Abstract
Corticosterone is known to modulate GABAergic synaptic transmission in the hypothalamic paraventricular nucleus. However, the underlying receptor mechanisms are largely unknown. In the anterior hypothalamic area (AHA), the sympathoinhibitory center that project GABAergic neurons onto the PVN, we examined the expression of glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) of GABAergic neurons using intact GAD65-eGFP transgenic mice, and the effects of corticosterone on the burst firing using adrenalectomized transgenic mice. GR or MR immunoreactivity was detected from the subpopulations of GABAergic neurons in the AHA. The AHA GABAergic neurons expressed mRNA of GR (42%), MR (38%) or both (8%). In addition, in brain slices incubated with corticosterone together with RU486 (MR-dominant group), the proportion of neurons showing a burst firing pattern was significantly higher than those in the slices incubated with vehicle, corticosterone, or corticosterone with spironolactone (GR-dominant group; 64 vs. 11~14%, p< 0.01 by χ2-test). Taken together, the results show that the corticosteroid receptors are expressed on the GABAergic neurons in the AHA, and can mediate the corticosteroid-induced plasticity in the firing pattern of these neurons. This study newly provides the experimental evidence for the direct glucocorticoid modulation of GABAergic neurons in the AHA in the vicinity of the PVN.
Keywords: Paraventricular nucleus, Glucocorticoid receptors, Burst firing, Single cell RT-PCR, Slice patch clamp
INTRODUCTION
Corticosteroid hormones are released by the adrenal cortex in response to stress, and they play major roles in maintaining the homeostasis of the body. Corticosteroid hormones act through two types of receptors: high affinity mineralocorticoid receptor (MR) and low affinity glucocorticoid receptor (GR; [1]). Both receptors mediate the classical genomic [2] as well as the rapid non-genomic effects of corticosteroid hormones [3,4].
One of major targets of corticosteroid hormones in the central nervous system is the hypothalamic paraventricualr nucleus (PVN), which plays a key role in the exertion of stress response [5,6]. The hypophysiotropic neuroendocrine cells in the PVN receive GABAergic input, which originates from the immediate vicinity of the PVN (peri-PVN) as well as from a series of adjacent hypothalamic and forebrain territories, including the anterior hypothalamic area (AHA; [6-8]). The corticotrophin releasing hormone (CRH) neurons in the medial parvocellular region of the PVN receive heavy GABAergic input, which accounts for approximately 78% of the total synaptic boutons in these neurons [9]. Removal of endogenous corticosterone, by adrenalectomy (ADX), can affect GABAergic transmission in the PVN. For example, ADX altered the excitability of neurosecretory PVN neurons in rats [10] and increased GABAergic transmission in the PVN [11,12]. Stress in rats, or in vitro exposure of the brain slice to corticosterone suppressed GABAergic transmission [13], and altered the expression of GABAA receptor subunits [14]. All these findings imply that GABAergic inputs into the PVN are the targets of corticosterone modulation. However, it is not yet known whether corticosterone acts directly on the GABAergic neurons that are projected into the PVN.
The aim of this study was to demonstrate the possible direct action of corticosteroid on GABAergic neurons that are projected into the PVN. Toward this end, we used GAD65-eGFP transgenic mice to identify the GABAergic neurons in the AHA [15,16], which is located ventrolateral to the PVN. The AHA is known to inhibit sympathetic tone [17] and sends its GABAergic neurons to the PVN [6-8]. It is also known that stress activates AHA GABAergic neurons [15]. In this study, we identified the expression of two types of corticosteroid receptors, GR and MR, on GABAergic neurons in the AHA, using single cell RT-PCR and immunohistochemistry. We also attempted to confirm the corticosteroid-receptor-mediated changes in the firing patterns of GABAergic neurons in the rat AHA, using patch clamp techniques in combination with in vitro slice incubation.
METHODS
Animals and slice preparation
The GAD65-eGFP transgenic mice are kindly provided by Dr. Szabo in Hungary [15]. Four- to six-week-old GAD65-eGFP transgenic mice, of either sex, were used. Mice were housed under conditions consisting of constant temperature and humidity and a 12 h light/dark cycle, with free access to food and water. For the electrophysiological experiment, the mice were bilaterally adrenalectomized, by dorsal approach, and 0.9% saline was provided after surgery [18]. Intact mice were used for single cell RT-PCR and immunohistochemistry to evaluate corticosteroid receptor expression. All the experiments were performed in accordance with the guidelines of the Laboratory Animal Care Advisory Committee of Seoul National University. The mice were decapitated in 7~12 days after the adrenalectomy, under the anesthesia induced by an injection of a mixture (ketamine:xylzaine=3:1, 0.05 ml/animal) of ketamine (50 mg/ml) and xylazine (23 mg/ml). The brains were quickly removed from the specimens' skulls, and placed in a slicing chamber filled with ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM) 126 NaCl, 26 NaHCO3, 5 KCl, 1.2 NaH2PO4, 2.4 CaCl2, 1.2 MgCl2, and 10 glucose. Coronal slices, each with a thickness of 300 µm, were cut, using a vibratome (Vibratome Company, St. Louis, MO, USA), and transferred to an incubation chamber at 30~32℃, then stabilized for 1 h [19]. The eGFP (+) neurons in the slices were used either to harvest the corticosteroid receptors or for electrophysiological recording [16].
