The hormonal form of vitamin D, 1,25-dihydroxyvitamin D (1,25(OH)2D) is a pluripotent seco-steroid whose actions extend far beyond its classical role in calcium homeostasis and bone metabolism. In particular, well-documented antiproliferative and immunomodulatory effects of 1,25(OH)2D have underlined its potential use as treatment for common cancers, as well as autoimmune and infectious diseases.1 Despite this, our understanding of the signaling pathways associated with non-classical responses to 1,25(OH)2D is remarkably poor. In recently published studies, we identified a novel mechanism that may act to integrate both immunomodulatory and antiproliferative activities of 1,25(OH)2D. Specifically, we have shown that 1,25(OH)2D is able to regulate the mammalian/mechanistic target of rapamycin (mTOR) signaling pathway by stimulating expression of DNA damage-inducible transcript 4 (DDIT4), also known as regulated in development and DNA damage response 1 (REDD1), a potent suppressor of mTOR activity.2 Given the role of mTOR as a “master regulator” of cell function,3 it seems likely that DDIT4-mediated inhibition of this pathway will also play a pivotal role in mediating cellular responses to 1,25(OH)2D, as well as provide new strategies for its use in disease therapy.
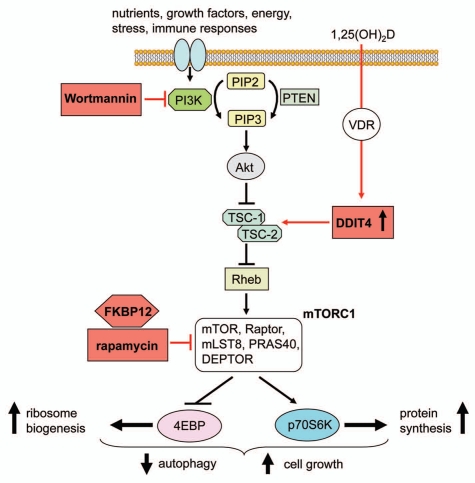
The mTOR system forms part of a pathway that integrates signals from environmental nutrients, energy, stress and growth factors and therefore plays a crucial role in directing cell proliferation, growth and differentiation. The mTOR serine/threonine kinase protein forms the catalytic sub-unit of two molecular complexes that mediate mTOR signaling—mTOR Complex 1 (mTORC1) and mTORC2. The mTORC2 system is closely associated with the actin cytoskeleton, cell polarity and phosphorylation of Akt (protein kinase B). By contrast, as outlined in Figure 1, mTORC1 responses are initiated by the phosphatidylinositol-3-kinase (PI3K) system, which is linked to the mTOR pathway by another serine/threonine kinase known as Akt, which phosphorylates and inhibits the tuberous sclerosis protein complex (TSC1/2), thereby suppressing the upstream small GTPase regulator Rheb (Ras homolog enriched in brain). This, in turn, promotes the kinase activity of the mTORC1 complex by binding to FKBP38, a member of the FK506-binding protein family that acts as an endogenous inhibitor of mTOR.4 The resulting activation of the mTORC1 complex then leads to phosphorylation of elongation factor 4E-binding protein (4E-BP) and 70 kDa ribosomal protein S6 kinase (p70S6K), leading, respectively, to increased ribosome biogenesis and protein synthesis. In this way, mTOR is able to influence cell cycle progression, growth and metabolism homeostasis as well as autophagy.3
Figure 1.
Vitamin D and the inhibition of mTOR signaling. Schematic representation showing signaling via mTORC1 (black arrows) and the targets for mTOR inhibitors (red arrows), including the active form of vitamin D, 1,25(OH)2D. mTORC1 integrates responses to various cell stimuli, leading to eventual phosphorylation of eukaryotic translation-initiation factor 4E-binding protein (4EBP) and p70S6 kinase 1 (p70S6K) and to promotion of mRNA translation, ribosomal biogenesis and the suppression of autophagy. DDIT4 (DNA-damage-inducible transcript 4, also known as REDD1) facilitates the assembly and activation of the tuberous sclerosis complex (TSC)1/2 complex for eventual suppression of downstream mTOR activity through actions on ras homolog enriched in the brain (Rheb). DDIT4 mRNA and protein is upregulated by 1,25(OH)2D treatment resulting in reduced phosphorylation of S6K1 and decreased cell proliferation and growth. Other abbreviations: PIP2, phosphatidylinositol-bisphosphate 2; PIP3, phosphatidylinositol-triphosphate; PTEN, phosphatase and tensin homolog; Akt, protein kinase B; mLST8, mTOR, Raptor, LST8 homolog; PRAS40, proline-rich Akt substrate 40 kDa; DEPTOR, DEP domain-containing mTOR-interacting protein.
