Abstract
Regulation of B cell receptor (BCR)-induced Ca2+ signaling by CD40 co-stimulation was compared in long-term BCR-stimulated immature (WEHI-231) and mature (Bal-17) B cells. In response to long-term pre-stimulation of immature WEHI-231 cells to α-IgM antibody (0.5~48 hr), the initial transient decrease in BCR-induced [Ca2+]i was followed by spontaneous recovery to control level within 24 hr. The recovery of Ca2+ signaling in WEHI-231 cells was not due to restoration of internalized receptor but instead to an increase in the levels of PLCγ2 and IP3R-3. CD40 co-stimulation of WEHI-231 cells prevented BCR-induced cell cycle arrest and apoptosis, and it strongly inhibited the recovery of BCR-induced Ca2+ signaling. CD40 co-stimulation also enhanced BCR internalization and reduced expression of PLCγ2 and IP3R-3. Pre-treatment of WEHI-231 cells with the antioxidant N-acetyl-L-cysteine (NAC) strongly inhibited CD40-mediated prevention of the recovery of Ca2+ signaling. In contrast to immature WEHI-231 cells, identical long-term α-IgM pre-stimulation of mature Bal-17 cells abolished the increase in BCR-induced [Ca2+]i, regardless of CD40 co-stimulation. These results suggest that CD40-mediated signaling prevents antigen-induced cell cycle arrest and apoptosis of immature B cells through inhibition of sustained BCR-induced Ca2+ signaling.
Keywords: B cell receptor, Ca2+, CD40, Reactive oxygen species, WEHI-231
INTRODUCTION
Intracellular Ca2+ signaling induced by engagement of B cell antigen receptor (BCR) controls diverse Ca2+-dependent B cell immune responses, such as B cell motility, immunological synapse formation, regulation of gene expression, and B cell fate determination [1-3]. Engagement of BCR initiates coordinated recruitment of a series of adaptor molecules and protein kinases, ultimately resulting in activation of PLCγ2, which produces inositol 1,4,5-triphosphate (IP3) and mobilizes stored Ca2+. Engagement of antigens with BCR triggers internalization of antigen-BCR complexes, which then are processed and ultimately presented in complex form with MHC-II on the cell surface membrane to recruit T cell help [4].
BCR-mediated Ca2+ signaling together with other signaling pathways determines B cell fate at certain stages of development [2]. In response to the same antigenic stimulus, mature B cells proliferate and undergo clonal expansion into memory B cells, whereas immature B cells cannot proliferate and instead undergo cell cycle arrest and apoptosis. Negative selection - the elimination of immature B cells upon exposure to self-antigens - is a pivotal immunological response for the avoidance of diverse autoimmune diseases. The molecular mechanisms underlying the different immune responses between mature and immature B cells to the same antigenic stimulus have been extensively studied. For example, up-regulation of p27Kip1 accumulation [5] and phospholipase A2-mediated disruption of mitochondrial membrane potential [6,7] have been suggested as the main causes of BCR-mediated cell cycle arrest and apoptosis in stimulated immature B cells. Nonetheless, the underlying mechanisms of negative selection of activated immature B cells are not entirely clear.
CD40 is a membrane receptor belonging to the tumor necrosis factor receptor (TNF-R) superfamily. CD40 is expressed not only on immune cells (B cells, macrophages, dendritic cells) but also on non-immune cells such as endothelial cells and keratinocytes [8]. The ligand of CD40 (CD40L) is expressed on the activated CD4+ T cell membrane. Binding of CD40L to CD40 receptor results in both humoral and cellular immune responses [8]. It is also well known that ligation of CD40 receptor expressed on B lymphocytes with CD40L can block BCR-induced cell cycle arrest and apoptosis in immature B cells, resulting in stabilization of mitochondrial membrane potential, upregulation of anti-apoptotic protein Bcl-xL [9,10], maintenance of NFκB activity [11], and down-regulation c-Myc and p27Kip1 [5]. In addition, CD40 ligation was reported to reduce autophagy [12] and ER stress-mediated apoptosis caused by BCR ligation [13]. Nonetheless, the detailed signaling mechanisms underlying CD40-mediated rescue of immature B cells from growth arrest and apoptosis remain to be elucidated.
