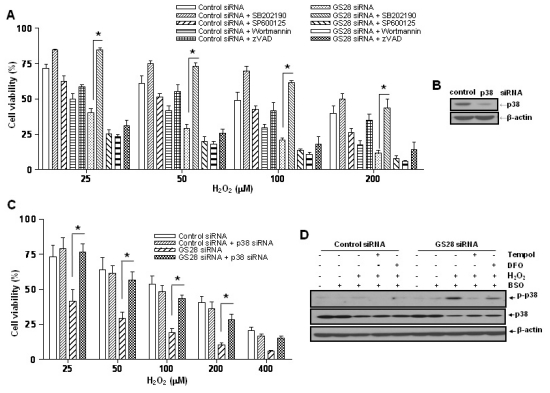
Since we showed that GS28 has a protective role against ROS-induced cellular damage, we examined the effects of GS28 on signaling pathways involved in H2O2-induced cytotoxicity. It is widely recognized that MAPK pathways are important in the regulation of cell survival and death in response to intracellular ROS levels. Cell viability was examined in GS28 siRNA-transfected cells by pretreatment with MAPK inhibitors (Fig. 3A). Pretreatment of the cells with a PI3K (wortmannin) or JNK (SP600125) inhibitor had no effect on cytotoxicity, but pretreatment with a p38 (SB202190) inhibitor significantly inhibited cytotoxicity. As shown in Fig. 3D, we also confirmed via immunoblot analysis that p38 MAPK was activated in GS28 siRNA-transfected cells treated with H2O2 for 3 h. These data suggest that GS28 has a protective role against H2O2-induced cell death through inhibition of p38 MAPK.
Fig. 3.
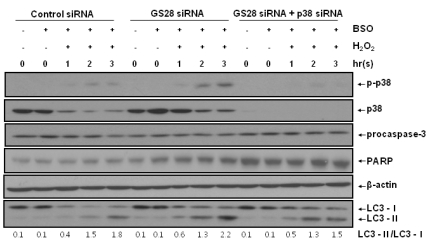
Involvement of p38 in the H2O2-induced cytotoxicity in GS28 siRNA-transfected cells. (A) siRNA-transfected cells were treated with H2O2 in the presence of BSO without or with pretreatment with chemical inhibitors. The inhibitors are 20 µM SB202190 (p38), 20 µM SP600125 (JNK), 200 nM wortmannin (PI3K), and 20 µM zVAD-fmk (pan-specific caspase). Cell viability was determined by an MTT reduction assay. The data represent the mean±SEM of 3 independent experiments. *p<0.05 versus the values in GS28 siRNA-transfected cells without pretreatment. (B) Efficiency of silencing gene expression was examined after transfection of p38 siRNA by immunoblot analysis of protein lysates. β-actin was used as loading controls. (C) H2O2-induced cytotoxicity was examined in GS28 siRNA-transfected cells without or with cointroduction of p38 siRNA. The cells were treated with H2O2 for 24 h in the presence of BSO. Cell viability was determined by an MTT reduction assay. The data represent the mean±SEM of 3 independent experiments. *p<0.05 versus the values in the cells transfected with GS28 siRNA alone. (D) Activation of p38 MAPK in GS28 siRNA-transfected cells treated with H2O2 was examined. siRNA-transfected cells were treated with 100 µM H2O2 for 3 h without or with pretreatment with 1 mM tempol and 50 µM deferoxamine (DFO). Phosphorylation of p38 was determined by immunoblot analysis. β-actin was used as a loading control.
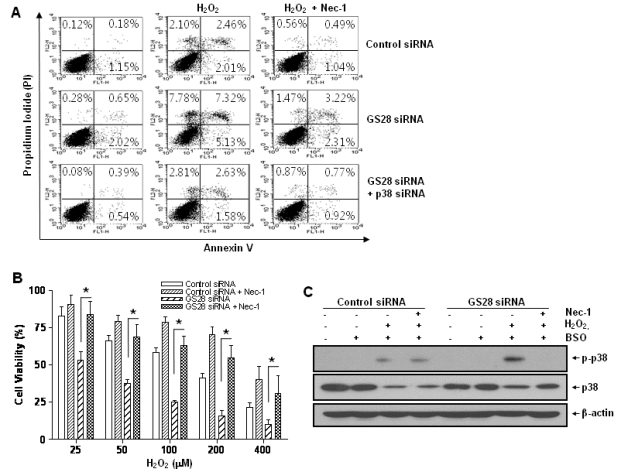
To examine whether or not H2O2-induced cytotoxicity was attributed to apoptosis, GS28 siRNA-transfected cells treated with H2O2 were analyzed by staining with annexin V and propidium iodide in flow cytometry. Apoptotic rates were low in both control and GS28 siRNA-transfected cells (Fig. 7A). We further examined cytotoxicity with molecular apoptotic markers. We could not detect the cleavage fragments of procaspase-3 and PARP in immunoblot analyses (Fig. 5). Pretreatment with zVAD-fmk, a pan-specific caspase inhibitor, had a minor inhibitory effect on H2O2-induced cytotoxicity in the cells, but the effect was not significant statistically (Fig. 3A). These data suggest that H2O2-induced cytotoxicity in the GS28 siRNA-transfected cells is shown primarily as non-apoptotic.
