Abstract
Introduction
Anaplastic thyroid cancer (ATC) is an undifferentiated, aggressive malignancy, for which there are no effective therapies. Though ATCs only make up less than 2% of all thyroid cancer cases, they represent over half of the thyroid cancer related deaths. Chrysin, a natural flavonoid, has recently been reported as a potential anti-cancer agent. However, the effect of this compound on ATC cells is not known. Thus, in this study, we evaluated the antiproliferative nature of Chrysin in ATC cells.
Methods
HTH7 and KAT18 cells, derived from patients with ATC, were treated with Chrysin (25–50 μM) for up to 6 days. Cell proliferation was measured every 2 days using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Western blot analysis for molecular makers of apoptosis was carried out to investigate the effect and mechanism of Chrysin on ATC.
Results
Chrysin inhibited proliferation of HTH7 and KAT18 in a dose and time dependent manner. HTH7 and KAT18 cells with Chrysin treatment showed a significant increase in cleaved caspase-3, cleaved PolyADP Ribose Polymerase (PARP), along with a decrease in cyclin D1, Mcl-1 and XIAP. Furthermore, the ratio of Bax to Bcl-2 expression in ATC cells revealed an increase after the treatment.
Conclusions
Chrysin inhibits growth in ATC cells via apoptosis in vitro. Therefore, the natural flavonoid Chrysin warrants further clinical investigation as a new potential drug for the treatment for ATC.
Keywords: anaplastic thyroid cancer, chrysin, apoptosis, proliferation
Introduction
Thyroid cancer accounts for about 2% of all the malignances in the United States 1. According to the National Cancer Institute, there will be 44,670 new cases of thyroid cancer and 1690 deaths due to this disease in the United States in 2010. Anaplastic thyroid cancer (ATC) is one of the four main thyroid cancer types with undifferentiated, fast growing, and very aggressive features. It is usually characterized for extensive local invasion and is always classified as TNM stage IV regardless of the tumor size, location, or metastasis 2. Though ATCs only make up less than 2% of all the cases of thyroid cancer 3, they represent over half of the thyroid cancer-related deaths, and the mean survival time for ATC patients is less than 6 months from diagnosis 4. In a national cancer database of 53,856 cases of thyroid carcinoma treated in the U.S, the ten-year overall relative survival rate of ATC is 14% 3.
The current standard treatments including surgery, chemotherapy, and radiation have not shown to be curative for ATC 5, 6. For instance, total thyroidectomy is only indicated for a few rare cases of localized ATC; and ATC is not responsive to radioactive iodine ablation since ATC cell has lost the capacity of concentrating or absorbing iodine though it is originated from thyroid follicular cells. Therefore, there is a critical need to develop novel approaches to treat patients with ATC.
Recently, Chrysin has emerged as a potential drug therapy for different cancers including leukemia and cervical cancer 7–9. Chrysin, an active natural bioflavonoid found in honey and many plant extracts, is first known for its anti-oxidant and anti-inflammatory effect. Later on, more and more studies have revealed that Chrysin inhibits cancer cell growth by inducing apoptosis 9. Apoptosis, defined as programmed cell death, is a very important regulatory mechanism for cell growth. This is a process in which cells undergo irreversible biological changes including cell shrinkage, chromosomal condensation, and genomic DNA fragmentation leading to cell death to maintain the homeostasis of the cell number. However, most cancer cells have lost the normal regulation of apoptosis. Therefore, inducing apoptosis is widely accepted as one of the most important approaches to treat cancers 10.
Though Chrysin has been shown as a promising anti-cancer agent, the effect of Chrysin on ATC has remained completely unknown. In this study, we aimed to evaluate the effects of Chrysin on ATC cell growth and investigate the underlying mechanism of apoptosis.