In the present study, we investigated whether or not CD40-mediated regulation of BCR-induced Ca2+ signaling plays an important role in the B cell response to chronic stimulation by antigens that induce B cell anergy or apoptosis. For this purpose, we used Bal-17 cells and WEHI-231 cells, which are representative models of mature and immature murine B cells, respectively. To mimic the B cell anergy-inducing state, or a state of unresponsiveness to previously encountered antigens, these two B cell lines were stimulated long-term (0.5~48 hr) with α-IgM antibody, after which changes in their [Ca2+]i in response to brief BCR ligation were compared. We analyzed the time courses of BCR-mediated [Ca2+]i, degree of BCR internalization, PLCγ2 and IP3R-3 expression, and BCR-induced cell cycle arrest and apoptosis. It has been reported that the ligation of CD40 with CD40L activates diverse signaling pathways including generation of reactive oxygen species (ROS) [8,14]. Therefore, we tested the effects of an antioxidant (NAC) on CD40-mediated regulation of BCR-induced Ca2+ signaling in immature WEHI-231 cells.
METHODS
Cell culture
WEHI-231 cells and Bal-17 cells were cultured in DMEM and RPMI media (Gibco), respectively. Culture media were supplemented with 10% inactivated fetal bovine serum (FBS, Gibco) and 50 µM 2-mecaptoethanol (Sigma). Cells were maintained in a 37℃ incubator with 5% CO2 for Bal-17 cells and 10% CO2 for WEHI-231 cells.
[Ca2+]i measurement
Cells (2×106) were harvested and washed with normal Tyrode solution. Cells were then loaded with 4 µM Fura 2-AM (Molecular Probes) at room temperature for 30 min. After loading, cells were washed twice before [Ca2+]i measurement in a 1 ml temperature-controlled stirring cuvette system (Photon Technology International, PTI, USA). [Ca2+]i measurements were carried out at 36℃. Briefly, the cells were alternatively excited (340 and 380 nm) at a frequency of 5 Hz, after which the fluorescence emitted at 510 nm was collected via a photomultiplier tube. The obtained fluorescence ratios (F340/F380) were used to calculate the [Ca2+] by treating the cells with ionomycin (Enzo Life Sciences) and EGTA (Sigma) at the end of each measurement [15,16]. Finally, data were analyzed and plotted using Felix32 (PTI) and Origin software (OriginLab). Normal Tyrode solution used for [Ca2+]i measurement contained (in mM), NaCl 140, KCl 5, MgCl2 1, CaCl2 1.5, glucose 10, and HEPES 10, at a pH of 7.4. For [Ca2+]i measurement in high-K+ solution, 130 mM NaCl was replaced with the same concentration of KCl. For the measurement of Ca2+ release from the intracellular Ca2+ stores, 2 mM EGTA was added into the measuring cuvette to chelate 1.5 mM of extracellular Ca2+. Addition of 4 µl of EGTA solution (0.5 M, pH 9) into 1 ml of Tyrode solution (pH 7.4) did not change the extracellular pH of the solution.
FACS analysis of surface IgM receptor (surface BCR)
About 2×105 cells were washed twice and incubated in pre-warmed Tyrode solution at room temperature. After ~1 hr, cells were washed in ice-cold phosphate buffer solution (PBS) before incubation with 1 µl of phycoerythrin-conjugated anti-mouse IgM (BioLegend) in 200 µl of FACS buffer (PBS containing 2% FBS) for 20 min at 4℃. The cells were then washed and re-suspended in FACS buffer for analysis with FACSCalibur (Becton Dickinson).
Immunoprecipitation (IP) and Western blot (WB)
Cells were lysed in lysis buffer containing 50 mM Tris-HCl (pH 7.4), 1% (v/v) Triton X-100, 150 mM NaCl, proteinase, and phosphatase inhibitors. At least 500 µg and 10 µg of protein were used for IP and WB, respectively. Cell lysate was round-rotated with 2 µg of anti-phosphotyrosine antibody (clone 4G10, Upstate) and protein G agarose (Upstate) at 4℃ for 2 hr before the G beads were collected, washed three times with lysis buffer, and re-suspended in 30 µl of loading buffer. After boiling at 100℃ for 5 min, 25 µl of bead-free supernatant was separated in SDS gel for Western blotting with PLCγ2 on PVDF membrane. Protein exposure was performed in the dark using an ECL kit (Amersham).
Apoptosis and cell cycle arrest assay
These assays utilized the DNA-staining dye propidium iodide (PI) as previously described [17,18]. Briefly, cells were washed once with ice-cold PBS before being re-suspended in hypotonic solution (0.1% sodium citrate, 0.1% Triton X-100). Five micrograms of PI (Sigma) was added, and cell tubes were incubated in the dark and on ice for 1 hr, followed by analysis with FACSCalibur. Cell cycle and apoptosis were analyzed on linear and logarithmic fluorescence scales, respectively.
Antibodies and reagents
Antibodies used included: anti-mouse IgM (α-IgM) (Jackson ImmunoResearch), anti-mouse CD40 (CD40L) (eBioscience), anti-PLCγ2 antibody (Cell Signaling), anti IP3R-3 (BD Biosciences), and anti-β-actin antibody (Sigma). Thapsigargin (Tg) and N-acetyl-L-cysteine (NAC) were purchased from Enzo Life Sciences and Sigma, respectively.