Fig. 7.
H2O2-induced necroptotic cell death via activations of p38 MAPK in GS28 siRNA-transfected cells. (A) Pattern of H2O2-induced cell death was examined in GS28 siRNA-transfected cells without or with cointroduction of p38 siRNA. siRNA-transfected cells were treated with H2O2 for 3 h in the presence of BSO without or with pretreatment with 40 µM necrostatin-1 (Nec-1). The cells were stained with annexin V and propidium iodide (PI) and analyzed by flow cytometry, as described in Materials and Methods. (B) H2O2-induced cell death was examined in GS28 siRNA-transfected cells without or with pretreatment with 40 µM Nec-1. The cells were treated with H2O2 for 24 h in the presence of BSO. Cell viability was determined by an MTT reduction assay. The data represent the mean±SEM of 3 independent experiments. *p<0.05 versus the values in the cells transfected with GS28 siRNA alone. (C) H2O2-induced necroptotosis via activations of p38 MAPK was examined in GS28 siRNA-transfected cells. siRNA-transfected cells were treated with 100 µM H2O2 for 3 h without or with pretreatment with 40 µM Nec-1. Phosphorylation of p38 was determined by immunoblot analysis. β-actin was used as a loading control.
Fig. 5.
Immunoblot analysis in GS28 siRNA-transfected cells treated with H2O2. siRNA-transfected cells were treated with 100 µM H2O2 for indicated times. Activation of p38, procaspase-3, and LC3 and cleavage of PARP in the cells were examined by immunoblot analysis using the specific antibodies as described in Materials and Methods. β-actin was used as a loading control.
Necroptotic cell death induced by H2O2
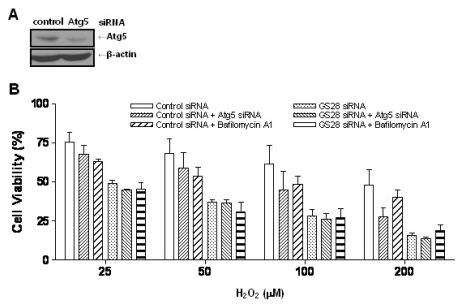
We were unable to observe any involvement of apoptotic processes in GS28 siRNA-transfected cells treated with H2O2. Therefore, we examined the autophagic process in cells treated with H2O2. To identify whether or not H2O2 induced autophagy, we observed the conversion of LC3-I into LC3-II, a marker of autophagy, by immunoblot. Increase of LC3-II conversion was shown in both control siRNA- and GS28 siRNA-transfected cells treated with H2O2 without a significant difference between them (Fig. 5), which imply that a defensive autophagic process to survive against H2O2 exposure is activated in the cells. To further examine autophagic cell death, we introduced Atg5 siRNA into cells to silence gene expression. The siRNA was efficient in silencing the expression of Atg5 proteins (Fig. 6A). Atg5 siRNA was unable to inhibit cytotoxicity in GS28 siRNA-transfected cells treated with H2O2 (Fig. 6B). Pretreatment of the cells with bafilomycin A1, a lysosomal inhibitor, also did not affect cytotoxicity. Recent studies suggest that necroptosis is another type of cell death induced by ROS. Nec-1 is a specific inhibitor on the process of necroptosis via inhibition of receptor-interacting protein 1 (RIP1) kinase activity [8]. In flow cytometry analysis, increase of ROS generation was completely inhibited by the pretreatment with Nec-1 in GS28 siRNA-transfected cells treated with H2O2 (Fig. 4). Cytotoxicity induced by H2O2 in the cells was completely inhibited by the pretreatment with Nec-1 in flow cytometry and a cell viability assay (Fig. 7A, 7B). The data suggest that H2O2-induced cytotoxicity in the GS28 siRNA-transfected cells is shown primarily to be necroptotic.
