Abstract
Folic acid (FA) supplementation before and during pregnancy has been associated with decreased risk of neural tube defects although recent reports suggest it may also increase the risk of other chronic diseases. We evaluated exposure to maternal FA supplementation before and during pregnancy in relation to aberrant DNA methylation at two differentially methylated regions (DMRs) regulating insulin-like growth factor 2 (IGF2) expression in infants. Aberrant methylation at these regions has been associated with IGF2 deregulation and increased susceptibility to several chronic diseases. Using a self-administered questionnaire, we assessed FA intake before and during pregnancy in 438 pregnant women. Pyrosequencing was used to measure methylation at two IGF2 DMRs in umbilical cord blood leukocytes. Mixed models were used to determine relationships between maternal FA supplementation before or during pregnancy and DNA methylation levels at birth. Average methylation at the H19 DMR was 61.2%. Compared to infants born to women reporting no FA intake before or during pregnancy, methylation levels at the H19 DMR decreased with increasing FA intake (2.8%, p = 0.03 and 4.9%, p = 0.04, for intake before and during pregnancy, respectively). This methylation decrease was most pronounced in male infants (p = 0.01). Methylation alterations at the H19 DMR are likely an important mechanism by which FA risks and/or benefits are conferred in utero. Because stable methylation marks at DMRs regulating imprinted genes are acquired before gastrulation, they may serve as archives of early exposures with the potential to improve our understanding of developmental origins of adult disease.
Key words: folic acid, epigenetics, IGF2, periconception, prenatal, exposure
Introduction
The benefit of daily folic acid (FA) supplementation during periconception on a decreased risk of neural tube defects1–5 led to recommendations that all women at risk for pregnancy take 400 µg of FA per day (µg/d) from fortified foods, supplements or both.3,6 Because the national unintended pregnancy rate in the US is 49%,7 mandatory FA fortification of milled grain was instituted in 1998.8 Since then, reported median folate intake among women of child-bearing age has increased to >450 µg/d with approximately 75% contributed by FA from fortified foods, supplements or both.9,10 Currently, a little over 50% of pregnant women report daily FA supplementation in the periconceptional period and another 35% report starting intake in the prenatal period.11 Reports following this increase in FA intake, link lower risk of involuntary abortion,12 pre-eclampsia,13 gestational hypertension14 and other congenital abnormalities15 to FA intake. However, more recently, periconceptional FA intake has also been associated with increased risk of wheezing and asthma in both mice and humans16–18 leading to the contention that FA at current doses may be detrimental to the long term health of the developing fetus.19–21
As the population coverage and dose of FA intake increase, monitoring its effects on human health will require an understanding of mechanisms underlying the benefits (hence potential risks) of FA supplement use. Although epigenetic mechanisms are hypothesized,48 empirical data are lacking. Folate is an essential cofactor for transfer of one-carbon moieties including methyl groups required for DNA methylation of cytosine-guanine (CpG) dinucleotides.23 In mice, prenatal supplementation with methyl group donor nutrients including FA, increases methylation at the cryptic promoter upstream of the Agouti gene and decreased gene expression.24 In humans, a major obstacle is the lack of stable epigenetic targets with known baseline levels also known to be responsive to FA exposure. Another obstacle is that epigenetic assays must be conducted in DNA from peripheral blood leukocytes, usually the only tissue type available when studying healthy human populations, yet epigenetic regulation varies by tissue and cell type.
Insulin-like growth factor-2 (IGF2) is a well characterized imprinted gene that is regulated by allele-specific methylation25 at two differentially methylated regions (DMRs). DMRs at imprinted loci are established in the gamete and early embryo and are maintained in all germ layers, resulting in systemic albeit subtle inter-individual variation in locus-specific epigenetic regulation.26,27 Moreover, because imprinted genes occur in clusters,28 and their regulation may be under network-level control,29 methylation status at a single DMR may be representative of multiple genes and, if aberrant, can provide clues on global epigenetic dysregulation. Aberrant methylation at the IGF2 DMR has been associated with elevated colon cancer risk and its temporal stability in adulthood has been shown.30–32 Aberrant methylation at the same IGF2 DMR was also found in 60-year old individuals exposed to severe caloric restriction in utero during the Dutch famine of 1944–45,33 illustrating that this DMR is epigenetically labile to early environmental influence. Dutch famine survivors have an excess risk of several common chronic diseases. Interestingly, a recent report suggests that moderate maternal FA intake (400 µg/day) during the periconceptional period—concomitant with epigenetic reprogramming in the zygote—can ameliorate aberrant methylation marks at the IGF2 DMR.34 However, the epigenetic effects of much higher FA exposure common among women in the US are unknown. Also unknown is whether these beneficial effects are confined to the periconceptional period, since half of US pregnancies are unplanned. In the current analyses, we utilize data from a multiethnic population of newborns to test the hypothesis that FA exposure is associated with DNA methylation changes at two DMRs regulating IGF2 expression, and that this association is limited to periconceptional exposure.
Results
The mean maternal age at enrollment was 29 years (standard deviation 6.2), with a range of 18 to 49 years. Forty-nine percent of pregnant women (n = 215) reported daily FA intake during the year before pregnancy (Table 1); and in 12% (n = 52) FA intake was categorized as high. Of the 223 who reported no FA intake during the year before pregnancy, 54% (n = 120) reported daily use during pregnancy; and 7% of women (n = 15) reported high FA intake during pregnancy. Moderate and high FA intake before pregnancy was reported more frequently by women aged 35 years or older, among those who had at least a college education, who were Caucasian and who had a BMI < 25 kg/m2. As expected, women reporting moderate or high FA intake were also more likely to have infants with a birth weight >2,500 grams (p value <0.05). Moderate or high FA intake during pregnancy was also reported more frequently among women with at least a college education, among Caucasians and current cigarette smokers (p value < 0.05). We adjusted for these factors in subsequent analyses.
Table 1.