Signaling via the mTOR pathway provides a versatile system for responding to a variety of cellular stimuli. Factors such as insulin or toll-like receptor ligands promote mTOR by inhibiting TSC1/2 and Rheb, leading, in turn, to enhanced cell growth or suppressed autophagy.3 Mechanical stress is also known to promote mTOR, but many other stimuli, such as osmotic stress, heat shock and reactive oxygen species, inhibit mTOR signaling via direct suppression of mTORC1. The role of DDIT4 as a regulator of mTOR is less well documented, although mTORC1 signaling under hypoxic conditions is known to involve hypoxia-inducible factor 1α-targeting of DDIT4.5 This parallel pathway for oxygen tension- and 1,25(OH)2D-mediated regulation of mTOR may be particularly important for regulation of skeletal homeostasis, as hypoxia is known to enhance bone regeneration.6 However, targeting of mTOR may also play a pivotal role in mediating nonclassical actions of 1,25(OH)2D. In our recently published report, we showed that siRNA knockdown of DDIT4 completely suppressed the antiproliferative effects of 1,25(OH)2D,2 suggesting that this may be a key anticancer mechanism for vitamin D. In this regard it is interesting to note previous studies showing that inhibition of tumor cell proliferation by a synthetic analog of 1,25(OH)2D was associated with decreased mTOR signaling.7 In a similar fashion, inhibition of mTOR signaling using the anticancer agent Everolimus (RAD-001) potentiated antiproliferative effects of 1,25(OH)2D,8 suggesting a possible strategy for combination therapy targeting of mTOR in the treatment of some cancers. Inhibition of mTOR also plays a key role in innate and adaptive immunity, most notably by promoting the formation of autophagosomes involved in bacterial killing and as a mediator of T-cell metabolism and function.9 Given that each of these facets of immunity is known to be enhanced by 1,25(OH)2D,10 it is tempting to speculate that mTOR is a “master regulator” target for the immunomodulatory effects of vitamin D.
Inhibition of mTOR signaling is already an established mechanism for the treatment of human diseases. The drug rapamycin is currently in use for suppression of transplantation rejection, whilst other mTOR inhibitors such as RAD-001 and Temsirolimus (CCI-779) have been approved for the treatment of renal cell carcinoma and transplantation rejection. However, perhaps the most provocative potential application for mTOR inhibitors stems from studies of a wide range of organisms including mammals,11 showing that suppression of mTORC1 by rapamycin is associated with increased longevity. With these observations in mind, it is interesting to speculate that the ability of 1,25(OH)2D to induce DDIT4 and suppress mTOR may further extend the therapeutic applications of vitamin D to include a role as a potential anti-aging factor.
Comment on: Lisse TS, et al. FASEB J. 2010;25:937–947. doi: 10.1096/fj.10-172577.
References
- 1.Plum LA, et al. Nat Rev Drug Discov. 2010;9:941–955. doi: 10.1038/nrd3318. [DOI] [PubMed] [Google Scholar]
- 2.Lisse TS, et al. FASEB J. 2011;25:937–947. doi: 10.1096/fj.10-172577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zoncu R, et al. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai X, et al. Science. 2007;318:977–980. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- 5.Ellisen LW. Cell Cycle. 2005;4:1500–1502. doi: 10.4161/cc.4.11.2139. [DOI] [PubMed] [Google Scholar]
- 6.Wan C, et al. Proc Natl Acad Sci USA. 2008;105:686–691. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Kelly J, et al. J Steroid Biochem Mol Biol. 2006;100:107–116. doi: 10.1016/j.jsbmb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, et al. Exp Hematol. 2010;38:666–676. doi: 10.1016/j.exphem.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Powell JD, et al. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams JS, et al. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison DE, et al. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]