Statistical analysis
The values given in the text are mean±S.E.M. with n, the sample size. Statistical significance was analyzed by Student's t-test.
RESULTS
CD40 co-stimulation inhibits sustained BCR-induced Ca2+ signaling of immature B cells
Mature Bal-17 cells and immature WEHI-231 cells were pre-treated with α-IgM antibody (5 µg/ml) in culture media for 0.5, 2, 4, 8, 24, and 48 hr. After removing the pre-treatment of α-IgM, B cells were briefly stimulated with α-IgM (5 µg/ml) for 150 sec, and the resulting BCR-induced [Ca2+]i increases were compared between the two cell lines.
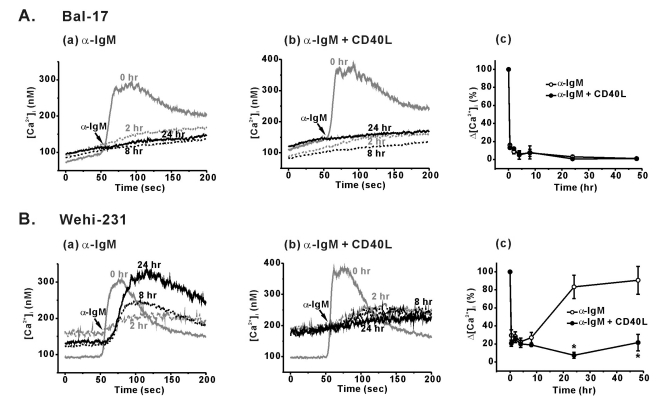
Following α-IgM pre-stimulation of mature Bal-17 cells, negligible BCR-induced [Ca2+]i increases were produced in response to incoming brief BCR ligation (Fig. 1Aa). After α-IgM pre-stimulation of Bal-17 cells for 2, 8, 24, and 48 hr, BCR-induced Δ[Ca2+]i values were 8.9±2.4, 7.4±3.9, 3.0±1.7, and 1.1±0.4% of non-treated control cells, respectively (n=4, Fig. 1Ac).
Fig. 1.
Differential BCR-induced Ca2+ responses and its modulation by CD40 co-stimulation in Bal-17 and WEHI-231 cells. B cells were pretreated with 5 µg/ml of α-IgM (Aa, Ba) or α-IgM+CD40L (0.5 µg/ml) (Ab, Bb) for 0.5, 2, 4, 8, 24, and 48 hr. After harvesting the cells, the pre-treatment agents were removed, and α-IgM (5 µg/ml)-triggered [Ca2+]i increases were measured in 1.5 mM [Ca2+]o containing normal Tyrode solution. Representative [Ca2+]i traces obtained from each B cell type are drawn, and the duration of α-IgM pre-stimulation (0, 2, 8, and 24 hr) are indicated inside the figure. (Ac, Bc) Δ[Ca2+]i values (difference between the resting and peak [Ca2+]i levels) were calculated and normalized against Δ[Ca2+]i values of un-stimulated control cells. Changes in Δ[Ca2+]i values are plotted against the duration of pre-stimulation. All experiments were repeated at least three times, and the detailed values are described in the text. *p<0.05.
Unlike mature B cells, immature WEHI-231 cells showed sustained BCR-induced Ca2+ signaling under identical α-IgM pre-stimulation. As shown in Fig. 1B, amplitudes of the BCR-induced [Ca2+]i transients markedly decreased during the early phase (0.5~8 hr) of α-IgM pre-stimulation. At 0.5, 2, 4, and 8 hr of α-IgM pre-stimulation, Δ[Ca2+]i values decreased to 29.6±6.1, 28.3±5.1, 22.5±4.2, and 27.0±5.9%, respectively (n=4, Fig. 1Bc). Unexpectedly, however, the initially suppressed BCR-induced [Ca2+]i responses spontaneously recovered to control level within 24 hr of pre-stimulation (Fig. 1Ba). After 24 and 48 hr of pre-stimulation, the Δ[Ca2+]i values actually recovered to 83.3±13 and 90.6±15.5% of the control, respectively (n=4, Fig. 1Bc). These spontaneously restored [Ca2+]i transients showed slow kinetics. The time it took to reach the peak of the restored [Ca2+]i transients was 53.5±2.5 sec, which is much slower than that of the control cells (28.0±0.5 sec) (p<0.01, n=7, Fig. 1Ba). Since slow [Ca2+]i kinetics is a feature of sub-maximally stimulated B cells, the strength of the restored Ca2+ signaling pathway was weakened.
