Abstract
Cytomegalovirus (CMV) infection promotes a broad T-cell response, with the resulting memory cells displaying diverse phenotypes. CMV establishes lifelong persistence/latency, and it is thought that viral antigens expressed during this period may regulate the expansion and/or maintenance of “inflationary” CD8 T-memory populations that display an effector memory phenotype. We show here that mouse CMV (MCMV)-specific inflationary memory T cells do not decrease in number after thymectomy, indicating that recent thymic emigrants are not strictly required for their maintenance. Furthermore, persistent MCMV replication in the salivary gland does not significantly impact the T-cell memory compartment, as surgical removal did not alter its composition. These results shed light upon the mechanisms required for maintenance of the large, MCMV-specific T-cell memory pool.
Human cytomegalovirus (HCMV/HHV5, a b-herpesvirus) establishes a largely asymptomatic infection in ≥50% of the healthy population. In contrast, immune-compromised individuals can suffer serious consequences when facing HCMV (e.g., newborns, transplant recipients, and AIDS patients) (1,2). While it is established that HCMV causes disease in settings in which immunity is decreased, increasing evidence indicates that CMV “shaping” of the immune system over a lifetime of infection may also be a cofactor for disease. This hypothesis stems partly from the fact that CMV-specific T cells expand to large numbers and compose a huge percentage of the circulating lymphocyte pool in infected persons, a process termed “memory inflation” (3–7).
Data gleaned from several model systems indicate that memory T cells are a heterogeneous population of cells requiring diverse stimuli for their generation and maintenance (8–10). The CMV-specific T-cell response is directed against many viral orfs (≥151), but ones that ultimately comprise the majority of the memory pool have a limited number of epitope specificities and display an effector memory phenotype (TEM, for example H-2Kb M38316–323, m139419–426, and IE3416–423) (3). In turn, the remainder of the CMV-specific memory T-cell pool is more diverse in specificity, has a central memory phenotype, and remains stable in number following its contraction after acute infection (TCM, for example H-2Db M45985–993) (6,11–14).
TEM differ from TCM in their maintenance requirements and functional properties, as they primarily reside in tissues and likely function as first lines of defense against mucosal infection (15–17). Heterologous epitopes derived from LCMV or influenza proteins expressed by a recombinant mouse CMV (MCMV) can give rise to inflationary TEM cells that are protective in a vaccine setting (19). Furthermore, a rhesus CMV vaccine-vector engineered to express SIV proteins promoted far superior protection by inducing cells with a TEM phenotype compared to TCM of the same specificity generated by different means (18). Consequently, even though it is not fully understood why some CMV orf-derived peptides induce TEM cells, there is a movement to utilize the fact that CMV induces large populations of these cells to clinical advantage. In this study, we address two fundamental questions regarding CMV-specific TEM cells: (1) are naïve recent thymic emigrants required to maintain the numbers of these cells, and (2) does persistent salivary gland replication play a role in their generation and/or maintenance.
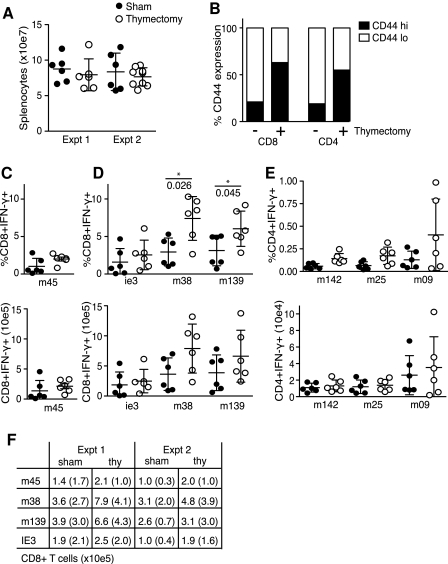