Single cell RT-PCR
Brain slices were placed in a recording chamber of an upright microscope (BX50WI, Olympus, Tokyo, Japan) and continuously perfused with oxygenated (95% O2 and 5% CO2) ACSF at 30~32℃. The eGFP (+) neurons that distributed in the anterior hypothalamic area (AHA) ventrolateral to the paraventricular nucleus, were harvested using a glass micropipette. Applying gentle negative pressure, the cytoplasm of the eGFP (+) neurons were aspirated into a glass micropipette and transferred to test tubes. Next, tubes containing the cytoplasm were immediately stored at -70℃ until the reverse transcription (RT) reaction was performed. The RT was performed using a total reaction volume of 20 µl. The reaction mixture was composed of 100 ng of random hexamer, 0.4 mM of dNTPs, a 1× first-strand buffer, 10 mM of DTT, 40 units of RNaseOUT™ Ribonuclease Inhibitor (Invitrogen, Carlsbad, CA), and 200 units of SuperScript® III (Invitrogen). After 5-min incubation at 25℃, the RT reaction was performed for 1-h at 45℃ and terminated by heating for 15-min at 70℃. In the (-) RT control, all reagents, except the reverse transcriptase were included.
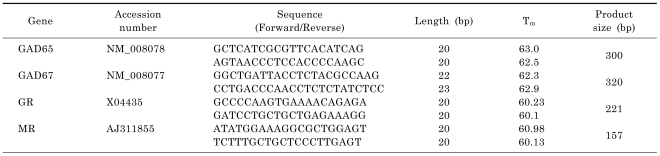
To determine if the eGFP (+) neurons were GABAergic, analyses were conducted on the mRNA of glutamic acid decarboxylase (GAD) 65 and 67, the enzymes representing the GABAergic neuronal markers [16]. Cell samples found not to be positive for either GAD65 or GAD67 were discarded in further analyses of GR and MR mRNA. The PCR conditions were optimized to detect GR and MR transcripts. Three to four microliters of cDNA samples were used for the PCR reaction. PCR was performed in a final volume of 25 µl containing 12.5 µl of 2× GoTaq® Green Master Mix (Promega, Madison, WI), 0.4 µM of sense primer and 0.4 µM of antisense primer. The thermal cycling conditions were as follows: 5-min of initial denaturation at 94℃; 45~50 cycles of denaturation at 94℃ for 30 sec; annealing at 57℃ for 30 sec; elongation at 72℃ for 45 sec; and 10 min of final elongation at 72℃. Information relating to the primers employed in this study is summarized in Table 1. The PCR products were run on a 1.8% agarose gel and visualized using ethidium bromide staining. The visualized PCR products were digitally photographed using a GelDoc UV transilluminator (BioRad, Hercules, CA).
Table 1.
Information on the primers used in the study
Immunohistochemistry
The mice were perfused with 0.01 M PBS and fixed with 4% paraformaldehyde. After postfixation at 4℃ overnight, the specimens' brains were cryoprotected in a 30% sucrose solution for two days. Using the cryostat, 25 µm brain sections, free floating in 0.01 M PBS, were collected. Sections were rinsed and incubated in a blocking buffer containing 5% normal donkey serum and 0.1% Triton X-100 for 2-h at room temperature. Then sections were incubated with primary antibodies (1:50 dilution) for 1-h at room temperature with mild shaking, followed by two days of incubation at 4℃. The primary antibodies, rabbit anti-GR polyclonal IgG (M-20) and goat anti-MR polyclonal IgG (N-17), were purchased from Santa Cruz (Santa Cruz Biotechnology, Santa Cruz, CA). Subsequently, sections were rinsed with 0.01 M PBS and incubated with Alexa Fluor 555 donkey anti-rabbit and anti-goat secondary antibodies (Molecular Probes, Eugene, OR) for 2-h at room temperature. After rinsing, sections were mounted and fluorescent photomicrographs were taken using confocal microscopes (Nikon TE-2000, Tokyo, Japan). The brightness and contrasts of the photomicrographs were adjusted using Adobe Photoshop.