To investigate the CD40-mediated regulation of BCR-induced Ca2+ signaling, B cells were pre-treated with CD40 ligand (CD40L, 0.5 µg/ml) along with α-IgM for the indicated time period. CD40 co-stimulation of Bal-17 cells did not affect the suppressed BCR-induced Ca2+ responses (Fig. 1Ab). In contrast, CD40 co-stimulation of WEHI-231 cells strongly prevented the spontaneous recovery of BCR-induced Ca2+ signaling (Fig. 1Bc). BCR-induced Δ[Ca2+]i values decreased to 6.5±3.4% (at 24 hr) and 19.7±5.9% (at 48 hr) of the control values upon CD40 co-stimulation.
We compared the BCR-induced increases in [Ca2+]i after exposure to high K+ (130 mM KCl)-containing solution in order to remove the influence of membrane potential (Fig. 2A). However, we observed the same patterns of restoration of Ca2+ signaling as described in Fig. 1B. Furthermore, CD40-mediated inhibition of BCR-induced [Ca2+]i increase was not changed by high-K+ solution. As another possibility, we compared the amounts of internal Ca2+ release and Ca2+ influx via store-operated Ca2+ (SOC) channels by depleting the stores using α-IgM (5 µg/ml) or thapsigargin (1 µM). However, the level of Ca2+ release and SOC activity did not change upon α-IgM pre-stimulation or CD40 co-stimulation (Fig. 2B~D).
Fig. 2.
Contribution of cell membrane potential, amounts of intracellular Ca2+ release and store-operated Ca2+ entry (SOC) on sustained BCR-induced Ca2+ responses in immature WEHI-231 cells. WEHI-231 cells were pretreated with 5 µg/ml of α-IgM or α-IgM+CD40L (0.5 µg/ml) for 24 hr. After harvesting the cells, the pre-treatment agents were removed, and α-IgM (5 µg/ml)-triggered [Ca2+]i increases were measured in 130 mM [KCl]o and 1.5 mM [Ca2+]o containing solution (A) or extracellular Ca2+-free Tyrode solution (B). The amounts of store-operated Ca2+ entry in response to the stimulation of the cells with α-IgM (B) or thapsigargin (C) were measured by the re-addition of 1.5 mM [Ca2+]o after depleting the stores in Ca2+-free Tyrode solution. (D) Δ[Ca2+]i values (difference between the resting and peak [Ca2+]i levels) from (A), (B) and (C) were calculated and plotted. Mean values±S.E.M. from at least 3 repeats were shown. #p<0.05.
BCR-induced apoptosis of WEHI-231 cells and its inhibition by CD40 co-stimulation
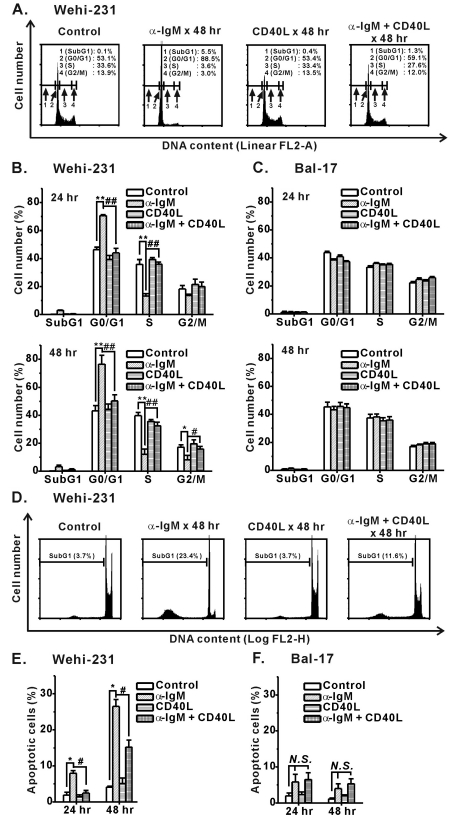
It is well known that CD40 co-stimulation prevents BCR-induced apoptosis of immature B cells, including WEHI-231 cells. In our experiment, CD40 co-stimulation of WEHI-231 cellṣ inhibited α-IgM-induced cell cycle arrest (G0/G1) and apoptosis (SubG1) (Fig. 3). Obvious cell cycle arrest and apoptosis of WEHI-231 cells occurred after 24~48 hr of α-IgM stimulation, which matches the recovery time of the BCR-induced [Ca2+]i response, as explained in Fig. 1B. Therefore, these results indicate that CD40-mediated signaling prevented BCR-induced cell growth inhibition and apoptosis of WEHI-231 cells via interference of sustained BCR-Ca2+ signaling.
Fig. 3.