Materials and Methods
Cell Culture
DNA profiling has determined that HTH7 (a kind gift from Dr. Rebecca Schweppe, University of Colorado, Denver, CO) and KAT18 cells are both derived from patients with ATC11. Both cell lines were maintained in standard RPMI 1640 medium (Invitrogen Life Technologies, Carlsbad, CA) with 10% fetal bovine serum (Sigma-Aldrich, St.Louis, MO), 100 IU/mL penicillin and 100 μg/ml streptomycin (Invitrogen Life Technologies). In addition, medium for KAT18 cells was supplemented with 1% non-essential amino acids and 1mM sodium pyruvate (Invitrogen Life Technologies). The cells were maintained in a humidified atmosphere of 5% CO2 in air at 37°C. Chrysin (MP boimedicals, Solon, OH) was dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich) at a stock concentration of 100 mmol/L and stored at 20°C. Fresh dilutions in cell culture medium were made for each experiment.
Cell Proliferation Assay
ATC cell proliferation was measured using the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay as previously described12. HTH7 and KAT18 cells were plated in quadruplicate on 24-well plates at the density of 20,000 cells/well and 15,000 cells/well respectively. Cells were incubated overnight under standard conditions. After incubation, cells were treated with culture media containing 25, 50 μM Chrysin or DMSO control. Treatment media was changed every two days. MTT assay was carried out up to six days, and cell proliferation was measured every two days. On the day of measurement, cells were incubated at standard conditions in 250μL of serum-free RPMI 1640 containing 0.5 mg/mL MTT. After 4 hours of incubation, 750 μL of dimethyl sulfoxide (DMSO) (Fischer Scientific, Pittsburg, PA) was added to each well and mixed thoroughly. Absorbance at 540nm was measured using a spectrophotometer (μQuant; Bio-Tek Instruments, Winooski, VT). Experiments were performed at least twice and the data were plotted as an average ± standard error of the mean (SEM).
Western Blot Analysis
HTH7 and KAT18 cells were plated in 100 × 15 mm tissue culture dishes and incubated at standard conditions overnight. After incubation, cells were treated with DMSO control, 25, or 50μM Chrysin for 2 days. Whole cell lysates were harvested as previously described 12. Protein concentration was quantified using the BCA Protein Assay Kit (Thermo Scientific, Waltham, MA) following the manufacturer’s instructions. Denatured cellular extracts were resolved by 8% 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), gels, transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA), blocked in 5% dry milk solution, then incubated with appropriate primary antibodies overnight at 4°C. Primary antibodies were diluted in 5% dry milk solution as following: 1:1000 for caspase-3 and XIAP, cyclinD1; 1:2000 for β-actin; 1:3000 for cleaved Poly-ADP ribose polymerase (PARP) (Cell Signaling Technology, Beverly, MA). Mcl-1, Bcl-2, and Bax primary antibodies (Cell Signaling Technology) were diluted at the ratio of 1:1000 in 5% BSA solution. PBS (Cellgro, Manassas, VA) with 0.1% Tween- 20 (Bio-Rad Laboratories) was used as solvent for both blocking milk solution and primary antibody dilution. After incubating with primary antibodies, membranes were incubated for 1.5 hours at room temperature with horseradish peroxidase conjugated rabbit or mouse antibodies depending on the source of the primary antibody. The protein signal was then detected by exposing the blots to x-ray films after developed with Immunstar (Bio-Rad Laboratories), SuperSignal West Pico, or SuperSignal West Femto (Pierce Biotechnology, Rockford, IL).
Statistical Analysis
Statistical analyses were performed utilizing analysis of variance testing (SPSS software version 10.0, SPSS; Chicago, IL). A p value < 0.05 was considered significant. Unless specifically noted, all data are represented as mean ± SEM.
Results and Discussion
Chrysin inhibits ATC cell proliferation
ATC is an undifferentiated, fast-growing malignancy, and there is a need for novel therapeutic strategies. Currently, there are many ongoing studies investigating the anti-cancer effects of Chrysin due to its non-toxic properties as a natural flavonoid. Yin et. al reported the growth inhibitory effects of Chrysin on an anaplastic thyroid cancer cell line (ARO81-1) in 1999 13. However, with a recent DNA profiling analysis, ARO81-1 has been identified as colon cancer cell line 11. To our knowledge, there are no studies which have examined the effects of Chrysin on ATC. Therefore, we were interested to determine the effect of Chrysin on ATC cell growth.