Selective activation of corticosteroid receptors and electrophysiological recording
To assess the effects of GR or MR activation on neuronal activity of AHA GABAergic neurons, endogenous corticosterone was removed by bilateral ADX, and GR or MR was activated experimentally by in vitro slice incubation according to Krugers et al [20]. One hour after the slice preparation, as described above, the brains from the ADX mice were treated for 20-min at 31℃ with one of the following: 1) vehicle (0.1% DMSO in ACSF, control group), 2) corticosterone (100 nM in ACSF, CORT group), 3) 100 nM corticosterone plus a GR antagonist, RU486 (500 nM in ACSF, MR-dominant group), or 4) 100 nM corticosterone plus an MR antagonist, spironolactone (100 nM in ACSF, GR-dominant group). After treating each condition for 20-min, the slices were left for at least 40-min in normal ACSF at 31℃ and, then, transferred to the recording chamber of an upright microscope (BX50WI, Olympus, Tokyo, Japan), which was continuously perfused with oxygenated ACSF (30~33℃). Patch pipettes were pulled from the borosilicate glass using a pipette puller (Model PP-83, Narishige, Japan). When filled with internal solutions, the resistance of the pipettes ranged from 4~6 MΩ. For the whole-cell recordings, the pipette solution (in mM) consisted of: 135 K-gluconate, 5 KCl, 0.5 CaCl2, 5 EGTA, 20 HEPES, and 5 MgATP. The cell membrane was ruptured and voltage responses to hyperpolarizing or depolarizing currents were measured in current-clamp mode of the whole-cell configuration. To determine the firing patterns of AHA GABAergic neurons, voltage responses to the depolarizing current pulses (20~60 pA, 500 ms) were measured at a holding potential of -75 to -85 mV [21,22]. In a given cell, injections of currents of different magnitude (20~60 pA) did not result in different firing pattern. The liquid junction potential of the K-gluconate-rich pipette solution (~14.3 mV) was corrected when the membrane potential of the cells was calculated. All recordings were obtained from eGFP (+) neurons in this study. Electrical recordings were acquired using Axopatch 200B (Axon Instruments, Sunnyvale, CA) and digitized using Digidata 1200 (Axon instruments). Data were analyzed using the Mini Analysis Program (Synaptosoft, Decatur, GA) and Clampfit 10.2 (Axon Instruments, CA, USA).
Statistical analysis
Statistical significance was determined using Student's t-test and one-way analysis of variance (ANOVA). An χ2-test was used to determine the neuronal populations for the firing patterns that were different between the groups. Data were presented as mean±S.E.M.
RESULTS
Expression of GR and MR mRNA in GABAergic neurons of the AHA
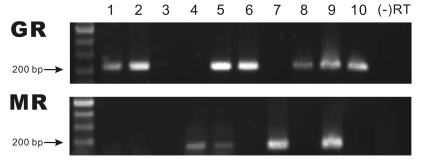
The expression of GR and MR mRNA transcripts in the eGFP(+) cells, located in the AHA, ventrolateral to the paraventricular nucleus, was analyzed using single cell RT-PCR. Fig. 1 shows representative results of the single cell RT-PCR analysis performed on the GABAergic neurons harvested in the AHA. Of the 26 total GABAergic neurons harvested from the AHA, 11 cells (42%) expressed GR and 10 cells (38%) expressed MR mRNA. Two cells (8%) co-expressed both GR and MR mRNA. The eGFP(+) cells that did not express either of two the GABAergic cell markers, GAD65 and GAD67, were excluded from this analysis. No amplified product was found in the (-) RT control. The sequences of the PCR products matched the targets in the GR and MR transcripts (data are not shown).
Fig. 1.
Single cell RT-PCR analyses for GR and MR mRNA transcripts in GABAergic neurons of the AHA. Each product represents the expression of GR or MR in 10 individual neurons. The number represents the expression of GR and MR in each cell. The expected size of the products of GR and MR is 221 bp and 157 bp, respectively. No product was amplified in the (-) RT control.