After thymectomy, the number of circulating naïve T cells gradually decreases due to the lack of recent thymic emigrants (RTE) (T½ naïve T cells ∼49 d) (20–24). To test how this might affect the CMV-specific T-cell pools, female young adult (6–8 wk) C57BL/6 (B6) mice were thymectomized or sham operated, and after a ∼10-d recovery period were infected intraperitoneally (IP) with 5 × 104 PFU of salivary gland propagated MCMV-Smith (ATCC VR-194) for ∼100 d. At that time, splenocytes were quantified and re-stimulated with 2 μg/mL of MCMV orf-derived peptides in the presence of 1 μg/mL brefeldin A for 5 h (CD8 T cells), or incubated first with peptide for 3 h and then for an additional 5 h in the presence of golgi-plug (BD, CD4 T cells). Virus-specific CD8 and CD4 T cells were identified by flow cytometry for intracellular IFN-γ expression. Re-stimulation with BFA alone resulted in <0.4% IFN-γ positive cells, and golgi-plug alone <0.03%. MCMV epitope-specific inflationary CD8 memory T cells (peptides derived from the MCMV proteins M38316–323, m139419–426, and IE3416–423), and stable CD8 memory T cells (M45985–993), as well as CD4 memory T cells (M25409–423, m14224–38, and m09133–147), were measured in a blinded fashion, segregating the groups after examining sacrificed mice for the presence of a thymus (3). The percentages of M45-specific CD8 T cells trended slightly higher in the spleens of thymectomized animals (Fig. 1C), but this was less dramatic when comparing total cell numbers (Fig. 1C), as spleens of thymectomized animals showed modestly reduced cellularity (Fig. 1A). The inflationary CD8 T populations specific for M38, M139, and IE3 consistently trended higher in thymectomized animals (Fig. 1D and F), while only the M09-specific CD4 T-cell response was slightly increased, leaving M142- and M25-specific CD4 T cells largely unaffected by the thymectomy (Fig. 1E).
FIG. 1.
The effect of thymectomy on CMV-specific memory T cells. Thymectomized (open circles) or sham operated (solid circles) wild-type (wt) B6 mice were MCMV infected. (A) On day 100, mice were sacrificed and splenocyte numbers were determined. (B) Splenocytes were stained for CD4, CD8, and CD44 expression. (C, D, and E) Splenocytes were stimulated in vitro with the indicated MCMV orf-derived peptides, and virus-specific CD8 (C and D) or CD4 (E) T cells were identified by flow cytometry for expression of IFN-γ. Results are displayed as percentages (top row) or numbers (bottom row) of peptide-specific CD8 or CD4 T cells/spleen, and are shown as mean ± SD of 6 mice/group. (F) Mean numbers and SD of epitope-specific CD8 T-cells/spleen at day 100 in two different experiments. CD4 T-cell determinations were done once. Statistical analysis was performed using the two-way Mann-Whitney U test.
Although HCMV-specific CD4 T cells may undergo memory inflation in infected patients (25), almost nothing is known about the phenotype of these cells. In MCMV-infected B6 mice, M142-specific CD4 T-cell responses show a canonical kinetic, peaking at ∼day 8 of primary infection, and contracting ∼90% by days 15–20. In contrast, M09-specific CD4 T cells only become detectable by ∼day 15 of infection, peak at ∼day 35, and then contract very slowly over the following months (26). However, even given the vastly different time course for development of these CD4 T-cell responses to MCMV, neither was found to depend upon RTE for their expansion and/or maintenance.