Frequency of folic acid supplementation before and during pregnancy by maternal and infant characteristics
| Folic acid use before pregnancy | Folic acid use during pregnancy | |||||
| None n = 223 | Moderate use n = 163 | High use n = 52 | None n = 103 | Moderate use n = 105 | High use n = 15 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Maternal age (years) | ||||||
| 18–20 | 19 (8.5) | 6 (3.7) | 2 (3.9) | 8 (7.8) | 10 (9.5) | 1 (6.7) |
| 21–24 | 60 (26.9) | 28 (17.2) | 4 (7.8) | 30 (29.1) | 26 (24.8) | 4 (26.7) |
| 25–29 | 71 (31.8) | 36 (22.1) | 15 (29.4) | 35 (30.0) | 35 (33.3) | 1 (6.7) |
| 30–34 | 36 (16.1) | 45 (27.6) | 14 (27.5) | 16 (15.5) | 14 (13.3) | 6 (46.7) |
| 35+ years | 37 (16.6) | 48 (29.5) | 16 (31.4) | 14 (13.6) | 20 (19.1) | 3 (20.0) |
| Maternal education** | ||||||
| Less than high school | 34 (15.3) | 12 (7.4) | 2 (3.9) | 17 (16.7) | 13 (12.4) | 4 (26.7) |
| High school/GED | 82 (36.9) | 28 (17.2) | 7 (13.7) | 47 (46.1) | 33 (31.4) | 2 (13.3) |
| Some college | 67 (30.2) | 42 (25.8) | 11 (21.6) | 33 (32.4) | 30 (28.6) | 4 (26.7) |
| College graduate | 24 (10.8) | 40 (24.5) | 13 (25.5) | 4 (3.9) | 19 (18.1) | 1 (6.7) |
| Graduate education | 15 (6.8) | 41 (25.2) | 18 (35.3) | 1 (1.0) | 10 (9.5) | 4 (26.7) |
| Maternal race** | ||||||
| African American | 137 (62.3) | 61 (37.4) | 18 (37.5) | 83 (81.4) | 48 (46.2) | 6 (42.9) |
| Caucasian | 72 (32.7) | 92 (56.4) | 29 (60.4) | 16 (15.7) | 49 (47.1) | 7 (50.0) |
| Asian | 4 (1.8) | 3 (1.8) | 0 (0) | 1 (1.0) | 3 (2.9) | 0 (0) |
| Native American | 1 (0.5) | 1 (0.6) | 0 (0) | 0 (0) | 0 (0.0) | 1 (7.1) |
| Other | 6 (2.7) | 6 (3.7) | 1 (2.1) | 2 (2.0) | 4 (3.9) | 0 (0) |
| Maternal BMI before pregnancy (kg/m2) | ||||||
| <25 | 93 (47.2) | 79 (53.0) | 30 (61.2) | 37 (40.7) | 46 (50.0) | 10 (71.4) |
| 25–29 | 39 (19.8) | 34 (22.8) | 9 (2.3) | 16 (17.6) | 21 (22.8) | 2 (14.3) |
| 30–34 | 27 (13.7) | 19 (12.8) | 5 (1.3) | 16 (17.6) | 10 (10.9) | 1 (7.1) |
| 35+ | 38 (19.3) | 17 (11.4) | 5 (1.3) | 22 (24.2) | 15 (16.3) | 1 (7.1) |
| Cigarette smoking** | ||||||
| Never smoked | 106 (47.5) | 96 (58.9) | 35 (68.6) | 54 (52.4) | 46 (43.8) | 6 (40.0) |
| Smoker | 100 44.8) | 51 (31.3) | 11 (21.6) | 42 (40.8) | 52 (49.5) | 6 (40.0) |
| Quit during pregnancy | 17 (7.6) | 16 (9.8) | 5 (9.8) | 7 (6.8) | 7 (6.7) | 3 (20.0) |
| Gestational age at delivery | ||||||
| <37 weeks | 41 (18.4) | 27 (16.8) | 6 (8.1) | 23 (22.3) | 15 (14.3) | 3 (20.0) |
| ≥37 weeks | 182 (81.6) | 135 (83.3) | 45 (12.4) | 80 (77.7) | 90 (85.7) | 12 (80.0) |
| Birth weight | ||||||
| ≤2,500 grams | 38 (17.4) | 16 (10.1) | 5 (9.8) | 19 (18.6) | 16 (15.5) | 3 (21.4) |
| >2,500 grams | 181 (82.7) | 143 (89.9) | 46 (90.2) | 83 (81.4) | 87 (84.5) | 11 (78.6) |
| Gender | ||||||
| Males | 110 (50.5) | 88 (54.7) | 23 (46.0) | 57 (55.9) | 45 (44.1) | 8 (57.1) |
| Females | 108 (49.5) | 73 (45.3) | 27 (54.0) | 45 (44.1) | 57 (55.9) | 6 (42.9) |
folic acid supplementation during pregnancy significantly varies by maternal or offspring characteristic and folic acid supplementation before pregnancy significantly varied by maternal or offspring characteristic (p < 0.05).
FA intake before and during pregnancy and IGF2 DMR methylation.
Overall, the average methylation level at the IGF2 DMR was 47.3% (SD = 4.5). Results from comparisons of single means were similar to those from mixed models; thus, multivariate results presented here (Table 3) are derived from mixed models. Table 2 shows unadjusted mean DNA methylation levels and standard deviations (SDs) by maternal FA use before and during pregnancy. Contrary to a large study that found significant methylation differences at this DMR between infants born to moderate FA users and non-FA users during periconception,34 methylation fractions were similar at all levels of maternal FA use before pregnancy. Infants born to women who reported no FA use before pregnancy had a methylation fraction (47.4%, SD = 8.3) comparable to that of infants born to moderate (47.6%, SD = 6.0) and high (46.4%, SD = 5.9) FA users (p = 0.603). Adjusting for cigarette smoking, race, sex, mode of delivery and educational level in mixed models (Table 3) did not alter these findings (p = 0.76 for moderate FA intake and p = 0.39 for high intake). This pattern was also similar among the 223 infants born to women who reported FA intake only after conception. Although previous reports suggest there may be sex differences in epigenetic response to nutrition during periconception,33,34 our study found no sex differences in methylation levels by maternal FA intake. We also found no differences in DNA methylation levels of infants born to women taking none, moderate and high doses of FA, whether taken before or only during pregnancy, despite studies suggesting genetic factors contribute to epigenetic alterations.46,47
Table 3.
*Adjusted H19 and IGF2 DMR methylation differences between offspring exposed and those not exposed to folic acid before and during pregnancy, stratified by sex and race
| Methylation % difference for FA intake before pregnancy (n = 428) | Methylation % difference for FA intake during pregnancy (n = 223) | |||||||
| IGF2 DMR | H19 DMR | IGF2 DMR | H19 DMR | |||||
| Folic acid intake | Difference | p value | Difference | p value | Difference | p value | Difference | p value |
| All participants | ||||||||
| Moderate | +0.28 | 0.76 | −1.96 | 0.03* | 0.75 | 0.59 | −2.87 | 0.02* |
| High | −1.15 | 0.39 | −2.76 | 0.04* | 0.25 | 0.93 | −4.90 | 0.05 |
| Males | ||||||||
| Moderate | −0.11 | 0.92 | −3.08 | 0.01* | −1.28 | 0.47 | −3.69 | 0.06 |
| High | −1.40 | 0.44 | −2.36 | 0.24 | −1.69 | 0.57 | −4.63 | 0.16 |
| Females | ||||||||
| Moderate | +0.22 | 0.89 | −0.66 | 0.62 | +1.92 | 0.46 | −3.27 | 0.09 |
| High | −1.90 | 0.37 | −3.43 | 0.08 | +6.38 | 0.36 | −2.59 | 0.56 |
| Black | ||||||||
| Moderate | −1.18 | 0.44 | −1.91 | 0.13 | 1.96 | 0.32 | −2.49 | 0.11 |
| High | −0.45 | 0.85 | −3.18 | 0.12 | −0.67 | 0.89 | −3.66 | 0.53 |
| White | ||||||||
| Moderate | 1.33 | 0.24 | −1.80 | 0.20 | −1.26 | 0.49 | −4.43 | 0.09 |
| High | −1.85 | 0.23 | −2.42 | 0.23 | −0.71 | 0.79 | −8.37 | 0.24 |
Statistically significant difference among methylation percent of infants born to non-FA users and users moderate and high doses of FA.