Distribution of GR and MR receptor proteins in the AHA
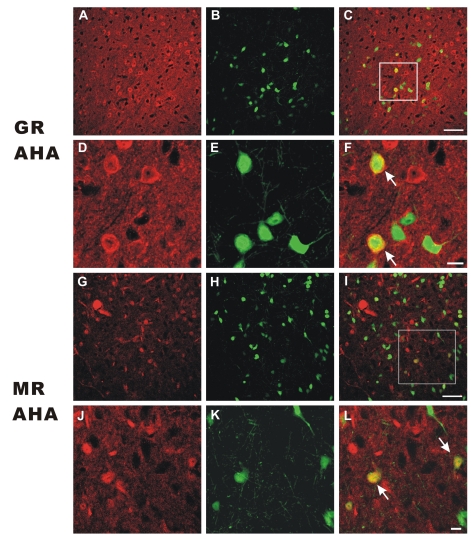
Immunofluorescence studies using specific antibodies for GR and MR proteins were performed to show the distribution of GR and MR proteins in the AHA (Fig. 2). In the AHA read, the GR-immunoreactivity (Fig. 2A, D) and MR-immunoreactivity (Fig. 2G, H) were observed as red (red), while the eGFP expressing neurons were observed as green, respectively. The neurons in the rectangular area in Fig. 2C and Fig. 2I were shown at higher magnification in 2D~2F, and 2J~2L, respectively. Interestingly, the GR immunofluorescence showed uneven distribution and was denser in the cytoplasmic or peripheral zone than in the nuclear or central zone of the AHA cells (Fig. 2A, D). However, the MR immunofluorescence showed a rather diffuse and even distribution (Fig. 2G, J). The green eGFP fluorescence was evenly labeled in the whole-cells, including the nucleus, cytoplasm, and proximal processes (Fig. 2E, K). The co-localization of GR- and MR-immunoreactivity in the eGFP(+) cells (yellow) was indicated at low (Fig. 2C, I) and high magnifications (Fig. 2F, L, as indicated by arrows), respectively. Collectively, the results show that a significant portion of GR- or MR-immunoreactive cells are co-localized with eGFP, and that GR-immunoreactivity is unevenly distributed within the GABAergic AHA cells (Fig. 2F).
Fig. 2.
GR and MR protein expression in the AHA. (A~F) GR-immunoreactivity in the AHA cells. GRimmunoreactivity is depicted in red (A, D) and the cell bodies of eGFP (+) neurons (B, E) in the AHA are shown in green. Co-localizations of GR-immunoreactivity on the cell bodies of eGFP (+) neurons are illustrated in yellow. (G~L) MR-immunoreactivity in the AHA cells. MR-immunoreactivity is depicted in red (G, J) and the cell bodies of eGFP (+) neurons (H, K) in the AHA are shown in green. Co-localizations of MR-immunoreactivity on the cell bodies of eGFP (+) neurons are illustrated in yellow. Panel A~C and G~I show 400× magnification of fluorescent photomicroscopes in the AHA (scale bar=50 µm). Panel D~F and J~L are higher magnifications of the rectangular areas in the C and I (scale bar=10 µm). Co-localization of GR- or MR-immunoreactivity and eGFP expressing neurons are indicated by arrows (F, L, respectively).
Effects of corticosteroid receptor activation on burst firing in GABAergic neurons
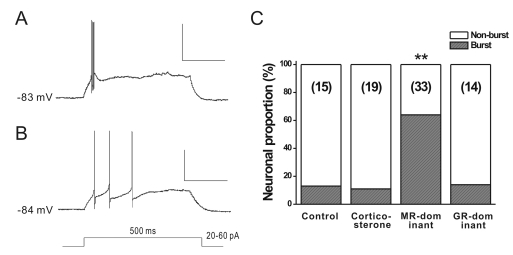
The results cited above indicate that MR and/or GR are expressed in the GABAergic cells of the AHA cells. To further prove the expression of functional corticosteroid receptors, we examined the effects of the activation of corticosteroid receptors on the firing properties of GABAergic neurons in AHA. We compared the burst firing [23-25] induced by injecting 20~60 pA of depolarizing current pulses (500-ms) from a holding potential of -75 mV to -85 mV [21,22]. Burst firing appeared in the cluster of spikes riding on a slow depolarization, as reported by Okuhara and Beck (1998). The mean interspike intervals (ISIs), between the initial two spikes, were significantly smaller in bursting than in non-bursting neurons (7.64±0.48, n=27 vs. 75.47±11.66 ms, n=44; p<0.01). Additionally, the mean ISI of the whole spikes in the bursting neurons (n=27) was also significantly smaller than that in the non-bursting neurons (n=44; 16.79±3.72 vs. 89.44±11.26 ms, p<0.01).
The proportion of neurons showing burst firing was different between the treatment groups (Fig. 3). Among the four treatment groups, the MR-dominant group showed a significantly higher proportion of burst firing neurons (64%, n=33) than the other groups; 13% in control (n=15), 11% in CORT (n=19), and 14% in GR-dominant groups, respectively (χ2-test, p<0.01). All the neurons fired at burst mode also showed low threshold spikes in response to hyperpolarizing pre-pulses (data not shown). In contrast, the resting membrane potentials (RMP) and input resistances (Rin) of AHA neurons in treatment groups were not significantly different from those of the control group (RMP, CTL vs. CORT vs. CORT+RU486 vs. CORT+Spiro, -63.2±1.1 vs. -63.2±1.0 vs. -62.5±0.6 vs. -65.4±1.0 mV; Rin, 399±25 vs. 546.3±67.7 vs. 448.2±30.5 vs. 423.8±43.2 MΩ; p>0.05 by one-way ANOVA). Taken together, these results indicate that corticosterone can directly modulate the firing properties of GABAergic neurons by activating the corticosteroid receptors expressed on the GABAergic neurons in the AHA.