During acute infections with vaccinia virus and LCMV Armstrong, the size of the CD8 TCM pool is maintained primarily by low-level, cytokine-driven homeostatic proliferation after virus is cleared (i.e., IL-7 and IL-15), and does not require either “lingering” viral antigen or newly-primed, naïve T cells (27,28). Consequently, the fact that M45-specific CD8 memory T-cell numbers do not decrease in thymectomized animals was not entirely surprising (10,29). In a thorough series of T-cell transfer experiments, Snyder et al. estimated the half-life of circulating MCMV-specific CD8 TEM at ∼45–60 d in both naïve and infected animals, and also proposed that the overall maintenance of this population requires both sporadic expansion and the contribution of RTE (30). Our experimental time course was equivalent to ∼2 half-lives of MCMV-specific CD8 TEM, and therefore we anticipated a potential reduction in their numbers following thymectomy (20,22–24). Instead, however, we observed a slight expansion of MCMV-specific inflationary T-cell numbers. In turn, the total circulating T-cell pool was composed of a significantly higher percentage of cells that displayed a “memory” phenotype 100 d after operation (∼20% or ∼3 × 10e6 CD44hiCD8+ T cells in sham operated versus ∼60% or ∼6 × 10e6 CD44hiCD8+ T cells in thymectomized mice; Fig. 1B). The apparent insensitivity of inflationary CD8 T cells specific for MCMV antigens to thymectomy differs from mice infected with LCMV clone 13 or polyoma virus, in which CD8 TEM and CD62lo memory populations are reduced, respectively (31,32). Consistently, memory T-cell populations specific for these pathogens show phenotypic differences from inflationary cells generated during MCMV infection. An example is that CD8 TEM that arise during LCMV clone 13 infection express high levels of PD-1 and become functionally exhausted, whereas this does not generally occur for inflationary CD8 TEM in either human or mouse CMV infection (33–35). One likely possibility is that the level, locale, and/or duration of antigen expression during the persistent phase of infection is a differentiating factor in regulating both the maintenance and phenotype of these cells during diverse infections. In the case of LCMV clone 13 and polyoma virus, as well as during bacterial infection with Leishmania major and Bacille Calmette-Guerin, the maintenance of these memory CD8 T cells is critically dependent upon continued exposure to antigen (31,36–38). However, during MCMV infection the precise role that prolonged antigen expression plays in maintaining the inflationary CD8 TEM remains incompletely understood.
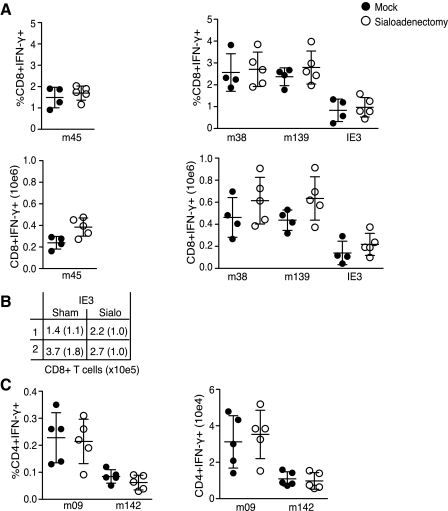
High levels of MCMV replication continue in the salivary gland (SG) for several weeks/months, suggesting this organ may contribute to shaping the virus-specific T-cell memory pool. To address this, a sialoadenectomy was performed to remove the SG, a minimally-invasive surgery from which the mice recover quickly with no alterations in weight or feeding behavior (39). Wild-type B6 mice were sialoadenectomized or sham operated on day 0 and subsequently infected IP with 5 × 104 PFU of MCMV. One hundred days later splenocytes were analyzed as already described.
M45-specific CD8 TCM numbers were comparable in the operated and sham groups. Somewhat surprisingly, CD8 T cells specific for peptides derived from M38, M139, and IE3 were also equivalent, with an apparent trend towards being slightly higher in number, although not reaching statistical significance (Fig. 2A and B). This result indicates that replication in the SG does not impact the development of either stable or inflationary MCMV-specific CD8 T-cell memory populations. Next, CD4 T-cell responses specific for peptides derived from MCMV orfs M142 and M09 were analyzed (Fig. 2C) (26). CD4 T cells are essential for the eventual control of MCMV in the SG (40), and are localized to this organ during times of replication, suggesting some of these cells may require SG-expressed antigen for their priming, expansion, and/or maintenance. However, similarly to what was observed for MCMV-specific CD8 T cells, neither the numbers nor the percentages of M142- or M09-specific CD4 T cells were altered in sialoadenectomized mice.
FIG. 2.
Salivary gland replication is not required for the development of CMV-specific memory T cells. (A and C) WT B6 mice were sialoadenectomized (open circles) or sham operated (solid circles) and MCMV infected. (A) One hundred days later, splenocytes were stimulated with the indicated viral peptides and virus-specific CD8 T cells were identified by flow cytometry. Results are displayed as percentages or numbers of peptide-specific CD8 T cells/spleen, and are shown as mean ± SD of 6 mice/group. (B) Mean numbers and SD (in parentheses) of IE3-specific CD8 T cells/spleen at day 100 and are shown as mean ± SD of 5 (top row, Expt 1) or 6 (bottom row, Expt 2) mice/group. (C) Splenocytes were stimulated with the indicated viral peptides, and MCMV-specific CD4 T cells expressing IFN-γ were identified by flow cytometry. Results are displayed as percentages or numbers of CD4 T cells/spleen. Statistical analysis was performed with the two-way Mann-Whitney U test.