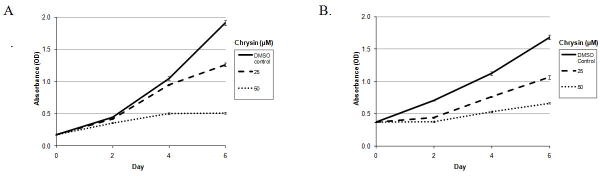
In our study, the MTT data showed that Chrysin inhibited proliferation of HTH7 and KAT18 cells in a dose and time dependent manner (Fig.1). Compared to control group, after the 2 day treatment, a statistically significant growth inhibition was observed in both the 25 and 50μM Chrysin conditions (p < 0.01). In particular, 4-day treatment of HTH7 cells with 25μM Chrysin suppressed cell growth by 9% while 50μM Chrysin suppressed cell growth by 50%. After the 6-day treatment, 25μM and 50μM Chrysin suppressed HTH7 cell growth by 34% and 73%, respectively (Fig.1A). Similar results were found for KAT18 cells treated with Chrysin (Fig.1B). 4-day treatment of KAT18 cells with 25μM Chrysin suppressed cell growth by 32% while 50μM Chrysin suppressed cell growth by 52%. After the 6-day treatment, 25μM, and 50μM Chrysin suppressed HTH7 growth by 37% and 60%, respectively.
Figure 1.
Chrysin inhibited proliferation of ATC cells in vitro. HTH7 cells (Fig.1A) and KAT18 cells (Fig. 1B) were treated with DMSO control, 25 μM, or 50 μM Chrysin for up to six days. Cell proliferation was measured every 2 days using MTT assay.
Chrysin induces apoptosis in ATC cells in vitro through activating irreversible cleavage of caspase proteins
The underlying mechanism for the observed growth inhibition was investigated by performing Western blot analysis for different apoptosis molecular markers of whole ATC cell lysates after 2-day Chrysin treatment.
Caspases are a conserved family of enzymes that commit cells to programmed cell death. Among the 11 human caspases which have been identified, caspase-3 is an effector caspase 14. Proteolytic activation of caspase-3 leads to the cleavage of PARP, a DNA repair protein as well as a protein to maintain genomic DNA integrity 15. An increase in cleaved PARP and cleaved caspase-3 levels are indicative of cells undergoing apoptosis. In this study, compared to the DMSO control group, both ATC cell lines showed an increase in cleaved caspase-3 together with an increase in cleaved PARP when treated with Chrysin in a dose-dependent manner (Fig. 2).
Figure 2.
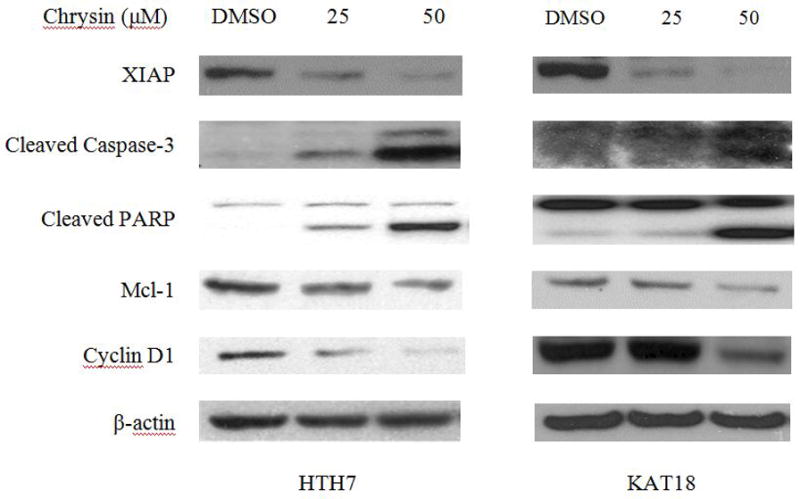
Chrysin induced apoptosis in ATC cells. 2 day treatment of Chrysin on HTH7 cells (A), and KAT18 (B) resulted in a dose-dependent increase in cleaved caspase-3, cleaved PARP, and a dose-dependent decrease in XIAP, Mcl-1, and Cyclin D1.