Fig. 3.
Proportion of AHA GABAergic neurons showing the burst firing at selective activation of corticosteroid receptors in vitro. (A, B) GABAergic neurons in the AHA showing burst action (A) or non-burst action potentials (B). Burst firing represents a cluster of action potentials riding on a slow depolarizing hump (A). The train of the action potential was evoked by 20~60 pA of depolarizing current pulses (500-ms duration) at a holding potential of ~-80 mV. (C) comparing the proportion of bursting neurons in each group. In response to depolarizing currents, the proportion of the burst firing neurons of the MR-dominant group (100 nM CORT+500 nM RU486) is significantly higher than that of the other three treatment groups (**p<0.01 by χ2-test). The total number of recorded neurons in each group is shown in parenthesis. CTL, control; CORT, corticosteroid; Spiro, spironolactone. Scale bars in A and B are 150 ms and 50 mV.
DISCUSSION
The present study demonstrated that both GR and MR are expressed on the GABAergic neurons in the AHA, using GAD65-eGFP transgenic mice. Single-cell RT-PCR provided evidence of GR and MR mRNA transcripts contained in individual GABAergic neurons, and immunohistochemical studies showed distribution of GR and MR proteins in GABAergic neuronal cell bodies. Furthermore, patch clamp studies revealed that activation of the corticosteroid receptors could affect the firing properties of GABAergic neurons in AHA, indicating that functional GR and MR, expressed on AHA GABAergic neurons, can mediate the action of corticosteroid hormones in healthy and diseased rats.
In view of enhanced proportion of burst firing pattern in MR-dominant conditions, our result is congruent with the report that MR activation enhances membrane electrical activity in neonatal rat cardiomyocytes [26]. Treatment with the MR agonist aldosterone increased the amplitude of T-type and L-type calcium currents as well as the mRNA of α1H subunit for the T-type Ca2+ channel, and the α1C and α2 subunits for the L-type Ca2+channels in neonatal rat cardiomyocytes [26]. Furthermore, aldosterone increases T-type calcium currents and the expression of mRNA coding α1H, T-channel isoforms. These increases were blunted by MR antagonist spironolactone [27,28]. Burst firing is known to be the result of activation of T-type calcium channels in the subthalamic, lateral geniculate and sensory thalamic nuclei [29-31]. Collectively, given the well-known roles of T-type calcium channels in modulating firing pattern, an increased expression or activity of T-type calcium channels of GABAergic neurons in the AHA seems to be the most likely mechanism underlying enhanced proportion of bursting firing pattern in MR-dominant conditions.
Previous studies indicate that the AHA can be involved in the regulation of the HPA axis [6-8] and/or sympathetic tone [17]. Therefore, it is likely that the corticosteroid receptors expressed in the AHA GABAergic cells can indirectly affect the HPA axis and/or sympathetic nervous system. In regulation of the HPA axis, studies have shown that the PVN is under a fine modulation of local GABAergic inputs as well as the direct inhibition of humoral feedback by corticosterone [6]. Among the GABAergic synapses in the medial parvocellular region, 78% of the GABAergic boutons terminate on the corticotrophin releasing hormone (CRH) neurons indicating the importance of neuronal regulation of the PVN by GABA [9]. Local GABAergic neurons integrate excitatory and inhibitory signals from upstream regions, including the hippocampus, the prefrontal cortex and the amygdale, and relay to the PVN neurons [32]. Bali et al [15], using GAD65-eGFP transgenic mice, showed that Fos expression was significantly increased, by acute ether stress in the subparaventricular zone, the AHA, the lateral septum and the bed nucleus of stria terminalis. All these studies indicate that local GABAergic neurons play a significant role in regulation of the HPA axis and/or sympathetic tone.
Corticosterone can bind both GR and MR with a different affinity [MR>>>GR; 1]. Our results showing that the excitability of the AHA GABAergic cells can be dually modulated by corticosteroid balance are in good agreement with this report [1]. In light of the dual modulation by corticosteroid balance, we can expect that AHA GABAergic cells may result in an enhanced neuronal excitability or GABA release, which in turn can induce sympathoinhibition of the AHA in MR-dominant conditions, while sympathoinhibition is reduced in GR-dominant conditions [13]. Therefore, our results indicate that corticosteroid hormones can modulate the sympathetic tone by acting on the local GABAergic cells in the AHA, which project to the brain areas containing the neurons that, when activated, increase sympathetic tone and blood pressure.