The surface marker phenotype of TEM cells suggests a recent encounter with peptide-MHC complex (41); however, the specific requirements for maintaining this phenotype are still incompletely understood, and almost certainly vary based on the specific pathogen or vaccination regimen, as already discussed. While the maintenance of the total CMV-specific CD8 TEM pool over time is thought to involve expression of persistent viral antigen (30), spread-defective MCMV mutants can induce both antibody and T-cell responses to some degree (42,43). Snyder et al. have recently shown that the immunodominance hierarchy of CD8 T cells is similar between wild-type and a spread-defective mutant MCMV at early times of infection, although the overall magnitude was lower (42,43). In turn, Mohr et al. demonstrated that a spread-defective MCMV mutant provides protection against viral challenge up to 20 wk later, indicating that the initial populations of cells infected by MCMV can function as efficient “antigen sources” to provide lasting immunity (42,43). However, neither of these studies analyzed the effect that these mutant viruses had on the inflationary populations of CD8 TEM specific for MCMV antigens.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants AI076864 and AI069298 to C.A.B, a DFG fellowship to A.I.L. (DFG# 1421/1-1), and NIH grants AI064586, AI075164, and AI090610, as well as the World Class University program, National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (R31-2008-000-10105-0) for C.D.S.
Author Disclosure Information
No competing financial interests exist.
References
- 1.Adler SP. Nigro G. Pereira L. Recent advances in the prevention and treatment of congenital cytomegalovirus infections. Semin Perinatol. 2007;31:10–18. doi: 10.1053/j.semperi.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Baldanti F. Lilleri D. Gerna G. Monitoring human cytomegalovirus infection in transplant recipients. J Clin Virol. 2008;41:237–241. doi: 10.1016/j.jcv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Sylwester AW, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holtappels R, et al. Two antigenic peptides from genes m123 and m164 of murine cytomegalovirus quantitatively dominate CD8 T-cell memory in the H-2d haplotype. J Virol. 2002;76:151–164. doi: 10.1128/JVI.76.1.151-164.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holtappels R, et al. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62L(lo) memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J Virol. 2000;74:11495–11503. doi: 10.1128/jvi.74.24.11495-11503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karrer U, et al. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 7.Khan N, et al. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol. 2004;173:7481–7489. doi: 10.4049/jimmunol.173.12.7481. [DOI] [PubMed] [Google Scholar]
- 8.Kalia V, et al. Differentiation of memory B and T cells. Curr Opin Immunol. 2006;18:255–264. doi: 10.1016/j.coi.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Stemberger C, et al. Stem cell-like plasticity of naive and distinct memory CD8+ T cell subsets. Semin Immunol. 2009;21:62–68. doi: 10.1016/j.smim.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Jameson SC. Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klenerman P. Hill A. T cells and viral persistence: lessons from diverse infections. Nat Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 12.Sierro S. Rothkopf R. Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur J Immunol. 2005;35:1113–1123. doi: 10.1002/eji.200425534. [DOI] [PubMed] [Google Scholar]
- 13.Munks MW, et al. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J Immunol. 2006;177:450–458. doi: 10.4049/jimmunol.177.1.450. [DOI] [PubMed] [Google Scholar]
- 14.Komatsu H, et al. Population analysis of antiviral T cell responses using MHC class I-peptide tetramers. Clin Exp Immunol. 2003;134:9–12. doi: 10.1046/j.1365-2249.2003.02266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 16.Wakim LM, et al. Cutting edge: local recall responses by memory T cells newly recruited to peripheral nonlymphoid tissues. J Immunol. 2008;181:5837–5841. doi: 10.4049/jimmunol.181.9.5837. [DOI] [PubMed] [Google Scholar]
