Abstract
Aims/hypothesis:
Islet amyloid polypeptide is originally isolated as the chief constituent of amyloid deposits in type 2 diabetic islets. Islet amyloid polypeptide hyposecretion was known in type 1 diabetics and this study aimed to detect possibly reduced islet amyloid polypeptide-positive cells in type 1 diabetic islets.
Results:
Non-diabetic control islets showed about 60% of islet cells were insulin cells, and 60% of insulin cells were positive for IAPP. In type 1 diabetic islets, islets were generally smaller than control islets, consisting of weaker positive cells for insulin and islet amyloid polypeptide. Medium-sized islets still retained some insulin positive cells, whereas islet amyloid polypeptide positive cells were much less or even absent, but some insulin-negative cells were weakly islet amyloid polypeptide positive. An occasional extra-large islet, representing regenerating islets, consisting of more than 100 islet cells revealed less than 35% insulin and 20% islet amyloid polypeptide positive cells with relatively increased glucagon and somatostatin cells. Both normal and type 1 diabetic islets revealed scattered, densely insulin and islet amyloid polypeptide positive sickle-shaped cytoplasm without granular appearance, consistent with degenerating insulin cells.
Methods:
Using commercially available rabbit anti-islet amyloid polypeptide antibody, immunostaning was performed on ten cases of type 1 diabetic pancreata and eight non-diabetic controls. Both control and type 1 diabetic pancreata were systematically immunostained for insulin, glucagon, somatostatin and islet amyloid polypeptide.
Conclusion/Interpretation:
Control islets consisted of about 60% insulin cells, and about 34% of islet cells were amyloid polypeptide positive with scattered and densely positive for insulin and islet amyloid polypeptide without granular appearance, consistent with degenerating β-cells. All islets, including occasional extra-large islets from type 1 diabetics, showed less insulin cells and less islet amyloid polypeptide positive cells with twice increased glucagon and somatostatin cells of the control islets, but some insulin-negative cells were positive for islet amyloid polypeptide, suggesting the presence of islet amyloid polypeptide in degenerating and extra large regenerating islets. Thus, this immunocytochemical staining revealed generally less islet amyloid positive cells in type 1 diabetic islets, corresponding to severe hyposecretion of islet amyloid polypeptide in type 1 diabetics.
Key words: immunocytochemistry, islet amyloid polypeptide, pancreatic islets, type 1 diabetes
Introduction
Islet amyloid polypeptide (IAPP) is a 37 amino acid polypeptide that is originally isolated as the chief constituent of islets from type 2 diabetics.1–4 IAPP is co-localized in β-granules and co-secreted into the blood stream with insulin in response to glucose- and amino acid-stimulated insulin secretion.3 In fetal islets, IAPP is co-localized with insulin-cells at 50% frequency whereas glucagon and somatostatin (SRIF) cells were co-localized at 1.4 and 2.6%, respectively, by immunofluorescence study.5 By double immuncocytochemical staining, Iki and Pour reported 50% of insulin cells positive for IAPP and none of glucagon cells were co-stained for IAPP in non-diabetic islets whereas there were some islets cells co-expressing both IAPP and SRIF cells in atrophic type 2 diabetic islets.6 A synthetic IAPP, Pranlintide25,28,29 (Pro-hIAPP) has been used for treating type 1 diabetics with insulin for a better glycemic control as type 1 diabetics presented with IAPP hyposecretion, and IAPP injection compliments insulin treatment to have a better glycemic control through suppressing glucagon secretion. This study was aimed to investigate the possible IAPP deficient pancreatic islets being responsible for severe IAPP hyposecretion in type 1 diabetics.
Results
Control islets.
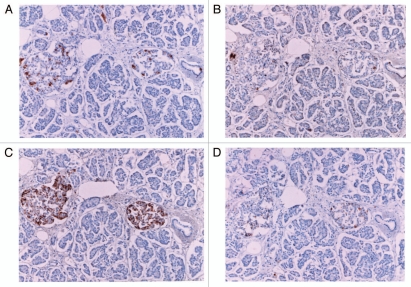
Large control islets consisted of major insulin cells, followed by glucagon and SRIF cells at a relative ratio of 6:3:1 whereas in medium sized islets, the relative ratio of three kinds of islet cells was 6:3:1.5 (Table 1 and Fig. 1). About 34% of islet cells were positively stained for IAPP and the ratio of IAPP positive cells to insulin cells was about 60% corresponding to the darkly insulin positive cells (Fig. 1). There were scattered, irregular, sickle-shaped cytoplasms often without nucleus, which were densely positive for both insulin and IAPP without granular appearance (Fig. 1A and B). Insulin and IAPP positive cells with generally abundant cytoplasm were mostly located in mid portion of the islets, revealing a variety of staining density whereas glucagon cells with uniformly dark stained small cytoplasm were in the outer margin of the islet and islet lobule with scanty cytoplasm (Fig. 1A and C). SRIF cells were located in mid portion of islets adjacent to insulin cells with an intermediate cytoplasmic size between insulin cells and glucagon cells (Fig. 1D).