**Referents are non-folic acid users, adjusted for maternal educational attained, race, mode of delivery and cigarette smoking. All participants are additionally adjusted for sex.
Table 2.
IGF2 DMR methylation levels by maternal folic acid supplementation before and during pregnancy
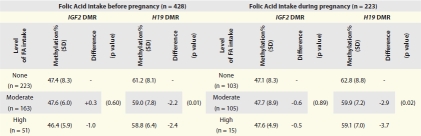 |
*p-values for methylation differences among folic acid supplement categories.
Periconceptional FA use and H19 DNA methylation.
The average methylation level at the H19 DMR was 61.2% (SD = 4.2). Table 2 compares unadjusted H19 DMR mean methylation levels and SDs by FA use before and during pregnancy. Compared to infants born to non-FA users before pregnancy (mean methylation = 61.2%, SD = 8.1), we found persistent and statistically significantly lower DNA methylation levels among infants born to moderate FA users (mean = 59%, SD = 7.8) who had a 2.2% lower methylation fraction and high FA users (mean = 58.8%, 6.4) who had a 2.4% lower methylation fraction (p = 0.001). Adjusting for maternal cigarette smoking, race, mode of delivery and education (Table 3) in multivariable models did not substantively alter these patterns (p = 0.03 and p = 0.04 for infants born to moderate and high FA users, respectively).
Prenatal-only FA use and H19 DNA methylation.
Given the high unintended pregnancy rate in the US, we determined if FA intake that began only after knowledge of pregnancy was also associated with lower methylation at the H19 DMR by repeating these analyses among the 223 infants born to women who began FA intake after pregnancy. We found that when compared to infants of non-FA users, methylation levels were 2.9 and 3.7% lower among born to moderate and high FA users, respectively (p = 0.02). These methylation differences were essentially unaltered after accounting for the potential confounding effect of cigarette smoking, race, sex, mode of delivery and educational attainment. The adjusted mean methylation in mixed models was 2.9% lower in offspring born to moderate FA users and 4.9% lower in those born to high FA exposure (p = 0.04), suggesting periconceptional and prenatal-only FA use independently predicted lower methylation levels at the H19 DMR. To further explore this conjecture, we constructed statistical models that simultaneously included both periconceptional and prenatal-only FA use, and found both to independently predict lower H19 DMR methylation (p = 0.05). To explore if these differences were driven by the 10 infants with extreme (>85%) methylation levels, we repeated these analyzed excluding the 10 infants and our findings were unchanged (p = 0.03).
Sex differences in H19 DNA methylation of infants born to FA and non-FA users.
Table 3 also shows sex-specific DNA methylation differences among infants born to women reporting no FA intake before and during pregnancy, and those born to moderate and high FA users, adjusted for cigarette smoking, race, mode of delivery and education. Compared to male infants of non-FA users, methylation levels of male infants born to women who reported moderate and high FA intake before pregnancy were 3.1% (p = 0.01) and 2.4% (p = 0.24) lower, respectively. Restricting our analyses to the 110 males born to women whose FA intake began during pregnancy resulted in DNA methylation that was 3.7 and 4.6% lower for moderate and high FA intake during pregnancy, respectively; however, this difference was no longer statistically significant (p = 0.06 and p = 0.16 for infants born to moderate and high FA users, respectively). Further exploration of the source of methylation variation using models that included moderate and high FA use as indicator variables revealed that moderate but not high FA use was associated with lower methylation at this DMR in males. No methylation differences were observed in female infants born to FA users and non-users before (p = 0.21) and during pregnancy (p = 0.22). Further testing for statistical interaction using the cross-product term derived from FA intake and sex did not reveal a statistically significant effect (p = 0.34).
Race/ethnic differences in H19 DNA methylation of infants of FA and non-FA users.
Because epigenetic perturbations could also vary by genetic factors, Table 3 also shows race differences in methylation levels of infants born to women taking different daily doses of FA, taken before or only during pregnancy. Whereas no methylation differences were observed among African Americans, the difference between infants born to non-FA users and FA users appeared limited to Caucasians. In this race/ethnic group, infants born to women reporting moderate FA intake during pregnancy only had a 4.4% lower methylation than infants of non-FA users, and 8.4% lower methylation in those born to women reporting high FA use (p = 0.09). Testing for interaction using the cross-product term derived from FA intake and race also found no evidence suggesting that methylation differences by FA exposure significantly varied by race.
Finally, using logistic regression analysis, we evaluated the association between FA intake and aberrant methylation at the H19 DMR and IGF2 DMR. Aberrant methylation at H19 was defined as methylation >75th percentile and methylation ≤25th percentile at the IGF2 DMR. Adjusting for education, mode of delivery, race and cigarette smoking we observed a lower risk of hypermethylation at the H19 DMR, with increasing maternal FA intake before pregnancy for both moderate (OR = 0.47; 95% CI = 0.22–0.91) and high (OR = 0.31; 95% CI = 0.11–0.67) FA intake, respectively (data not shown). Similar findings were observed in relation to FA exposure during pregnancy. We found no evidence for an association between maternal FA intake and IGF2 DMR hypomethylation.
Discussion
DNA methylation profiles at DMRs of imprinted genes, of which the best characterized is the IGF2/H19 imprinted domain, are epigenetically labile to early environmental influences.27 We48 and others49,50 have proposed that in the study of developmental origins of adult disease, DNA methylation shifts at these loci can serve as ‘biosensors’ or ‘archives’ of the early environment. In this study, we examined maternal FA intake before and during pregnancy in relation to variation in DNA methylation obtained from leukocytes of newborns at two DMRs regulating IGF2 expression. Overall, we found higher than the expected (∼50%) methylation levels at the H19 DMR, and lower than expected methylation levels at the IGF2 DMR. While there was no evidence for methylation differences at the IGF2 DMR, infants born to FA users had significantly lower H19 DMR methylation compared to infants of non-FA users, a difference that appeared pronounced in male infants and in those born to Caucasian women. Findings from our study begin to address the practical question of whether women planning a pregnancy should adhere to recommended FA intake post-FA fortification.