- 17.Woodland DL. Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 18.Hansen SG, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karrer U, et al. Expansion of protective CD8+ T-cell responses driven by recombinant cytomegaloviruses. J Virol. 2004;78:2255–2264. doi: 10.1128/JVI.78.5.2255-2264.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parretta E, et al. Kinetics of in vivo proliferation and death of memory and naive CD8 T cells: parameter estimation based on 5-bromo-2′-deoxyuridine incorporation in spleen, lymph nodes, and bone marrow. J Immunol. 2008;180:7230–7239. doi: 10.4049/jimmunol.180.11.7230. [DOI] [PubMed] [Google Scholar]
- 21.Monaco AP. Wood ML. Russell PS. Adult thymectomy: Effect on recovery from immunologic depression in mice. Science. 1965;149:432–435. doi: 10.1126/science.149.3682.432. [DOI] [PubMed] [Google Scholar]
- 22.Hellerstein MK. Measurement of T-cell kinetics: recent methodologic advances. Immunol Today. 1999;20:438–441. doi: 10.1016/s0167-5699(99)01529-7. [DOI] [PubMed] [Google Scholar]
- 23.Sprent J. Tough DF. Lymphocyte life-span and memory. Science. 1994;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 24.Tough DF. Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wikby A, et al. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol. 2002;37:445–453. doi: 10.1016/s0531-5565(01)00212-1. [DOI] [PubMed] [Google Scholar]
- 26.Arens R, et al. Cutting edge: murine cytomegalovirus induces a polyfunctional CD4 T cell response. J Immunol. 2008;180:6472–6476. doi: 10.4049/jimmunol.180.10.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surh CD. Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Boyman O, et al. Homeostatic proliferation and survival of naive and memory T cells. Eur J Immunol. 2009;39:2088–2094. doi: 10.1002/eji.200939444. [DOI] [PubMed] [Google Scholar]
- 29.Wherry EJ, et al. Homeostatic proliferation but not the generation of virus specific memory CD8 T cells is impaired in the absence of IL-15 or IL-15Ralpha. Adv Exp Med Biol. 2002;512:165–175. doi: 10.1007/978-1-4615-0757-4_22. [DOI] [PubMed] [Google Scholar]
- 30.Snyder CM, et al. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vezys V, et al. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J Exp Med. 2006;203:2263–2269. doi: 10.1084/jem.20060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller NE, et al. Role of thymic output in regulating CD8 T-cell homeostasis during acute and chronic viral infection. J Virol. 2005;79:9419–9429. doi: 10.1128/JVI.79.15.9419-9429.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wherry EJ, et al. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci USA. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.La Rosa C, et al. Programmed death-1 expression in liver transplant recipients as a prognostic indicator of cytomegalovirus disease. J Infect Dis. 2008;197:25–33. doi: 10.1086/523652. [DOI] [PubMed] [Google Scholar]
- 35.Petrovas C, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dudani R, et al. Multiple mechanisms compensate to enhance tumor-protective CD8(+) T cell response in the long-term despite poor CD8(+) T cell priming initially: comparison between an acute versus a chronic intracellular bacterium expressing a model antigen. J Immunol. 2002;168:5737–5745. doi: 10.4049/jimmunol.168.11.5737. [DOI] [PubMed] [Google Scholar]
- 37.Belkaid Y, et al. CD4+ CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 38.Uzonna JE, et al. Immune elimination of Leishmania major in mice: implications for immune memory, vaccination, and reactivation disease. J Immunol. 2001;167:6967–6974. doi: 10.4049/jimmunol.167.12.6967. [DOI] [PubMed] [Google Scholar]
- 39.Elitok B, et al. The effect of dexamethasone on gastric mucosal changes following sialoadenectomy in rat. J Endocrinol Invest. 2005;28:700–703. doi: 10.1007/BF03347552. [DOI] [PubMed] [Google Scholar]
- 40.Jonjic S, et al. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J Exp Med. 1989;169:1199–1212. doi: 10.1084/jem.169.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sallusto F. Geginat J. Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 42.Snyder CM, et al. Cross-presentation of a spread-defective MCMV is sufficient to prime the majority of virus-specific CD8+ T cells. PLoS One. 2010;5:e9681. doi: 10.1371/journal.pone.0009681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohr CA, et al. A spread-deficient cytomegalovirus for assessment of first-target cells in vaccination. J Virol. 2010;84:7730–7742. doi: 10.1128/JVI.02696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]