Inhibition of α-IgM-induced cell cycle arrest and apoptosis of immature WEHI-231 cells by CD40 co-stimulation. WEHI-231 and Bal-17 cells were stimulated with α-IgM or α-IgM+CD40L for 24 and 48 hr, after which FACS analysis was performed to quantify the degree of cell cycle arrest (A~C) and apoptosis (D~F). Cell cycle and apoptosis were analyzed on linear FL2-area (FL2-A, total cell PI fluorescence) and logarithmic FL2-height (FL2-H, maximum PI fluorescence emission) scales, respectively. All experiments were repeated three times. Mean±S.E.M.. *,#p<0.05, **,##p<0.01, N.S., no significance.
CD40 co-stimulation enhances BCR internalization
To understand the underlying mechanisms of the differential Ca2+ responses in mature and immature B cells, we investigated changes in BCR internalization. In addition, changes in the expression levels of PLCγ2 and IP3R were measured by Western blotting. We paid particular attention to the molecules corresponding to the time course of BCR-induced Ca2+ signaling in WEHI-231 cells.
As shown in Fig. 4, significant and sustained internalization of surface BCR was observed in both cell lines after α-IgM pre-stimulation. Neither cell type showed spontaneous restoration of internalized BCR back to the plasma membrane. Therefore, the spontaneous recovery of BCR-induced Ca2+ signaling observed in WEHI-231 cells was not mediated by an increased number of surface receptors. The degree of BCR internalization was much greater in WEHI-231 cells than Bal-17 cells. After α-IgM pre-stimulation, less than 15% of the surface receptors remained on the plasma membrane in WEHI-231 cells, whereas ~50% of the receptors were present on Bal-17 cells. This was contrary to our expectations, since Bal-17 cells maintaining a higher number of receptors showed a negligible [Ca2+]i increase (Fig. 1A). These results indicate that immature B cells were more sensitive to BCR ligation, and thus a smaller number of receptors could produce physiologically relevant Ca2+ signals.
Fig. 4.
Effects of CD40 co-stimulation on internalization of surface BCR triggered by α-IgM pre-stimulation. WEHI-231 (A) and Bal-17 (B) cells were stimulated with α-IgM alone or α-IgM+CD40L for the indicated times, followed by surface BCR measurement indicated by PE fluorescence emission (FL2-H) in FACS machine. Effects of CD40 co-stimulation on α-IgM-triggered internalization of BCR were quantified and normalized (% surface BCR) to that of un-treated cells and then plotted against the indicated times of stimulation in WEHI-231 (C) and Bal-17 (D) cells. Mean±S.E.M. values of three repeats are plotted. *p<0.05.
CD40 co-stimulation of WEHI-231 cells slightly increased BCR internalization. Normalized surface BCR levels in co-stimulated WEHI-231 cells decreased to 11.7±1.1, 9.1±1.0, 9.3±1.1, and 8.1±0.1% at 2, 8, 24, and 48 hr of pre-stimulation, respectively (Fig. 4C). CD40-induced increase in receptor internalization was much more prominent in Bal-17 cells (Fig. 4D). Normalized surface BCR levels at 8, 24, and 48 hr of α-IgM pre-exposure were 45.2±2.9, 48.5±1.2, and 50.4±0.3%, respectively, in Bal-17 cells. However, upon CD40 co-stimulation, the values decreased to 36.2±1.8, 36.8±2.1, and 31.0±1.4% (p<0.05, n=3, Fig. 4D). These results suggest that increased internalization of surface BCR was responsible for the CD40-mediated suppression of BCR-induced Ca2+ signaling.