The mechanisms by which FA intake decreases hypermethylation at the H19 DMR are unknown. However, the imprinted expression of paternally expressed IGF2 and the maternally expressed H19 are coordinately regulated by sequences encompassing the H19 DMR that include CTCF binding sites.51,52 CTCF is a zinc-finger protein that binds to the unmethylated maternal copy, creating a boundary that blocks access of enhancers to the IGF2 promoter on the maternal allele. Aberrant gain of CpG methylation within these CTCF sites on the maternal allele53,54 and IGF2 DMR methylation losses on the maternal allele30,43 have both been independently associated with loss of IGF2 imprinting. Using DNA obtained from 17-month old children, favorable DNA methylation shifts in response to periconceptional FA intake were recently reported at the IGF2 DMR.34 We provide the first evidence supporting recent assertions55 that FA use favorably shifts the methylation profile at the H19 DMR, post-conception. While it is unlikely that many phenotypic consequences arise from a single gene effect, especially if they represent an adaptive response, imprinted genes occur in clusters28 and their regulation may be networked.29 Therefore, our findings raise the possibility that epigenetic reprogramming may be malleable during fetal development, and this holds prospects for therapeutic and public health intervention. The potential malleability of fetal epigenetic marks also suggests epigenetic regulation (and dysregulation) can be monitored via methylation alterations at DMRs regulating imprinted gene expression. However, for these DMRs to be useful ‘biosensors’ or ‘archives’ of early exposures,48,49 it will also be important to further characterize environmental factors associated DNA methylation shifts at these regulatory sequences, and their stability during the lifespan of the individual.
Our findings are consistent with the interpretation that FA intake during fetal development is causally related to subtle and persistent favorable methylation alterations at DMRs regulating imprinted genes. Thus, despite FA supplement use exceeding the current tolerable upper limit for adults by ∼10% of pregnant women,35 and population-wide FA fortification inherent in the US population, our data suggest that the beneficial epigenetic effects of FA may not be confined to folate-deficient populations. Our findings do not support the contention that maternal FA use at current doses is associated with epigenetic dysregulation at birth, the putative explanation for increased risk of chronic conditions in individuals exposed to FA in utero.16,18 Findings of sex-specific epigenetic response to the perceived environment are consistent with the Dutch study that showed gender-specific IGF2 DMR methylation loss related to severe periconceptional caloric restriction.33
The significance of the relatively small effect in the magnitude of methylation levels found in our infants is still unclear, especially when compared to much larger methylation differences between mice exposed and those non-exposed to methyl group donor nutrients, including FA, during the periconceptional period,57 or in human malignancies. However, this should be expected, given the exposure heterogeneity of human populations. Moreover, the magnitude of the effect on aberrant methylation in our study are strikingly similar to the 4.5% methylation difference at the IGF2 DMR found in 17-month old Dutch children exposed versus unexposed to FA during periconception.34 This methylation difference is also of a similar magnitude to that found in adults who were periconceptionally exposed and those unexposed to the Dutch famine of 1944–45,33 compared to their same-sex siblings. As adults, Dutch famine survivors are at significantly higher risk of neurological disorders,58 obesity in early and late adulthood,59,60 breast cancer,61 infertility62 and metabolic disorders that include type-2 diabetes and dyslipidemia.63–66 Modest aberrant methylation (shifts from 50%) at the H19 or IGF2 DMR has been previously shown to be associated with increased expression of IGF2,25,37–44,67 and increased susceptibility to several chronic diseases,30,31,37,68,69 presumably via loss of IGF2 imprinting. Modest methylation differences in early life observed in our study may therefore represent an adaptation of the metabolic pathway that contributes substantially to lowering the risk of chronic diseases in adulthood.
A major strength of this multiethnic study is that we evaluated epigenetic response to a ubiquitous exposure, FA, whose increased intake has generated concerns. We have shown that FA-related DNA methylation shifts at a DMR regulating IGF2, a well studied imprinted gene, whose epigenetic profile is largely known, and should be similar across cell types. Whereas the inclusion of erythrocyte folate measurements may provide answers to the broader question of an appropriate FA dose as it represents the sum of folate intake from all sources, findings from our study address the practical question of whether women planning a pregnancy should adhere to recommended FA acid supplementation post-FA fortification. One possible limitation of this study is that DNA obtained from unfractionated blood with multiple cell types may have multiple epigenetic profiles depending on the cell population analyzed. Therefore, we fractionated UCB from 30 specimens and analyzed the major cell types for methylation at the same H19 and IGF2 DMRs. The purity of each fraction was determined by cell counts of polymorphonuclear and mononuclear cells. We observed similar methylation profiles at the IGF2 DMRs across cell fractions, suggesting that DMR methylation profiles measured using unfractionated UCB, do not differ by cell type. The ability to self-administer the questionnaire in this highly literate population (where 80% had at least high school diploma) may have improved the accuracy of responses to FA intake.
A limitation this study shares with other clinical and epidemiological studies is that we relied on women recalling periconceptional FA supplementation, raising the possibility that we over-estimated FA intake, as this is a socially desirable behavior. However, the prevalence of periconceptional (64%) and prenatal (53%) FA intake estimated from the present study is similar to that of the US70,71 and other developed countries,72 suggesting social desirability may not have unduly affected our findings. Moreover, in the event that FA intake was over-reported, such over-reporting would be expected to be similar by methylation status, with the net effect of attenuating the methylation differences observed. Another limitation is that white blood cell (WBC) counts were not evaluated and controlled for in analyses. However, neither FA intake nor folate levels have been shown to be associated with WBC counts. Moreover, statistically adjusting for race and birth weight, which may contribute to inter-individual variation of WBCs, did not alter our findings. Third, even if accurately recalled, our data collection procedures could not capture exact FA doses or total folate intake. However, FA supplementation in women of child bearing age is estimated to account for ∼70% of supplemental folate consumed in the US. Future analyses will require measurement of FA, together with estimates of total folate and synthetic folate consumed during the course of pregnancy. Another limitation that will require further investigation in future studies is that imprinted genes may function in a network and thus may be coordinately regulated;29 we have not demonstrated that FA supplementation affects DMRs regulating other imprinted genes in these analyses. Such studies will require larger sample sizes to account for multiple comparisons.
In summary, we found FA use before and during pregnancy associated with lower methylation levels at DNA sequences regulating IGF2 expression, a decrease that may differ by sex and race/ethnicity. Our findings do not support the hypothesis that FA taken at current doses before and during pregnancy is associated with epigenetic dysregulation previously linked to increases in risk chronic diseases, such as cancer. If confirmed in larger studies, where prospectively measured FA use and concomitant circulating folate levels, as well as other imprint regulatory regions, are also evaluated, these findings would suggest that mitotically heritable DNA methylation shifts at DMRs can potentially serve as genome-wide “biosensors” or “archives” of early FA exposure, useful in monitoring potential non-genotoxic effects of population-wide FA fortification programs.