In addition, a significant reduction in X-linked Inhibitor of Apoptosis Protein (XIAP) levels was also observed in Chrysin-treated ATC cells in a dose-dependent manner (Fig. 2). XIAP is one of the eight mammalian IAPs. Caspase -3 cleavage has been reported to be inhibited mainly by XIAP 16. Thus, the reduction of XIAP levels in Chrysin treated cells provided more evidence for the induction of apoptosis.
Cells keep the homeostasis of the anti-apoptotic and pro-apoptotic regulators of apoptosis to maintain the proper survival and turnover. One of the important anti-apoptotic proteins is Myeloid cell leukemia 1 (Mcl-1). In this study, we found a reduction of Mcl-1 in both ATC cell lines in response to increasing concentrations of Chrysin treatment (Fig.2). It is believed that Mcl-1 binds to Bcl-2 homologous antagonist killer (Bak), and Bcl-2 associated protein X (Bax), which results in a decrease in cytochrome c release into cytoplasm. Cytochrome c is known to activate proteolytic activity of caspases. Thus, a reduction in Mcl-1 may result in more cytochrome c activation which in turn accelerate the apoptosis 17. On the other hand, Cyclin D1 is a proto-oncogene which stimulate cells transition from G1 to S phase 18 and 19. The down-regulation of cyclin D1 in this study (Fig.2) implied ATC cells proliferation was inhibited by Chrysin treatment is due to apoptosis.
We also compared the ratio of Bcl-2 and Bax between Chrysin treated ATC cells and controls. Bcl-2 is a pro-survival protein which represses apoptosis. Bax, on the contrary, promotes apoptosis. It has been suggested that the increase in Bax to Bcl-2 ratio indicates that cells are more susceptible to apoptosis 20,21. In this study, we found a dose-dependent increase in Bax to Bcl-2 ratio in both HTH7 and KAT18 cells when they were treated with Chrysin (Fig.3).
Figure 3.

Two-day Chrysin treatment on ATC cells caused an increase in the ratio of Bax to Bcl-2 in whole cell lysates. HTH7 (A) and KAT18 cells were treated with Chrysin for 2 days, and whole cell lysates were analyzed by Western blotting. To quantify the protein expression levels and ratio of Bax, Bcl-2, the bands on the X-ray films were scanned and the intensity of the bands was measured using ImageJ (ImageJ 1.43u, National Institute of Health). An increase in the ratio of intensity of Bax over Bcl-2 with Chrysin treatment was detected.
In summary, we have shown in this study that Chrysin induced apoptosis in ATC cells. With increasing doses of Chrysin treatment, more cleaved caspase-3 and cleaved PARP levels were observed. The increase in these proteins is an indication that cells have committed to the irreversible programmed cell death process. Furthermore, we also found that the levels XIAP, Mcl-1, Cyclin D1 decreased, together with the increase of the ratio of Bax to Bcl-2 following treatment with Chrysin. Our findings showed consistency with previous studies investigating the effects of Chrysin on the other cancers such as cervical cancer and leukemia9. In cervical cancer, Chrysin induces apoptosis through activating NFkappaB/p65 22. Besides, Chrysin causes DNA fragmentation and down-regulation of XIAP and inactivation of Akt in leukemia cells 23.
Therefore, at the molecular level, we have found evidences of apoptosis induction by Chrysin on ATC cells. Because induction of apoptosis is generally accepted as the important approaches to treat cancers, Chrysin can be further evaluated for in vivo studies. Thus, Chrysin warrants further translational and clinical investigations as a new potential drug for the treatment of ATC.
Acknowledgments
The authors thank the other members of the Chen’s laboratory for their help and technical support with this project. This study was supported by NIH-T35 DK062709, NIH-R01-CA109053, NIH-R01-CA121115, and the American Cancer Society MEN2 Thyroid Professorship.