Table 1.
IAPP immunostaining for pancreatic islets from type 1 diabetics
| Large Islets | Medium Islets | |||||||||||
| Total Islet | % Ins | % Glu | % SRIF | % IAPP | % IAPP/Ins | Total Islet | % Ins | % Glu | % SRIF | % IAPP | % IAPP/Ins | |
| Case 1. 18/M | 51.2 | 15.7 | 66.5 | 19 | 18.2 | 154 | 18.8 | 15.4 | 52.4 | 32.2 | 19.8 | 125 |
| Case 2. 35/F | 75.1 | 0 | 66.6 | 33.5 | 8.5 | NC | 23 | 0 | 61.1 | 38.8 | 15.7 | NC |
| Case 3. 39/M | 50 | 0 | 67.2 | 32.8 | 12.1 | NC | 18.2 | 0 | 63.8 | 36.2 | 16.7 | NC |
| Mean (n = 3) | 58.2 | 5.2 | 66.7 | 28.4 | 12.9 | NC | 12.9 | 20 | 5.1 | 59.2 | 35.7 | NC |
| SE | 8.2 | 5.2 | 0.2 | 4.7 | 2.8 | NC | 1.5 | 2.7 | 3.4 | 1.9 | 1.2 | NC |
| Case 4. 43/F | 57.6 | 37.6 | 46.6 | 16.5 | 16.7 | 44.4 | 27.8 | 41 | 43 | 16.4 | 20.8 | 52 |
| Case 5. 50/F | 82 | 38.3 | 46.3 | 15.4 | 18.3 | 49.9 | 30.5 | 43 | 39.5 | 17.2 | 31 | 67.6 |
| Case 6. 52/F | 80.9 | 44.2 | 43.3 | 13.4 | 24.9 | 68.5 | 30.1 | 40.3 | 44.7 | 14.4 | 11.7 | 74.6 |
| Ex large | 123.5 | 42.5 | 46.8 | 10 | 19.7 | 48.8 | ||||||
| Case 7. 61/F | 83.1 | 36.4 | 35.4 | 27.7 | 23.7 | 59.2 | 30.7 | 39.2 | 33.1 | 27.9 | 21.8 | 57 |
| Ex large | 133.5 | 32.2 | 39.2 | 28.2 | 16.5 | 51.5 | ||||||
| Case 8. 65/F | 39 | 30.7 | 41.3 | 27.3 | 13.3 | 46.7 | 22.4 | 28 | 49.1 | 22..4 | 15 | 60.3 |
| Case 9. 75/F | 87.1 | 32.8 | 51 | 16.6 | 15.5 | 52.4 | 21 | 35.8 | 39.7 | 24.4 | 26.8 | 74.7 |
| Ex large | 115.4 | 32.2 | 53.9 | 14.2 | 12.2 | 43.1 | ||||||
| Case 10. 77/F | 71.8 | 39.8 | 48.9 | 10.7 | 20.4 | 52.1 | 22.3 | 39.7 | 46.8 | 13.6 | 16.7 | 43 |
| Ex large | 121 | 41 | 47 | 11.7 | 26.7 | 65.3 | ||||||
| Mean (n = 7) | 71.6 | 36.1 | 44.7 | 19.6 | 19 | 53.3 | 26.4 | 38.1 | 42.2 | 19.5 | 20.5 | 61.2 |
| SE | 6.5 | 2.4 | 2 | 3.5 | 1.6 | 3.1 | 1.6 | 1.8 | 2 | 2.1 | 2.5 | 4.4 |
| Ex large (n = 4) | ||||||||||||
| Mean | 123.2 | 37 | 46.7 | 16 | 18.7 | 52.2 | ||||||
| SE | 3.8 | 2.7 | 3 | 4.1 | 3 | 4.7 | ||||||
| Controls (n = 8) | ||||||||||||
| Mean | 71.3 | 58.3 | 29.1 | 11.4 | 34 | 60.8 | 23.6 | 57.6 | 27 | 14.6 | 37.8 | 65.7 |
| SE | 7.2 | 3.1 | 2.6 | 1 | 2.8 | 6.4 | 1.5 | 2.7 | 2.1 | 0.7 | 1.8 | 3.8 |
Ins, insulin; Glu, glucagon; S RIF, somtostatin; IAPP, islet amyloid polypeptide; Ex large, extra large islet; NC, not calculable.