Materials and Methods
Identification and enrollment of study participants.
Pregnant women were enrolled as part of the Newborn Epigenetics Study (NEST), a multiethnic birth cohort of infants born to mothers recruited from prenatal clinics serving Duke Maternal Fetal Medicine and Durham Regional Hospital, Durham County, NC. Identification and enrollment procedures have been detailed elsewhere in reference 35. Eligibility criteria were: age ≥18 years, English speaking and an intent to use one of two Durham County obstetric facilities, to enable specimen collection at delivery. Between April 2005 and June 2009, 1,101 eligible pregnant women had been identified through clinic appointment logs of which 940 (85%) gave informed consent and enrolled in NEST. Gestational ages at enrollment ranged from 19–42 weeks. This report is limited to the first 438 (46%) deliveries in which methylation analyses were conducted. The 438 mothers and infants included in these analyses were comparable to the 940 with respect to maternal age (p = 0.55), education (p = 0.94), race/ethnicity (p = 0.39) and sex of infant (p = 0.81). This study was approved by the Duke University Medical Center Institutional Review Board.
Collection of covariable data.
Depending on the literacy and willingness of participants a self or interviewer-administered, standardized questionnaire was used to solicit information on socio-demographic characteristics, current and past morbidity, intake of prescribed and over-the-counter medication and dietary supplementation before and during pregnancy. At delivery, medical records were used to verify some information obtained from the self-administered questionnaire and to obtain additional maternal peri-partum information including blood pressure and body temperature (a proxy for infection) and offspring information including sex, anthropometric measures and Apgar score.
Ascertainment of FA intake.
Preconception and prenatal FA supplementation was ascertained from the self-administered questionnaire. Questions were, “In the 12 months before this pregnancy, did you take the following dietary supplements?”—designed to include habitual FA intake that includes the peri-conception period and; “Since you found out you were pregnant this time, did you take the following dietary supplements?”—designed to include only the prenatal period. A list of dietary supplements that included multivitamins, multivitamins with additives, FA/folacin, vitamin B6 (pyridoxine), vitamin B12 (cobalamin) and “other” was provided against which Yes/No responses were solicited. Women reporting any use were asked the brand name, the frequency of intake and trimester when intake started, and if started during pregnancy.
“Non-users” of FA were those who reported no FA supplementation. The “Moderate FA users” comprised participants who reported either intake of an “over-the-counter daily multivitamin” (∼400 µg/day FA) and “additional FA” (∼800 µg/day FA) or “prenatal vitamins only” (∼600–1,000 µg/day). “High FA users” were those who reported a combination of prenatal vitamins including those prescribed (∼600–1,000 µg/day of FA) and “over-the-counter multivitamins” (∼400 µg/day) and/or multivitamins with “additional FA” (∼400 µg/day). The Moderate FA and High FA user categories correspond to within and above the tolerable upper limit for adults (TUL) recommended by the Institute of Medicine, respectively.3,6
Specimen collection and processing.
Within minutes of delivery, the umbilical vein was punctured and umbilical cord blood (UCB) samples were collected into K3EDTA-treated vacutainer tubes and inverted gently to mix the anticoagulant with UCB. Specimens were transported to the laboratory where they were centrifuged to isolate plasma and buffy coat, aliquoted and stored at −80°C. DNA was subsequently extracted from buffy coat using Puregene reagents according to the manufacturer's protocol (Qiagen, Valencia, CA).
Methylation analyses.
UCB leukocyte DNA specimens from the first 438 deliveries were analyzed for methylation patterns at two DMRs regulating IGF2 expression. Genomic DNA was modified by treatment with sodium bisulfite in 96-well format36,37 to convert unmethylated cytosines to uracils, leaving methylated cytosines unchanged. Pyrosequencing was performed using a Pyromark Q96 MD Pyrosequencing instrument (Qiagen; Valencia, CA).
One of the DMRs evaluated is an intragenic DMR located upstream of the imprinted promoters of IGF2 exon 3, hereafter referred to as IGF2 DMR. Methylation was measured at three CpG dinucleotides at the IGF2 DMR (chr 11p15.5; CpG site 1: 2,169,518; CpG site 2: 2,169,515; and CpG site 3: 2,169,499; NCBI Human Genome Build 37/hg19). This DMR has been previously evaluated by Cui et al.30 and Heijmans et al.33 The second DMR is intergenic, and is located upstream of the neighboring maternally expressed H19, within a sequence motif (the first of six) known to bind the CTCF zinc finger protein within the IGF2/H19 imprint control center, and is hereafter referred to as the H19 DMR. Methylation was measured at four CpG dinucleotides at the H19 DMR (chr 11p15.5; CpG site 1: 2,024,261, CpG site 2: 2,024,259, CpG site 3: 2,024,257 and CpG site 4: 2,024,254; NCBI Human Genome Build 37/hg19).30 Each region has previously been shown to normally exhibit parent-of-origin-dependent differential methylation (∼50% methylation overall) that is stably altered in response to environmental cues, including severe maternal caloric restriction.33 CpG hypermethylation at the H19 DMR,30,37 and CpG hypomethylation at the IGF2 DMR30 have been associated with increased IGF2 transcriptional activity and loss of imprinting, and had been found in multiple types of cancer.37–44 Pyrosequencing assays were designed using PSQ Assay Design Software. For the IGF2 DMR, 50 ng of bisulfite-modified DNA was amplified using 2 U Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA) and 0.15 µM each forward (5′-GGA GGG GGT TTA TTT TTT TAG GAA G-3′) and reverse (5′-[Biotin]-AAC CCC AAC AAA AAC CAC TAA ACA C-3′) primers in a 40 µl reaction volume with 0.2 mM dNTPs and 3 mM MgCl2. PCR conditions were 94°C for 3 m, then 5 cycles of 94°C for 30 s, 70°C for 30 s, 72°C for 30 s; then 5 cycles of 94°C for 30 s, 68°C for 30 s, 72°C for 30 s; then 50 cycles of 94°C for 30 s, 66°C for 30 s, 72°C for 30 s; followed by a 5 m extension at 72°C. Biotin-labeled single stranded amplicons were isolated according to protocol using the Qiagen Pyromark Q96 Work Station and underwent pyrosequencing with 0.5 µM primer 5′-GGG GTT TAT TTT TTT AGG A-3′. For the H19 DMR, 40 ng of bisulfite-modified DNA was amplified using 1 U Platinum Taq DNA polymerase and 0.15 µM each forward (5′-TTT GTT GAT TTT ATT AAG GGA G-3′) and reverse (5′-[Biotin] CTA TAA ATA AAC CCC AAC CAA AC-3′) primers in a 20 µl reaction volume with 0.1 mM dNTPs and 3 mM MgCl2. PCR conditions were 94°C for 3 m, then 5 cycles of 94°C for 30 s, 64°C for 30 s, 72°C for 30 s; then 5 cycles of 94°C for 30 s, 61°C for 30 s, 72°C for 30 s; then 35 cycles of 94°C for 30 s, 58°C for 30 s, 72°C for 30 s; followed by a 5 m extension at 72°C. The biotin-labeled single stranded amplicons were isolated as described above and underwent pyrosequencing using 0.5 µM of primer 5′-GTG TGG AAT TAG AAG T-3′. All primers were obtained from Sigma (St. Louis, MO).