Fig. 6.
Non-autophagic cell death by H2O2 in GS28 siRNA-transfected cells. (A) Efficiency of silencing gene expression was examined after transfection of Atg5 siRNA by immunoblot analysis of protein lysates. β-actin was used as loading controls. (B) H2O2-induced cytotoxicity was examined in GS28 siRNA-transfected cells without or with cointroduction of Atg5 siRNA. The cells were treated with H2O2 for 24 h in the presence of BSO. Cell viability was determined by an MTT reduction assay. Effect of pretreatment with 50 nM bafilomycin A1 also was examined in GS28 siRNA-transfected cells treated with H2O2. The data represent the mean±SEM of 3 independent experiments.
Necroptotic cell death by H2O2 via p38 MAPK activation
We have shown the activation of p38 MAPK in GS28 siRNA-transfected cells treated with H2O2 (Fig. 3D) and the inhibition of cytotoxicity with pretreatment of the cells with a p38 chemical inhibitor (Fig. 3A). The increase of p38 activation in the cells was inhibited by pretreatment with tempol or DFO (Fig. 3D), which implies that p38 MAPK activation is dependent on the level of intracellular ROS. To further confirm these molecular mechanisms, we introduced p38 siRNA into cells to silence gene expression. This siRNA was efficient in silencing gene expression (Fig. 3B) and was able to significantly inhibit ROS generation (Fig. 4) and cytotoxicity (Fig. 3C, 7A) in the GS28 siRNA-cotransfected cells treated with H2O2. Finally, we observed complete inhibition of p38 activation by pretreatment with Nec-1 in the GS28 siRNA-transfected cells treated with H2O2 (Fig. 7C). Collectively, these data indicate that necroptotic signals in GS28 siRNA-transfected cells treated with H2O2 are mediated by sequential activations in RIP1/p38/ROS and also suggest that GS28 has a protective role in necroptotic cell death induced by H2O2 via inhibition of p38 MAPK in GSH-depleted neuronal cells.
DISCUSSION
A number of studies suggest that oxidative stress can be a final common pathway in various forms of neuronal cell death, including a variety of acute and chronic neurological diseases as well as in normal aging [13]. GSH is considered to be the most prevalent and important intracellular anti-oxidant for reducing oxidative stresses. Astrocytes seem to play a role in protecting neurons from oxidative stress by establishing an anti-oxidative defense system because GSH is more abundant within astrocytes [14]. Reduced GSH status was reported in various diseases including a number of neurological disorders [15]. In our previous study, depletion of GSH with BSO significantly enhanced cytotoxicity by treatment of osteoblasts and glial cells with oxidative stresses [10,16].
We isolated a new gene, GS28, responding to oxidative stress in rat ischemic hippocampal tissues using a differential screening method. In this study, we examined the role of GS28 and its molecular mechanisms in SK-N-SH cell death induced by H2O2 as an oxidative stress. GS28 has been described as a member of soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) [17]. Mammalian SNAREs known to participate in vesicular transport include GS28, Bet1, Sec22b, and syntaxin 5. SNAREs have been implicated in several trafficking steps, including vesicular transport from the endoplasmic reticulum (ER) to the Golgi, intra-Golgi transport, and homotypic vacuole fusion [17]. All reports have been interested in roles of GS28 in vesicular transport, and little is known about the possible involvement of GS28 in pathological conditions. A recent study demonstrated that the deletion of GS28 in C. elegans induces reduced seam cell numbers and a missing ray phenotype during post-embryonic development, suggesting GS28 has roles in cell proliferation and differentiation [18]. There has been no report on a role for GS28 in cellular oxidative stress response.