Figure 1.
Control islets. Insulin cells were the major islet cells (about 60%) with abundant cytoplasm of variable staining density, followed by glucagon cells with densely stained, smaller cytoplasm (about 30%) and SRIF cells (about 10%). IAPP positive cells with abundant cytoplasm accounted for about 30% of total islet cells. Both insulin and IAPP immunostaining revealed scattered, dense staining in the irregular, sickle-shaped cytoplasm with lesser staining density for IAPP than that of insulin (*). L, large islet, M, medium sized islet. (A) Insulin, (B) IAPP, (C) glucagon and (D) SRIF immunostained.
Type 1 diabetic islets.
Cases 1–3 had a history of type 1 diabetes for 10–15 years and Cases 2 and 3 died of diabetic ketoacidosis whereas Cases 4–10 had 20–60 year history of type 1 diabetes and died of chronic diabetic complications including diabetic nephropathy and multiple organ failure following macro- and micro-atherosclerosis (Table 1).
Type 1 diabetic islets were divided into insulin-absent islets from three patients younger than 40 years of age and insulin-poor islets from seven patients older than 40 years of age (Table 1). In three patients younger than 40 years of age (Cases 1–3), islets consisted of β-cells with less than 16% of total islet cells or no insulin positive cells whereas glucagon cells were the major cells at 67% and the remaining 20–30% were SRIF cells, twice the ratio of control islets (Table 1 and Fig. 2). In Case 1, IAPP positive cells were about the same as insulin cells whereas in Cases 2 and 3 there were some IAPP positive cells among the totally insulin absent islets (Table 1 and Fig. 2). In Cases 4, 5 and 8, glucagon cells were major cells at 41–46%, more than insulin cells at 30–34% (Table 1). IAPP positive cells were weaker stained than insulin staining and represented 44–69% that of insulin cells (Table 1 and Fig. 3). Four cases (Cases 6, 7, 9 and 10) revealed a mixture of extra-large, large and medium sized islets and extra-large islets, consisted of more than 100 islet cells, containing more islet cells than the control islets (Table 1). In large and medium sized islets, insulin cells revealed abundant cytoplasm and glucagon cells revealed scanty cytoplasm, and both insulin and glucagon cell numbers were about the same whereas IAPP positive cells were much less at about 17–27% of total islet cells, about 1/3 less than that of control islets (Table 1 and Fig. 4). The ratio of IAPP positive cells to insulin cells was less in diabetic islets than control islets except Cases 6, 7, 9 and 10 in whom the ratio was about the same of that of the controls (Table 1). About 10% of diabetic islets consisted of more than 100 islet cells in Cases 6, 7, 9 and 10, containing more islet cells than large control islets (Table 1). The majority were glucagon cells and insulin cells were weakly stained with fewer secretary granules than control β-cells (Fig. 4). IAPP positive staining was even weaker and less granular than insulin staining and some densely IAPP positive staining was noted in sickle-shaped cytoplasms, often without nucleus, which appeared to be degenerating or dying islet cells with more IAPP positive staining than insulin staining in regenerating extra-large islets (Fig. 4). SRIF cells were relatively increased with somewhat abundant cytoplasm (Fig. 4).
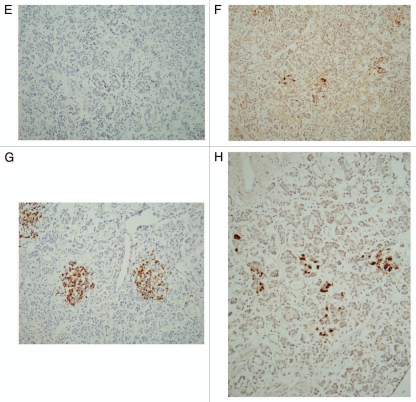
Figure 2A–D.