The percent methylation for each of the CpGs within the target sequence was calculated using PyroQ CpG Software (Qiagen). Bisulfite conversion efficiency was confirmed to be greater than 95.5% by measuring incorporation of cytosine and thymine for multiple predetermined non-CpG cytosine positions within each sequence run. Validation of the pyrosequencing assays was performed using defined mixtures of plasmids containing the fully methylated and unmethylated version of the bisulfite modified sequence as previously described in reference 45. All methylation analyses were performed in duplicate. Methylation data were 98% complete.
Statistical analyses.
Covariates included in the analyses were as follows: maternal age (18–20 years, 21–22 years, 25–29 years, 30–34 years, >35 years), body mass index (BMI) (kg/m2) before pregnancy, cigarette smoking (never smoked, smoked during early pregnancy but stopped or current smoker), gestational age (<37 weeks or ≥37 weeks), birth weight (<2,500 grams or ≥2,500 grams) and gender of the offspring (male or female), mother's education (less than high school, high school/GED, some college, college graduate and graduate education), and mother's race/ethnicity (African American, Caucasian or other).
We evaluated whether methylation percentages at each CpG dinucleotide were normally distributed using Kolmogorov-Smirnov tests. Principal components and confirmatory factor analyses were used to determine whether methylation at the three CpGs at the IGF2 DMR, and the four CpGs at the H19 DMR were correlated highly enough for each region to be represented by a single mean. Cronbach's alpha values for CpGs were 86% and 87% at the IGF2 DMR and H19 DMR, respectively. Correlation coefficients for the IGF2 and H19 DMRs ranged from 81–83% and 93%–96%, respectively. Chi-square tests were used to determine whether categorical maternal and offspring characteristics varied by FA intake. Using a single mean, F-tests were used to compare unadjusted and adjusted least squares mean methylation levels of offspring born to women at the three levels of FA intake: no FA intake, moderate and high FA intake before pregnancy. Variables adjusted for were cigarette smoking, education, mode of delivery and race/ethnicity. Because there may be gender33 and genetic differences in the epigenetic response to early exposures, we stratified these analyses by gender and race. Despite high correlations among CpG dinucleotides at each DMR, we repeated these analyses using mixed models, to allow unstructured model entry of individual CpGs. In final models, we included cross-product terms for maternal FA intake by infant's gender and maternal race.
As ∼50% of US pregnancies are unplanned, we evaluated potential malleability of these methylation marks among infants of post-conception-only FA users by repeating the multivariate analyses among offspring of 223 non-FA users during the year before pregnancy. The year before pregnancy sufficiently encompasses the periconceptional period. In the multivariate models, we also includes post-conceptional and periconceptional FA intake into the same models. Finally, we used logistic regression models to evaluate the association between IGF2 and H19 DMR methylation and FA intake. Methylation fractions were dichotomized at the 75th percentile (defined as hypermethylation) and were compared to methylation levels at and 25th percentile (defined as hypomethylation) at the IGF2 DMR, since hypermethylation at H19 and hypomethylation at the IGF2 DMR have been associated with deregulation of IGF2 expression. All statistical analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC).
Acknowledgments
We thank the parents and children of the Newborn Epigenetics Study, research nurse Tammy Bishop and research assistants Stacy Murray, Carole Grenier, Darby Kroyer, Natasha Duggan and Suba Narasimhan for enrolling and tracing participants; and the obstetrics faculty and staff at Duke University and Durham regional Hospitals, Durham, NC.
Abbreviations
- FA
folic acid
- DMR
differentially methylated regions
- CpG
cytosine-guanine
- IGF2
insulin-like growth factor-2
Financial Support
The Newborn Epigenetics Study (NEST) was funded through the US National Institutes of Health (agreement # R21ES014947, R01ES016772, K01CA104517) and the Duke Comprehensive Cancer Center (agreement ACS-IRG 83-006). Dr. Cathrine Hoyo was supported by the National Cancer Institute (KO1-CA104517) and Dr. Susan Murphy was supported by the Duke Comprehensive Cancer Center and Duke Environmental Health Sciences Research Center.
References
- 1.Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med. 1999;341:1485–1490. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- 2.Czeizel AE, Dudas I. Prevention of the first occurence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 3.Control CfD, editor. Effectiveness in Disease and Injury Prevention Use of Folic Acid for Prevention of Spina Bifida and Other Neural Tube Defects -- 1983–1991. MMWR Morb Mortal Wkly Rep Atlanta: Centers for Disease Control; 1991. [PubMed] [Google Scholar]
- 4.Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- 5.Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285:2981–2986. doi: 10.1001/jama.285.23.2981. [DOI] [PubMed] [Google Scholar]
- 6.(US) IoM. Institute of Medicine. Dietary reference intakes from thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, patothenic acid, biotin and choline. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- 7.Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health. 2006;38:90–96. doi: 10.1363/psrh.38.090.06. [DOI] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration. Food Standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Federal Register. 1996;61:8781–8797. [Google Scholar]
- 9.Yang QH, Carter HK, Mulinare J, Berry RJ, Friedman JM, Erickson JD. Race-ethnicity differences in folic acid intake in women of childbearing age in the United States after folic acid fortification: findings from the National Health and Nutrition Examination Survey, 2001–2002. Am J Clin Nutr. 2007;85:1409–1416. doi: 10.1093/ajcn/85.5.1409. [DOI] [PubMed] [Google Scholar]
- 10.Crane NT, Wilson DB, Cook DA, Lewis CJ, Yetley EA, Rader JI. Evaluating food fortification options: general principles revisited with folic acid. Am J Public Health. 1995;85:660–666. doi: 10.2105/ajph.85.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmichael SL, Shaw GM, Yang W, Laurent C, Herring A, Royle MH, et al. Correlates of intake of folic acid-containing supplements among pregnant women. Am J Obstet Gynecol. 2006;194:203–210. doi: 10.1016/j.ajog.2005.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George L, Mills JL, Johansson AL, Nordmark A, Olander B, Granath F, et al. Plasma folate levels and risk of spontaneous abortion. JAMA. 2002;288:1867–1873. doi: 10.1001/jama.288.15.1867. [DOI] [PubMed] [Google Scholar]