CD40 co-stimulation decreases PLCγ2 and IP3R-3 expression
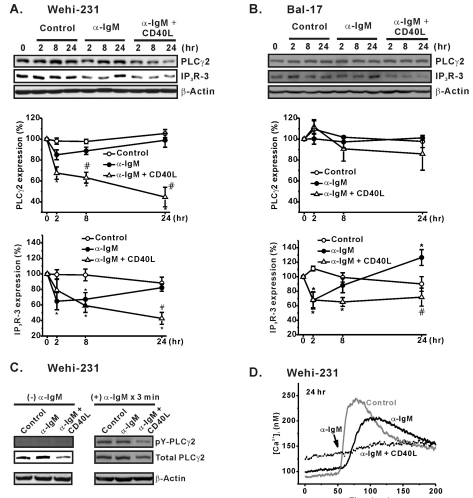
In the context of strength of BCR-induced Ca2+ signaling, changes in the expression of PLCγ2 and IP3R-3, which are key downstream molecules of BCR, were determined. PLCγ2 is the key enzyme that generates IP3 from PIP2 and releases stored Ca2+ by opening endoplasmic reticular IP3R. Among the three IP3R subtypes identified in B cells, we selected subtype 3 (IP3R-3) since it supposedly is important to the mediation of BCR-induced apoptosis in immature WEHI-231 cells [19]. Following α-IgM pre-stimulation of WEHI-231 cells, the total amount of PLCγ2 decreased to 85.1±4.7% at 2 hr, followed by an increase back to 98.9±9.6% at 24 hr. The same pattern was observed for IP3R-3 expression. IP3R-3 expression decreased to 64.8±11.1% at 2 hr and was then up-regulated to 82.3±4.7% at 24 hr after pre-stimulation (Fig. 5A). Importantly, CD40 co-stimulation of WEHI-231 cells strongly decreased the expression of PLCγ2 and IP3R-3, and it abolished spontaneous restoration of the molecules (Fig. 5A). Therefore, the biphasic changes in PLCγ2 and IP3R-3 expression correlated well with the biphasic recovery time courses of the BCR-induced Ca2+ responses in WEHI-231 cells. The total amount of PLCγ2 in WEHI-231 cells strongly governed overall PLCγ2 activity, which was indicated by the amount of tyrosine phosphorylated PLCγ2 (Fig. 5C) and the amplitudes of the BCR-induced [Ca2+]i transients (Fig. 5D). Although similar biphasic recovery of IP3R-3 expression and its inhibition by CD40 co-stimulation was observed in Bal-17 cells, the PLCγ2 level was not affected by either α-IgM or CD40 co-stimulation after 24 hr (Fig. 5B).
Fig. 5.
Effects of CD40 co-stimulation on expression of PLCγ2 and IP3R-3. WEHI-231 (A) and Bal-17 (B) cells were treated long-term with α-IgM in the absence or presence of CD40 co-stimulation. Extracted total protein content was subjected to Western blotting to measure PLCγ2 and IP3R-3 expression. β-Actin was used as a protein loading control. The quantified intensities of PLCγ2 (PLC32/β-actin) and IP3R-3 (IP3R-3/β-actin) were normalized (% expression) to those of un-treated cells and then plotted against the indicated times of stimulation. Mean±S.E.M. of three independent experiments are shown in A and B. *p<0.05 compared to untreated cells. #p<0.05 compared to α-IgM-treated cells. (C) WEHI-231 cells were treated for 24 hr with α-IgM or α-IgM+CD40L. The harvested cells were re-suspended in NT solution and stimulated with α-IgM (5 µg/ml) for 3 min at 37℃. The α-IgM-stimulated cells were immunoprecipitated to measure the amount of tyrosine-phosphorylated PLCγ2 (pY-PLCγ2). Densities of pY-PLCγ2 were compared against those of total PLCγ2 and β-actin. (D) The amounts of pY-PLCγ2 in response to 3 min of BCR ligation in C were clearly determined based on the amplitudes of α-IgM-triggered [Ca2+]i increases in WEHI-231 cells.
CD40-mediated ROS signaling regulates BCR-Ca2+ signaling of WEHI-231 cells
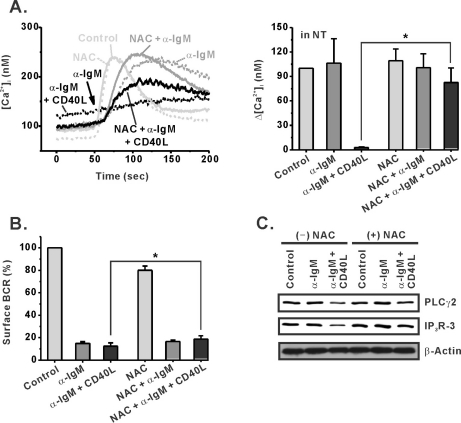
It has been reported that CD40 ligation in WEHI-231 cells produces reactive oxygen species (ROS), which play a role in CD40 signaling [14]. To investigate the requirement for ROS in CD40-mediated regulation of sustained Ca2+ signaling, we treated WEHI-231 cells with the antioxidant NAC (10 mM) prior to CD40 co-stimulation. As clearly shown in Fig. 6A, NAC strongly prevented CD40-induced suppression of BCR-Ca2+ signaling and induced spontaneous recovery of Ca2+ signaling. In addition, NAC-induced recovery of Ca2+ signaling in WEHI-231 cells was not only associated with reduced BCR internalization (Fig. 6B) but also with increased expression of PLCγ2 and IP3R-3 (Fig. 6C). In control cells and α-IgM pre-stimulated cells, however, NAC did not affect the amplitudes of [Ca2+]i transients, surface BCR expression, or the expression of PLCγ2 and IP3R-3 (Fig. 6A~C). These results suggest that CD40-mediated ROS generation played an important role in the regulation of BCR-induced Ca2+ signaling in immature B cells.