In this study, we found that GS28 siRNA-transfected cells are more vulnerable to H2O2-induced cytotoxicity than control siRNA-transfected cells under GSH-depleted conditions. This finding indicates that the level of intracellular GSH is important in H2O2-induced cell death and that GS28 has a protective role in cell death. Therefore, it is possible that inhibition of GSH synthesis in tumor cells is effective for patients undergoing radiation or chemotherapy. Increase of intracellular ROS in GS28 siRNA-transfected cells exposed to H2O2 was responsible for cell death, which was inhibited by an iron chelator and a ROS scavenger. Activation of p38 MAPK mediated the ROS increase in cells. The common type of neuronal cell death by ROS is apoptosis, which is also important in I/R injury [19]. When we examined involvement of apoptosis in H2O2-exposed cells, it was not detected in a cell viability assay, flow cytometry, and immunoblot analysis. Furthermore, we could not observe activation of the autophagic process. Ultrastructural morphology of the cells using transmission electron microscopy did not show any evidence suggesting apoptotic or autophagic cell death (data not shown). Pretreatment of the cells with Nec-1 showed the complete inhibition of H2O2-induced cytotoxicity, ROS generation, and p38 activation, which implies that the cell death induced by H2O2 in GSH-depleted conditions is necroptotic.
Necroptosis and its molecular mechanisms have been recently well introduced by Vandenabeele et al. [20]. Necroptosis is a type of cell death manifesting regulated necrosis by an intrinsic death program distinct from apoptosis. It was reported that oxidative stresses induce necroptosis in neuronal and non-neuronal cells [21,22]. Key molecules and processes in necroptosis are characterized as initiators and effectors [20]. One important initiator molecule is RIP1, which is part of the TNF receptor 1 (TNFR1) complex. When caspase activation is prevented, RIP1 is activated by phosphorylation and induces generation of effectors, ROS, via signaling molecules, including MAPKs. We demonstrated that RIP1 inhibitor (Nec-1) blocked H2O2-induced cytotoxicity in GS28 siRNA-transfected cells and inhibited p38 MAPK activation and ROS generation in the cells. The p38 inhibition by its siRNA blocked ROS generation in the cells. These findings correspond to the molecular mechanisms characterized in necroptosis. However, we did not examine contribution of p38 to RIP1 activity because an antibody against phospho-RIP1 is not commercially available. Recent studies have suggested the contribution of p38 activation to necroptosis, as induction of necroptosis via p38 activation and ROS generation, occurs in retinal ganglion cells stimulated with light and in hepatocytes exposed to a pollutant, 3-nitrofluoranthene [23,24]. GS28 is a member of SNAREs implicated in vesicular transport in ER and Golgi, and involvement of SNAREs in necroptosis is unknown. Future work should include examination of the intracellular localization of GS28 in different conditions including oxidative stress which will aid in the characterization of additional roles for GS28 in cells. In addition, the role of GS28 and its precise molecular mechanisms in ROS-induced necroptosis should also be determined.
In this study, we found that GS28 siRNA-transfected neuronal cells were more sensitive to H2O2 than control cells under GSH-depleted conditions. Exposure to H2O2 in the cells induced p38 MAPK activation via RIP1, which leads to generation of intracellular ROS. Molecular parameters suggesting apoptotic or autophagic cell death were not observed in the cells. These data suggest that GS28 has a protective role against ROS-induced necroptotic cell death via inhibition of p38 MAPK in GSH-depleted neuronal cells.
ACKNOWLEDGEMENTS
This work was supported by the Korea Science and Engineering Foundation (KOSEF) through the MRC for Cell Death Disease Research Center (CDDRC) at The Catholic University of Korea (R13-2002-005-03002-0).