In the regulation of sympathetic tone, the activation of neurons in the AHA is known to inhibit the sympathetic nervous system in normotensive rats [see review 17]. Direct injection of norepinephrine, epinephrine and clonidine into the AHA results in dose-related decreases in blood pressure and heart rate in rats [33-35]. Furthermore, in spontaneously hypertensive rats, hypertension induced by a high NaCl diet is associated with a reduced norepinephrine release in the AHA [17,36,37]. It is also known that angiotensin II and/or type 1 angiotensin II receptors in the AHA are involved in the pathogenesis of salt-sensitive hypertension in rats [38-40]. Therefore, our findings on the corticosteroid receptors in AHA neurons suggest that glucocorticoids released in stress can directly affect the sympathetic nervous system at the level of the hypothalamus, providing novel evidence supporting the interaction between the sympathoadrenal system and the hypothalamic-pituitary-adrenocortical system [41].
It is well known that activation of GR or MR receptors induces a translocation of receptors from the cytoplasmic to the nuclear regions [42-44]. In the present study, the distribution of the immunofluorescence of GR was more cytoplasmic than was the immunofluorescence of MR in the AHA cells of normal mice. This observation is consistent with previous reports in that MR immunoreactivity is distributed in both the cytoplasmic and nuclear regions of hippocampal and hypothalamic neurons [43,44]. However, the observation is not in agreement with Han et al [44], which showed that subcellular distribution of GR immunoreactivity was more nuclear in comparison with the subcellular distribution of MR immunoreactivity. This discrepancy could arise from the differences in the properties of the neurons studied: AHA GABAergic cells vs. hippocampal (CA1~CA3) or hypothalamic neurons (paraventricular and arcuate nuclei). It is also possible that the discrepancy was due to the primary GR antibody used in this study (M20), because Sarabdjitsingh et al [45] recently showed that the M20 antibody poorly detected nuclear GR immunoreactivity in the hippocampal CA1 and the dentate gyrus neurons. Further study is needed to understand the role of GR in AHA GABAergic cells.
In conclusion, our results demonstrate that the AHA GABAergic neurons located in the peri-PVN area can be a direct target of corticosteroid hormones. Our findings may provide novel mechanisms of corticosteroid regulation of the HPA axis and the sympathetic tone that can be mediated in the AHA in normal and disease states.
ACKNOWLEDGEMENTS
This research was supported by the Basic Research Promotion Fund of Korea Research Foundation (KRF-2008-314-E00230).
ABBREVIATIONS
- ACSF
artificial cerebrospinal fluid
- ADX
adrenalectomy
- AHA
anterior hypothalamic area
- CORT
corticosterone
- CRH
corticotrophin releasing hormone
- GAD
glutamic acid decarboxylase
- GR
glucocorticoid receptor
- ISIs
interspike intervals
- MR
mineralocorticoid receptor
- PVN
hypothalamic paraventricualr nucleus
References
- 1.De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 2.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 3.Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci. 2002;16:381–385. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- 6.Cullinan WE, Ziegler DR, Herman JP. Functional role of local GABAergic influences on the HPA axis. Brain Struct Funct. 2008;213:63–72. doi: 10.1007/s00429-008-0192-2. [DOI] [PubMed] [Google Scholar]
- 7.Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1993;332:123–143. doi: 10.1002/cne.903320109. [DOI] [PubMed] [Google Scholar]
- 8.Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- 9.Miklós IH, Kovács KJ. GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy. Neuroscience. 2002;113:581–592. doi: 10.1016/s0306-4522(02)00147-1. [DOI] [PubMed] [Google Scholar]
- 10.Yang JH, Li LH, Lee S, Jo IH, Lee SY, Ryu PD. Effects of adrenalectomy on the excitability of neurosecretory parvocellular neurones in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2007;19:293–301. doi: 10.1111/j.1365-2826.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang JH, Li LH, Shin SY, Lee S, Lee SY, Han SK, Ryu PD. Adrenalectomy potentiates noradrenergic suppression of GABAergic transmission in parvocellular neurosecretory neurons of hypothalamic paraventricular nucleus. J Neurophysiol. 2008;99:514–523. doi: 10.1152/jn.00568.2007. [DOI] [PubMed] [Google Scholar]