- 13.Wen SW, Chen XK, Rodger M, White RR, Yang Q, Smith GN, et al. Folic acid supplementation in early second trimester and the risk of preeclampsia. Am J Obstet Gynecol. 2008;198:45. doi: 10.1016/j.ajog.2007.06.067. e1-7. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Diaz S, Werler MM, Louik C, Mitchell AA. Risk of gestational hypertension in relation to folic acid supplementation during pregnancy. Am J Epidemiol. 2002;156:806–812. doi: 10.1093/aje/kwf129. [DOI] [PubMed] [Google Scholar]
- 15.Godwin KA, Sibbald B, Bedard T, Kuzeljevic B, Lowry RB, Arbour L. Changes in frequencies of select congenital anomalies since the onset of folic acid fortification in a Canadian birth defect registry. Can J Public Health. 2008;99:271–275. doi: 10.1007/BF03403753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haberg SE, London SJ, Stigum H, Nafstad P, Nystad W. Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child. 2009;94:180–184. doi: 10.1136/adc.2008.142448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitrow MJ, Moore VM, Rumbold AR, Davies MJ. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol. 2009;170:1486–1493. doi: 10.1093/aje/kwp315. [DOI] [PubMed] [Google Scholar]
- 18.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118:3462–3469. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Ulrich CM. Folate and cancer prevention: a closer look at a complex picture. Am J Clin Nutr. 2007;86:271–273. doi: 10.1093/ajcn/86.2.271. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Bostick RM, Friedman JM, Flanders WD. Serum folate and cancer mortality among U.S. adults: findings from the Third National Health and Nutritional Examination Survey linked mortality file. Cancer Epidemiol Biomarkers Prev. 2009;18:1439–1447. doi: 10.1158/1055-9965.EPI-08-0908. [DOI] [PubMed] [Google Scholar]
- 21.Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr. 2008;87:517–533. doi: 10.1093/ajcn/87.3.517. [DOI] [PubMed] [Google Scholar]
- 22.Barker DJ. Developmental origins of adult health and disease. J Epidemiol Community Health. 2004;58:114–115. doi: 10.1136/jech.58.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Depeint F, Bruce WR, Shangari N, Mehta R, O'Brien PJ. Mitochondrial function and toxicity: role of B vitamins on the one-carbon transfer pathways. Chem Biol Interact. 2006;163:113–132. doi: 10.1016/j.cbi.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy SK, Jirtle RL. Imprinting evolution and the price of silence. BioEssays. 2003;25:577–588. doi: 10.1002/bies.10277. [DOI] [PubMed] [Google Scholar]
- 26.Woodfine K, Huddleston JE, Murrell A. Quantitative analysis of DNA methylation at all human imprinted regions reveals preservation of epigenetic stability in adult somatic tissue. Epigenetics Chromatin. 4:1. doi: 10.1186/1756-8935-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waterland RA, Kellermayer R, Laritsky E, Rayco-Solon P, Harris RA, Travisano M, et al. Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles. PLoS Genet. 6:e1001252. doi: 10.1371/journal.pgen.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards CA, Rens W, Clarke O, Mungall AJ, Hore T, Graves JA, et al. The evolution of imprinting: chromosomal mapping of orthologues of mammalian imprinted domains in monotreme and marsupial mammals. BMC Evol Biol. 2007;7:157. doi: 10.1186/1471-2148-7-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varrault A, Gueydan C, Delalbre A, Bellmann A, Houssami S, Aknin C, et al. Zac1 regulates an imprinted gene network critically involved in the control of embryonic growth. Dev Cell. 2006;11:711–722. doi: 10.1016/j.devcel.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Cui H, Cruz-Correa M, Giardiello FM, Hutcheon DF, Kafonek DR, Brandenburg S, et al. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science. 2003;299:1753–1755. doi: 10.1126/science.1080902. [DOI] [PubMed] [Google Scholar]
- 31.Cruz-Correa M, Cui H, Giardiello FM, Powe NR, Hylind L, Robinson A, et al. Loss of imprinting of insulin growth factor II gene: a potential heritable biomarker for colon neoplasia predisposition. Gastroenterology. 2004;126:964–970. doi: 10.1053/j.gastro.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 32.Cruz-Correa M, Zhao R, Oviedo M, Bernabe RD, Lacourt M, Cardona A, et al. Temporal stability and age-related prevalence of loss of imprinting of the insulin-like growth factor-2 gene. Epigenetics. 2009;4 doi: 10.4161/epi.4.2.7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, Lindemans J, Siebel C, Steegers EA, et al. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One. 2009;4:e7845. doi: 10.1371/journal.pone.0007845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoyo C, Murtha AP, Schildkraut JM, Forman MR, Calingaert B, Demark-Wahnefried W, et al. Folic acid supplementation before and during pregnancy in the Newborn Epigenetics Study (NEST) BMC Public Health. 2011;11:46. doi: 10.1186/1471-2458-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Z, Wen Y, Shandilya R, Marks JR, Berchuck A, Murphy SK. High throughput detection of M6P/IGF2R intronic hypermethylation and LOH in ovarian cancer. Nucleic Acids Res. 2006;34:555–563. doi: 10.1093/nar/gkj468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy SK, Huang Z, Wen Y, Spillman MA, Whitaker RS, Simel LR, et al. Frequent IGF2/H19 domain epigenetic alterations and elevated IGF2 expression in epithelial ovarian cancer. Mol Cancer Res. 2006;4:283–292. doi: 10.1158/1541-7786.MCR-05-0138. [DOI] [PubMed] [Google Scholar]
- 38.Xu W, Fan H, He X, Zhang J, Xie W. LOI of IGF2 is associated with esophageal cancer and linked to methylation status of IGF2 DMR. J Exp Clin Cancer Res. 2006;25:543–547. [PubMed] [Google Scholar]
- 39.Murrell A, Ito Y, Verde G, Huddleston J, Woodfine K, Silengo MC, et al. Distinct methylation changes at the IGF2-H19 locus in congenital growth disorders and cancer LOI of IGF2 is associated with esophageal cancer and linked to methylation status of IGF2 DMR. PLoS One. 2008;3:e1849. doi: 10.1371/journal.pone.0001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui H, Niemitz EL, Ravenel JD, Onyango P, Brandenburg SA, Lobanenkov VV, et al. Loss of imprinting of insulin-like growth factor-II in Wilms' tumor commonly involves altered methylation but not mutations of CTCF or its binding site. Cancer Res. 2001;61:4947–4950. [PubMed] [Google Scholar]