ABBREVIATIONS
- GS28
golgi SNAP receptor complex 1
- H2O2
hydrogen peroxide
- ROS
reactive oxygen species
- GSH
glutathione
- BSO
buthionine sulfoximine
- H2DCF-DA
2',7'-dichlorodihydrofluorescein diacetate
- DFO
deferoxamine mesylate
- Nec-1
necrostatin-1
- siRNA
small interfering RNA
- RIP1
receptor-interacting protein 1
References
- 1.Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 2.Inoue M, Sato EF, Nishikawa M, Park AM, Kira Y, Imada I, Utsumi K. Mitochondrial generation of reactive oxygen species and its role in aerobic life. Curr Med Chem. 2003;10:2495–2505. doi: 10.2174/0929867033456477. [DOI] [PubMed] [Google Scholar]
- 3.Reiter RJ. Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB J. 1995;9:526–533. [PubMed] [Google Scholar]
- 4.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 5.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 6.Kim KC, Lee C. Curcumin induces downregulation of E2F4 expression and apoptotic cell death in HCT116 human colon cancer cells; involvement of reactive oxygen species. Korean J Physiol Pharmacol. 2010;14:391–397. doi: 10.4196/kjpp.2010.14.6.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitanaka C, Kuchino Y. Caspase-independent programmed cell death with necrotic morphology. Cell Death Differ. 1999;6:508–515. doi: 10.1038/sj.cdd.4400526. [DOI] [PubMed] [Google Scholar]
- 8.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 9.Azad MB, Chen Y, Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 2009;11:777–790. doi: 10.1089/ars.2008.2270. [DOI] [PubMed] [Google Scholar]
- 10.Son MJ, Lee SB, Byun YJ, Lee HO, Kim HS, Kwon OJ, Jeong SW. Sodium nitroprusside induces autophagic cell death in glutathione-depleted osteoblasts. J Biochem Mol Toxicol. 2010;24:313–322. doi: 10.1002/jbt.20340. [DOI] [PubMed] [Google Scholar]
- 11.Jang H, Choi SY, Cho EJ, Youn HD. Cabin1 restrains p53 activity on chromatin. Nat Struct Mol Biol. 2009;16:910–915. doi: 10.1038/nsmb.1657. [DOI] [PubMed] [Google Scholar]
- 12.Kim SY, Lee MY, Cho KC, Choi YS, Choi JS, Sung KW, Kwon OJ, Kim HS, Kim IK, Jeong SW. Alterations in mRNA expression of ribosomal protein S9 in hydrogen peroxide-treated neurotumor cells and in rat hippocampus after transient ischemia. Neurochem Res. 2003;28:925–931. doi: 10.1023/a:1023283628454. [DOI] [PubMed] [Google Scholar]
- 13.Bains JS, Shaw CA. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Brain Res Rev. 1997;25:335–358. doi: 10.1016/s0165-0173(97)00045-3. [DOI] [PubMed] [Google Scholar]
- 14.Peuchen S, Bolaños JP, Heales SJ, Almeida A, Duchen MR, Clark JB. Interrelationships between astrocyte function, oxidative stress and antioxidant status within the central nervous system. Prog Neurobiol. 1997;52:261–281. doi: 10.1016/s0301-0082(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 15.Lang CA, Naryshkin S, Schneider DL, Mills BJ, Lindeman RD. Low blood glutathione levels in healthy aging adults. J Lab Clin Med. 1992;120:720–725. [PubMed] [Google Scholar]
- 16.Byun YJ, Kim SK, Kim YM, Chae GT, Jeong SW, Lee SB. Hydrogen peroxide induces autophagic cell death in C6 glioma cells via BNIP3-mediated suppression of the mTOR pathway. Neurosci Lett. 2009;461:131–135. doi: 10.1016/j.neulet.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Zhang T, Hong W. Ykt6 forms a SNARE complex with syntaxin 5, GS28, and Bet1 and participates in a late stage in endoplasmic reticulum-Golgi transport. J Biol Chem. 2001;276:27480–27487. doi: 10.1074/jbc.M102786200. [DOI] [PubMed] [Google Scholar]
- 18.Maekawa M, Inoue T, Kobuna H, Nishimura T, Gengyo-Ando K, Mitani S, Arai H. Functional analysis of GS28, an intra-Golgi SNARE, in Caenorhabditis elegans. Genes Cells. 2009;14:1003–1013. doi: 10.1111/j.1365-2443.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- 19.Lewén A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 20.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum DM, Degterev A, David J, Rosenbaum PS, Roth S, Grotta JC, Cuny GD, Yuan J, Savitz SI. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J Neurosci Res. 2010;88:1569–1576. doi: 10.1002/jnr.22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berghe TV, Vanlangenakker N, Parthoens E, Deckers W, Devos M, Festjens N, Guerin CJ, Brunk UT, Declercq W, Vandenabeele P. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 2010;17:922–930. doi: 10.1038/cdd.2009.184. [DOI] [PubMed] [Google Scholar]
- 23.Ji D, Kamalden TA, del Olmo-Aguado S, Osborne NN. Light- and sodium azide-induced death of RGC-5 cells in culture occurs via different mechanisms. Apoptosis. 2011;16:425–437. doi: 10.1007/s10495-011-0574-4. [DOI] [PubMed] [Google Scholar]
- 24.Asare N, Låg M, Lagadic-Gossmann D, Rissel M, Schwarze P, Holme JA. 3-Nitrofluoranthene (3-NF) but not 3-aminofluoranthene (3-AF) elicits apoptosis as well as programmed necrosis in Hepa1c1c7 cells. Toxicology. 2009;255:140–150. doi: 10.1016/j.tox.2008.10.021. [DOI] [PubMed] [Google Scholar]