Fig. 6.
An antioxidant, NAC, inhibits the effect of CD40 co-stimulation in WEHI-231 cells. An antioxidant, NAC (10 mM), was treated 30 min before stimulation of WEHI-231 cells with α-IgM or α-IgM+CD40L. After 24 hr of stimulation in the absence or presence of NAC, the cells were harvested and subjected to measurement of BCR-induced [Ca2+]i increase in NT solution (A), analysis of surface BCR level (B), and Western blotting for PLCγ2 and IP3R-3 expression (C). Mean±S.E.M. *p<0.05.
DISCUSSION
In this report, we demonstrated that immature WEHI-231 cells and mature Bal-17 cells differentially respond to long-term α-IgM stimulation and produce quite distinct BCR-induced Ca2+ signaling profiles. In mature Bal-17 cells, total loss of Ca2+ signaling occurred in response to chronic antigenic exposure. Based on these results, we can speculate that the loss of responsiveness was mainly due to sustained BCR internalization. In contrast to mature Bal-17 cells, immature WEHI-231 cells were characterized by unique biphasic Ca2+ signaling in response to the same antigen. The initially suppressed BCR-Ca2+ signaling in WEHI-231 cells spontaneously recovered back to basal level at later phase of antigenic stimulation. We speculate that this recovery of Ca2+ signaling was mainly caused by biphasic restoration of expression of PLCγ2 and IP3R-3, which are key BCR downstream molecules. In the meantime, CD40 co-stimulation abolished the recovery of Ca2+ signaling. We speculate that this was caused by a decrease in the expression of surface BCR, PLCγ2, and IP3R-3. Finally, the ROS scavenger NAC strongly inhibited the effect of CD40 ligation by increasing expression of surface BCR, PLCγ2, and IP3R-3.
It has been reported that immature B cells exhibit higher sensitivity than mature B cells in response to BCR ligation. Immature B cells show larger Ca2+ mobilization when triggered by the same concentration of antigen, and they require lower concentration of antigen overall [20]. Here, we revealed that under chronic antigenic stimulation, responses of WEHI-231 cells to BCR ligation (Ca2+ signals) were high. However, this was not the case for mature Bal-17 cells. The sustained [Ca2+]i increase in WEHI-231 cells can be attributed to many factors. First of all, as shown in Fig. 4, spontaneous increase in surface IgM receptor number during antigenic pre-stimulation period was excluded as a possible mechanism. We also excluded the possibility of changes in cell membrane potential, the amounts of internal Ca2+ release and Ca2+ influx via store-operated Ca2+ (SOC) channels (Fig. 2).
At present, our findings suggest that immature WEHI-231 cells are not able to terminate BCR-mediated signaling upon prolonged BCR engagement. Therefore, continued BCR-mediated signaling may lead to exhaustive activation and contribute to activation-induced growth arrest and apoptosis in immature B cells.
The ligation of CD40 with CD40L activates many signaling pathways including ROS [8,14]. Our results clearly show that NAC, a ROS scavenger, affects regulation of Ca2+ signaling by CD40 co-stimulation in immature B cells (Fig. 6). Since our data were obtained simply by a pharmacological intervention of ROS generation, there is great potential for future molecular studies on the regulation of BCR-induced Ca2+ signaling by CD40-mediated ROS generation especially in immature B cells. Since we used a non-specific ROS scavenger (NAC), further studies using selective intervention of ROS generating enzymes (NADPH oxidases, 5-lipoxygenases) are needed to reveal the CD40-induced ROS generating systems. In variety of cells, ROS-dependent activation of ERK and p38 MAPK was clearly demonstrated [21]. Importantly, it has been suggested that CD40-mediated signaling requires ROS for the activation of JNK, p38, and Akt [14]. Therefore, it is necessary to demonstrate the roles of these signaling molecules on CD40-mediated inhibition of BCR-induced Ca2+ signaling, especially in immature B cells.
ACKNOWLEDGEMENTS
This work was supported by a Korea Research Foundation grant funded by the Korean Government (KRF-2008-314-E00008).