- 12.Verkuyl JM, Joëls M. Effect of adrenalectomy on miniature inhibitory postsynaptic currents in the paraventricular nucleus of the hypothalamus. J Neurophysiol. 2003;89:237–245. doi: 10.1152/jn.00401.2002. [DOI] [PubMed] [Google Scholar]
- 13.Verkuyl JM, Karst H, Joëls M. GABAergic transmission in the rat paraventricular nucleus of the hypothalamus is suppressed by corticosterone and stress. Eur J Neurosci. 2005;21:113–121. doi: 10.1111/j.1460-9568.2004.03846.x. [DOI] [PubMed] [Google Scholar]
- 14.Verkuyl JM, Hemby SE, Joëls M. Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. Eur J Neurosci. 2004;20:1665–1673. doi: 10.1111/j.1460-9568.2004.03568.x. [DOI] [PubMed] [Google Scholar]
- 15.Bali B, Erdélyi F, Szabó G, Kovács KJ. Visualization of stress-responsive inhibitory circuits in the GAD65-eGFP transgenic mice. Neurosci Lett. 2005;380:60–65. doi: 10.1016/j.neulet.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Shin SY, Yang JH, Lee H, Erdélyi F, Szabó G, Lee SY, Ryu PD. Identification of the adrenoceptor subtypes expressed on GABAergic neurons in the anterior hypothalamic area and rostral zona incerta of GAD65-eGFP transgenic mice. Neurosci Lett. 2007;422:153–157. doi: 10.1016/j.neulet.2007.05.060. [DOI] [PubMed] [Google Scholar]
- 17.Oparil S, Chen YF, Peng N, Wyss JM. Anterior hypothalamic norepinephrine, atrial natriuretic peptide, and hypertension. Front Neuroendocrinol. 1996;17:212–246. doi: 10.1006/frne.1996.0006. [DOI] [PubMed] [Google Scholar]
- 18.Kim E, Seo S, Chung H, Park S. Role of glucocorticoids in fasting-induced changes in hypothalamic and pituitary components of the growth hormone (GH)-axis. Korean J Physiol Pharmacol. 2008;12:217–223. doi: 10.4196/kjpp.2008.12.5.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han TH, Lee K, Park JB, Ahn D, Park JH, Kim DY, Stern JE, Lee SY, Ryu PD. Reduction in synaptic GABA release contributes to target-selective elevation of PVN neuronal activity in rats with myocardial infarction. Am J Physiol Regul Integr Comp Physiol. 2010;299:R129–R139. doi: 10.1152/ajpregu.00391.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krugers HJ, Alfarez DN, Karst H, Parashkouhi K, van Gemert N, Joëls M. Corticosterone shifts different forms of synaptic potentiation in opposite directions. Hippocampus. 2005;15:697–703. doi: 10.1002/hipo.20092. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg JH, Lacefield CO, Yuste R. Global dendritic calcium spikes in mouse layer 5 low threshold spiking interneurones: implications for control of pyramidal cell bursting. J Physiol. 2004;558:465–478. doi: 10.1113/jphysiol.2004.064519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawaguchi Y, Shindou T. Noradrenergic excitation and inhibition of GABAergic cell types in rat frontal cortex. J Neurosci. 1998;18:6963–6976. doi: 10.1523/JNEUROSCI.18-17-06963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, McEnery MW, Shin HS. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha(1G) T-type Ca(2+) channels. Neuron. 2001;31:35–45. doi: 10.1016/s0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Han TH, Sonner PM, Stern JE, Ryu PD, Lee SY. Molecular characterization of T-type Ca(2+) channels responsible for low threshold spikes in hypothalamic paraventricular nucleus neurons. Neuroscience. 2008;155:1195–1203. doi: 10.1016/j.neuroscience.2008.06.055. [DOI] [PubMed] [Google Scholar]
- 26.Lalevée N, Rebsamen MC, Barrére-Lemaire S, Perrier E, Nargeot J, Bénitah JP, Rossier MF. Aldosterone increases T-type calcium channel expression and in vitro beating frequency in neonatal rat cardiomyocytes. Cardiovasc Res. 2005;67:216–224. doi: 10.1016/j.cardiores.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Lesouhaitier O, Chiappe A, Rossier MF. Aldosterone increases T-type calcium currents in human adrenocarcinoma (H295R) cells by inducing channel expression. Endocrinology. 2001;142:4320–4330. doi: 10.1210/endo.142.10.8435. [DOI] [PubMed] [Google Scholar]
- 28.Rossier MF, Lesouhaitier O, Perrier E, Bockhorn L, Chiappe A, Lalevée N. Aldosterone regulation of T-type calcium channels. J Steroid Biochem Mol Biol. 2003;85:383–388. doi: 10.1016/s0960-0760(03)00201-2. [DOI] [PubMed] [Google Scholar]