References
- 1.Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol) 2010;22(6):486–97. doi: 10.1016/j.clon.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neff RL, Farrar WB, Kloos RT, Burman KD. Anaplastic thyroid cancer. Endocrinol Metab Clin North Am. 2008;37(2):525–38. xi. doi: 10.1016/j.ecl.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S. 1985–1995 [see commetns] Cancer. 1998;83(12):2638–48. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Cornett WR, Sharma AK, Day TA, et al. Anaplastic thyroid carcinoma: an overview. Curr Oncol Rep. 2007;9(2):152–8. doi: 10.1007/s11912-007-0014-3. [DOI] [PubMed] [Google Scholar]
- 5.Zarebczan B, Chen H. Multi-targeted approach in the treatment of thyroid cancer. Minerva Chir. 2010;65(1):59–69. [PMC free article] [PubMed] [Google Scholar]
- 6.Pinchot SN, Sippel RS, Chen H. Multi-targeted approach in the treatment of thyroid cancer. Ther Clin Risk Manag. 2008;4(5):935–47. doi: 10.2147/tcrm.s3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang T, Chen X, Qu L, et al. Chrysin and its phosphate ester inhibit cell proliferation and induce apoptosis in Hela cells. Bioorg Med Chem. 2004;12(23):6097–105. doi: 10.1016/j.bmc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Monasterio A, Urdaci MC, Pinchuk IV, et al. Flavonoids induce apoptosis in human leukemia U937 cells through caspase- and caspase-calpain-dependent pathways. Nutr Cancer. 2004;50(1):90–100. doi: 10.1207/s15327914nc5001_12. [DOI] [PubMed] [Google Scholar]
- 9.Khoo BY, Chua SL, Balaram P. Apoptotic effects of chrysin in human cancer cell lines. Int J Mol Sci. 2010;11(5):2188–99. doi: 10.3390/ijms11052188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5(11):876–85. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 11.Schweppe RE, Klopper JP, Korch C, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93(11):4331–41. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunnimalaiyaan M, Ndiaye M, Chen H. Apoptosis-mediated medullary thyroid cancer growth suppression by the PI3K inhibitor LY294002. Surgery. 2006;140(6):1009–14. doi: 10.1016/j.surg.2006.06.040. discussion 1014–5. [DOI] [PubMed] [Google Scholar]
- 13.Yin F, Giuliano AE, Van Herle AJ. Growth inhibitory effects of flavonoids in human thyroid cancer cell lines. Thyroid. 1999;9(4):369–76. doi: 10.1089/thy.1999.9.369. [DOI] [PubMed] [Google Scholar]
- 14.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5(11):897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 15.Krishnakumar R, Kraus WL. The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell. 2010;39(1):8–24. doi: 10.1016/j.molcel.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006;7(10):988–94. doi: 10.1038/sj.embor.7400795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS Letters. 2010;584(14):2981–2989. doi: 10.1016/j.febslet.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 18.Klein EA, Assoian RK. Transcriptional regulation of the cyclin D1 gene at a glance. J Cell Sci. 2008;121(Pt 23):3853–7. doi: 10.1242/jcs.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saha A, Kuzuhara T, Echigo N, et al. Apoptosis of human lung cancer cells by curcumin mediated through up-regulation of “growth arrest and DNA damage inducible genes 45 and 153”. Biol Pharm Bull. 2010;33(8):1291–9. doi: 10.1248/bpb.33.1291. [DOI] [PubMed] [Google Scholar]
- 20.Manetto V, L Rossana, Cordon-Cardo Carlosl, Krajewski Stan, Rosai Juan, Reed John, Eusebi Vincenzo. Bcl-2 and Bax expression in thyroid tumours: an immunohistochemical and Western blot analysis. Virchows Archiv: an international journal of pathology. 1996;430(2):125. doi: 10.1007/BF01008033. [DOI] [PubMed] [Google Scholar]
- 21.Oltval ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell. 1993;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 22.von Brandenstein MG, Ngum Abety A, Depping R, et al. A p38-p65 transcription complex induced by endothelin-1 mediates signal transduction in cancer cells. Biochim Biophys Acta. 2008;1783(9):1613–22. doi: 10.1016/j.bbamcr.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Woo KJ, Jeong YJ, Park JW, Kwon TK. Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells. Biochem Biophys Res Commun. 2004;325(4):1215–22. doi: 10.1016/j.bbrc.2004.09.225. [DOI] [PubMed] [Google Scholar]