ABBREVIATIONS
- BCR
B cell antigen receptor
- [Ca2+]i
intracellular Ca2+ concentration
- PLCγ2
phospholipase C-γ2
- α-IgM
anti-IgM antibody
- CD40L
CD40 ligand
- NAC
N-acetyl-L-cysteine
- ROS
reactive oxygen species
- IP3R
inostol 1,4,5-trisphosphate receptor
- EGTA
ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid
References
- 1.Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002;2:945–956. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- 2.Scharenberg AM, Humphries LA, Rawlings DJ. Calcium signalling and cell-fate choice in B cells. Nat Rev Immunol. 2007;7:778–789. doi: 10.1038/nri2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koncz G, Bodor C, Kövesdi D, Gáti R, Sármay G. BCR mediated signal transduction in immature and mature B cells. Immunol Lett. 2002;82:41–49. doi: 10.1016/s0165-2478(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 4.Harwood NE, Batista FD. New insights into the early molecular events underlying B cell activation. Immunity. 2008;28:609–619. doi: 10.1016/j.immuni.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Donjerković D, Zhang L, Scott DW. Regulation of p27Kip1 accumulation in murine B-lymphoma cells: role of c-Myc and calcium. Cell Growth Differ. 1999;10:695–704. [PubMed] [Google Scholar]
- 6.Chakraborti T, Das S, Mondal M, Roychoudhury S, Chakraborti S. Oxidant, mitochondria and calcium: an overview. Cell Signal. 1999;11:77–85. doi: 10.1016/s0898-6568(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 7.Katz E, Deehan MR, Seatter S, Lord C, Sturrock RD, Harnett MM. B cell receptor-stimulated mitochondrial phospholipase A2 activation and resultant disruption of mitochondrial membrane potential correlate with the induction of apoptosis in WEHI-231 B cells. J Immunol. 2001;166:137–147. doi: 10.4049/jimmunol.166.1.137. [DOI] [PubMed] [Google Scholar]
- 8.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 9.Katz E, Lord C, Ford CA, Gauld SB, Carter NA, Harnett MM. Bcl-(xL) antagonism of BCR-coupled mitochondrial phospholipase A(2) signaling correlates with protection from apoptosis in WEHI-231 B cells. Blood. 2004;103:168–176. doi: 10.1182/blood-2003-07-2473. [DOI] [PubMed] [Google Scholar]
- 10.Lee HH, Dadgostar H, Cheng Q, Shu J, Cheng G. NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc Natl Acad Sci USA. 1999;96:9136–9141. doi: 10.1073/pnas.96.16.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mineva ND, Rothstein TL, Meyers JA, Lerner A, Sonenshein GE. CD40 ligand-mediated activation of the de novo RelB NF-kappaB synthesis pathway in transformed B cells promotes rescue from apoptosis. J Biol Chem. 2007;282:17475–17485. doi: 10.1074/jbc.M607313200. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe K, Ichinose S, Hayashizaki K, Tsubata T. Induction of autophagy by B cell antigen receptor stimulation and its inhibition by costimulation. Biochem Biophys Res Commun. 2008;374:274–281. doi: 10.1016/j.bbrc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Yan BC, Adachi T, Tsubata T. ER stress is involved in B cell antigen receptor ligation-induced apoptosis. Biochem Biophys Res Commun. 2008;365:143–148. doi: 10.1016/j.bbrc.2007.10.137. [DOI] [PubMed] [Google Scholar]
- 14.Lee JR. Reactive oxygen species play roles on B cell surface receptor CD40-mediated proximal and distal signaling events: effects of an antioxidant, N-acetyl-L-cysteine treatment. Mol Cell Biochem. 2003;252:1–7. doi: 10.1023/a:1025529704480. [DOI] [PubMed] [Google Scholar]
- 15.Tsien RY. New tetracarboxylate chelators for fluorescence measurement and photochemical manipulation of cytosolic free calcium concentrations. Soc Gen Physiol Ser. 1986;40:327–345. [PubMed] [Google Scholar]
- 16.Malgaroli A, Milani D, Meldolesi J, Pozzan T. Fura-2 measurement of cytosolic free Ca2+ in monolayers and suspensions of various types of animal cells. J Cell Biol. 1987;105:2145–2155. doi: 10.1083/jcb.105.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nunez R. DNA measurement and cell cycle analysis by flow cytometry. Curr Issues Mol Biol. 2001;3:67–70. [PubMed] [Google Scholar]
- 18.Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- 19.Khan AA, Soloski MJ, Sharp AH, Schilling G, Sabatini DM, Li SH, Ross CA, Snyder SH. Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5-trisphosphate receptor. Science. 1996;273:503–507. doi: 10.1126/science.273.5274.503. [DOI] [PubMed] [Google Scholar]
- 20.Benschop RJ, Brandl E, Chan AC, Cambier JC. Unique signaling properties of B cell antigen receptor in mature and immature B cells: implications for tolerance and activation. J Immunol. 2001;167:4172–4179. doi: 10.4049/jimmunol.167.8.4172. [DOI] [PubMed] [Google Scholar]
- 21.Lee YJ, Kim NY, Suh YA, Lee C. Involvement of ROS in curcumin-induced autophagic cell death. Korean J Physiol Pharmacol. 2011;15:1–7. doi: 10.4196/kjpp.2011.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]