- 29.Beurrier C, Congar P, Bioulac B, Hammond C. Subthalamic nucleus neurons switch from single-spike activity to burst-firing mode. J Neurosci. 1999;19:599–609. doi: 10.1523/JNEUROSCI.19-02-00599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhan XJ, Cox CL, Rinzel J, Sherman SM. Current clamp and modeling studies of low-threshold calcium spikes in cells of the cat's lateral geniculate nucleus. J Neurophysiol. 1999;81:2360–2373. doi: 10.1152/jn.1999.81.5.2360. [DOI] [PubMed] [Google Scholar]
- 31.Bessaïh T, Leresche N, Lambert RC. T current potentiation increases the occurrence and temporal fidelity of synaptically evoked burst firing in sensory thalamic neurons. Proc Natl Acad Sci USA. 2008;105:11376–11381. doi: 10.1073/pnas.0801484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacol Biochem Behav. 2002;71:457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- 33.Borkowski KR, Finch L. Cardiovascular changes in anaesthetised rats after the intra-hypothalamic administration of adrenaline. Clin Exp Hypertens. 1978;1:279–291. doi: 10.3109/10641967809068609. [DOI] [PubMed] [Google Scholar]
- 34.Pitts DK, Beuthin FC, Commissaris RL. Cardiovascular effects of perfusion of the rostral rat hypothalamus with clonidine: differential interactions with prazosin and yohimbine. Eur J Pharmacol. 1986;124:67–74. doi: 10.1016/0014-2999(86)90125-1. [DOI] [PubMed] [Google Scholar]
- 35.Poole S. Cardiovascular responses of rats to intrahypothalamic injection of carbachol and noradrenaline. Br J Pharmacol. 1983;79:693–700. doi: 10.1111/j.1476-5381.1983.tb10006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wyss JM, Chen YF, Jin H, Gist R, Oparil S. Spontaneously hypertensive rats exhibit reduced hypothalamic noradrenergic input after NaCl loading. Hypertension. 1987;10:313–320. doi: 10.1161/01.hyp.10.3.313. [DOI] [PubMed] [Google Scholar]
- 37.Chen YF, Meng QC, Wyss JM, Jin H, Oparil S. High NaCl diet reduces hypothalamic norepinephrine turnover in hypertensive rats. Hypertension. 1988;11:55–62. doi: 10.1161/01.hyp.11.1.55. [DOI] [PubMed] [Google Scholar]
- 38.Yang RH, Jin H, Chen SJ, Wyss JM, Oparil S. Blocking hypothalamic AT1 receptors lowers blood pressure in salt-sensitive rats. Hypertension. 1992;20:755–762. doi: 10.1161/01.hyp.20.6.755. [DOI] [PubMed] [Google Scholar]
- 39.Oparil S, Yang RH, Jin HG, Chen SJ, Meng QC, Berecek KH, Wyss JM. Role of anterior hypothalamic angiotensin II in the pathogenesis of salt sensitive hypertension in the spontaneously hypertensive rat. Am J Med Sci. 1994;307(Suppl 1):S26–S37. [PubMed] [Google Scholar]
- 40.Kubo T, Yamaguchi H, Tsujimura M, Hagiwara Y, Fukumori R. An angiotensin system in the anterior hypothalamic area anterior is involved in the maintenance of hypertension in spontaneously hypertensive rats. Brain Res Bull. 2000;52:291–296. doi: 10.1016/s0361-9230(00)00266-5. [DOI] [PubMed] [Google Scholar]
- 41.Kvetnanský R, Pacák K, Fukuhara K, Viskupic E, Hiremagalur B, Nankova B, Goldstein DS, Sabban EL, Kopin IJ. Sympathoadrenal system in stress. Interaction with the hypothalamic-pituitary-adrenocortical system. Ann N Y Acad Sci. 1995;771:131–158. doi: 10.1111/j.1749-6632.1995.tb44676.x. [DOI] [PubMed] [Google Scholar]
- 42.Usuku T, Nishi M, Morimoto M, Brewer JA, Muglia LJ, Sugimoto T, Kawata M. Visualization of glucocorticoid receptor in the brain of green fluorescent protein-glucocorticoid receptor knockin mice. Neuroscience. 2005;135:1119–1128. doi: 10.1016/j.neuroscience.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 43.Ito T, Morita N, Nishi M, Kawata M. In vitro and in vivo immunocytochemistry for the distribution of mineralocorticoid receptor with the use of specific antibody. Neurosci Res. 2000;37:173–182. doi: 10.1016/s0168-0102(00)00112-7. [DOI] [PubMed] [Google Scholar]
- 44.Han F, Ozawa H, Matsuda K, Nishi M, Kawata M. Colocalization of mineralocorticoid receptor and glucocorticoid receptor in the hippocampus and hypothalamus. Neurosci Res. 2005;51:371–381. doi: 10.1016/j.neures.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 45.Sarabdjitsingh RA, Meijer OC, de Kloet ER. Specificity of glucocorticoid receptor primary antibodies for analysis of receptor localization patterns in cultured cells and rat hippocampus. Brain Res. 2010;1331:1–11. doi: 10.1016/j.brainres.2010.03.052. [DOI] [PubMed] [Google Scholar]