- 41.Cui H, Onyango P, Brandenburg S, Wu Y, Hsieh CL, Feinberg AP. Loss of imprinting in colorectal cancer linked to hypomethylation of H19 and IGF2. Cancer Res. 2002;62:6442–6446. [PubMed] [Google Scholar]
- 42.Nakagawa H, Chadwick RB, Peltomaki P, Plass C, Nakamura Y, de La Chapelle A. Loss of imprinting of the insulin-like growth factor II gene occurs by biallelic methylation in a core region of H19-associated CTCF-binding sites in colorectal cancer. Proc Natl Acad Sci USA. 2001;98:591–596. doi: 10.1073/pnas.011528698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan MJ, Taniguchi T, Jhee A, Kerr N, Reeve AE. Relaxation of IGF2 imprinting in Wilms tumours associated with specific changes in IGF2 methylation. Oncogene. 1999;18:7527–7534. doi: 10.1038/sj.onc.1203096. [DOI] [PubMed] [Google Scholar]
- 44.Takai D, Gonzales FA, Tsai YC, Thayer MJ, Jones PA. Large scale mapping of methylcytosines in CTCF-binding sites in the human H19 promoter and aberrant hypomethylation in human bladder cancer. Hum Mol Genet. 2001;10:2619–2626. doi: 10.1093/hmg/10.23.2619. [DOI] [PubMed] [Google Scholar]
- 45.Wong HL, Byun HM, Kwan JM, Campan M, Ingles SA, Laird PW, et al. Rapid and quantitative method of allele-specific DNA methylation analysis. Biotechniques. 2006;41:734–739. doi: 10.2144/000112305. [DOI] [PubMed] [Google Scholar]
- 46.Kerkel K, Spadola A, Yuan E, Kosek J, Jiang L, Hod E, et al. Genomic surveys by methylation-sensitive SNP analysis identify sequence-dependent allele-specific DNA methylation. Nat Genet. 2008;40:904–908. doi: 10.1038/ng.174. [DOI] [PubMed] [Google Scholar]
- 47.Richards EJ. Inherited epigenetic variation--revisiting soft inheritance. Nat Rev Genet. 2006;7:395–401. doi: 10.1038/nrg1834. [DOI] [PubMed] [Google Scholar]
- 48.Hoyo C, Murphy SK, Jirtle RL. Imprint regulatory elements as epigenetic biosensors of exposure in epidemiological studies. J Epidemiol Community Health. 2009;63:683–684. doi: 10.1136/jech.2009.090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heijmans BT, Tobi EW, Lumey LH, Slagboom PE. The epigenome: archive of the prenatal environment. Epigenetics. 2009;4:526–531. doi: 10.4161/epi.4.8.10265. [DOI] [PubMed] [Google Scholar]
- 50.Mathers JC. Early nutrition: impact on epigenetics. Forum Nutr. 2007;60:42–48. doi: 10.1159/000107066. [DOI] [PubMed] [Google Scholar]
- 51.Bell AAC, Felsenfeld GG. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature. 2000;405:482–485. doi: 10.1038/35013100. [DOI] [PubMed] [Google Scholar]
- 52.Kanduri C, Holmgren C, Pilartz M, Franklin G, Kanduri M, Liu L, et al. The 5′ flank of mouse H19 in an unusual chromatin conformation unidirectionally blocks enhancer-promoter communication. Curr Biol. 2000;10:449–457. doi: 10.1016/s0960-9822(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 53.Ulaner GAGA, Vu THTH, Li TT, Hu JFJ-F, Yao XMX-M, Yang YY, et al. Loss of imprinting of IGF2 and H19 in osteosarcoma is accompanied by reciprocal methylation changes of a CTCF-binding site. Hum Mol Genet. 2003;12:535–549. doi: 10.1093/hmg/ddg034. [DOI] [PubMed] [Google Scholar]
- 54.Nakagawa HH, Chadwick RRB, Peltomaki PP, Plass CC, Nakamura YY, de La Chapelle AA. Loss of imprinting of the insulin-like growth factor II gene occurs by biallelic methylation in a core region of H19-associated CTCF-binding sites in colorectal cancer. Proc Natl Acad Sci USA. 2001;98:591–596. doi: 10.1073/pnas.011528698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomes MV, Waterland RA. Individual epigenetic variation: when, why, and so what? Nestle Nutr Workshop Ser Pediatr Program. 2008;62:141–150. doi: 10.1159/000146257. discussion 151-5. [DOI] [PubMed] [Google Scholar]
- 56.Waterland RA. Do maternal methyl supplements in mice affect DNA methylation of offspring? J Nutr. 2003;133:238. doi: 10.1093/jn/133.1.238. author reply 9. [DOI] [PubMed] [Google Scholar]
- 57.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr. 1998;68:899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 58.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 59.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 60.Elias SG, Peeters PH, Grobbee DE, van Noord PA. Breast cancer risk after caloric restriction during the 1944–1945 Dutch famine. J Natl Cancer Inst. 2004;96:539–546. doi: 10.1093/jnci/djh087. [DOI] [PubMed] [Google Scholar]
- 61.Elias SG, Onland-Moret NC, Peeters PH, Rinaldi S, Kaaks R, Grobbee DE, et al. Urinary endogenous sex hormone levels in postmenopausal women after caloric restriction in young adulthood. Br J Cancer. 2004;90:115–117. doi: 10.1038/sj.bjc.6601513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 63.Gluckman P, Hanson M, Beedle A. Early life events and their consequences for later disease: A life history and evolutionary perspective. Am J Hum Biol. 2007;19 doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- 64.Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol. 2005;20:345–352. doi: 10.1016/j.reprotox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 65.Yajnik CS. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J Nutr. 2004;134:205–210. doi: 10.1093/jn/134.1.205. [DOI] [PubMed] [Google Scholar]
- 66.Takano Y, Shiota G, Kawasaki H. Analysis of genomic imprinting of insulin-like growth factor 2 in colorectal cancer. Oncology. 2000;59:210–216. doi: 10.1159/000012163. [DOI] [PubMed] [Google Scholar]
- 67.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feinberg AP. An epigenetic approach to cancer etiology. Cancer J. 2007;13:70–74. doi: 10.1097/PPO.0b013e31803c6e3b. [DOI] [PubMed] [Google Scholar]
- 69.D'Angelo D, Williams L, Morrow B, Cox S, Harris N, Harrison L, et al. Preconception and interconception health status of women who recently gave birth to a live-born infant--Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 26 reporting areas, 2004. MMWR Surveill Summ. 2007;56:1–35. [PubMed] [Google Scholar]
- 70.Jasti S, Siega-Riz AM, Cogswell ME, Hartzema AG, Bentley ME. Pill count adherence to prenatal multivitamin/mineral supplement use among low-income women. J Nutr. 2005;135:1093–1101. doi: 10.1093/jn/135.5.1093. [DOI] [PubMed] [Google Scholar]
- 71.Vollset SE, Gjessing HK, Tandberg A, Ronning T, Irgens LM, Baste V, et al. Folate supplementation and twin pregnancies. Epidemiology. 2005;16:201–205. doi: 10.1097/01.ede.0000152914.84962.13. [DOI] [PubMed] [Google Scholar]