Type 1 diabetic islets, Case 1 (A–D) and Case 2 (E–H) from patients younger than 40 years of age. Small insulin cells with scanty cytoplasm were minor components and glucagon cells, which were diffusely located in the islets, were the major components at about 66%, whereas IAPP positive cells revealed small and scanty cytoplasm similar to that of insulin cells although IAPP immunostaining was less densely stained than insulin staining in Case 1. (A and E) Insulin, (B and F) IAPP, (C and G) glucagon and (D and H) SRIF immunostained. Type 1 diabetic islets, Case 2 (E–H) from patients younger than 40 years of age. There were no insulin-positive cells in Case 2 but were residual IAPP positive cells in insulin-negative islet cells. SRIF cells were located in mid portion of islets, location of which roughly corresponds to IAPP positive cells. (A and E) Insulin, (B and F) IAPP, (C and G) Glucagon and (D and H) SRIF immunostained.
Figure 3.
Type 1 diabetic islets, Case 5. Insulin cells were less numerous than in control islets at moderate granular staining. IAPP positive cells were much less than insulin cells with granular appearance. Both glucagon and SRIF cells were more numerous than in control islets. (A) Insulin, (B) IAPP, (C) Glucagon and (D) SRIF immunostained.
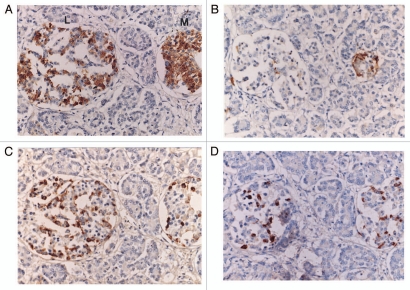
Figure 4.
Type 1 diabetic large (A–D) and extra-large islets (E–H), Case 7 from a patient older than 40 years of age. There were more insulin positive cells than IAPP positive cells with stronger staining for insulin than IAPP staining and major glucagon cells were of about equal numbers in large and medium sized islets (A–D) with glucagon cells diffusely distributed. (A and E) Insulin, (B and F) IAPP, (C and G) Glucagon and (D and H) SRIF immunostained. Type 1 diabetic extra-large islets (E–H), Case 7 from a patient older than 40 years of age. In extra-large islets (E–H), weakly stained, granular insulin cells with abundant cytoplasm were less than glucagon and SRIF cells. Both insulin and IAPP immunostaining was weakly granular with scattered irregular sickle-shaped densely dark cytoplasmic staining without granular appearance (*). IAPP immunostained cells with weakly granular staining were relatively more than insulin cells. (A and E) Insulin, (B and F) IAPP, (C and G) Glucagon and (D and H) SRIF immunostained.
Discussion
By immunocytochemical staining, IAPP was co-localized in β-cells but the presence of IAPP in non-β-cells has been debated and has not been settled in normal islets and diabetic islets.6 Glucagon and SRIF cells have been co-localized with IAPP in fetal islets5 and the other authors also co-localized SRIF cells with IAPP in fetal rat islets7 and in mouse gastric mucosa,8 respectively. Despite co-secretion of insulin and IAPP in the blood stream, IAPP is co-localized with β-cells at 50%5,6 with less densely immunocytochemical staining than that of insulin staining in control islets (Fig. 1). This less IAPP immunostaining in control islets corresponds to less IAPP serum levels at a molar ration of 1:20 compared to insulin levels.9 In type 1 and insulin-requiring type 2 diabetics, IAPP is absent or decreased in serum, thus absent serum IAPP levels correspond to the absent or decreased IAPP in pancreatic islets.9 IAPP also signals the stomach to regulate the gastric emptying and reduces food intake by centrally stimulating satiety12 as circulating IAPP binds to receptors in the hindbrain and increases satiety, delays gastric emptying and suppressing glucagon secretion.13 Insulin replacement therapy in type 1 diabetes is non-physiologic but pharmacologic and thus causes peripheral hyperinsulinemia to achieve normal insulin concentration in the liver and this non-physiological peripheral hyperinsulinemia results in hypoglycemia, increased food intake with weight gain, and insufficient regulation of postprandial glucose excursions.13 A synthetic IAPP, Pramlitide was approved by the FDA for treating type 1 and insulin-requiring type 2 diabetics for a better glycemic control by mainly suppressing postprandial serum glucose surge through suppressing glucagon secretion although a molecular ratio of Pramlitide was much less than that of exogenous insulin.14,15
The pathophysiological significance of IAPP in type 1 diabetes is four folds: first, serum IAPP is markedly decreased or absent in type 1 diabetics; second, IAPP positive cells are markedly decreased or absent in type 1 diabetic islets, which has not been documented to our knowledge; third, markedly decreased or absent serum IAPP is thus due to decreased or absent IAPP positive islet cells, and fourth, concomitant insulin and synthetic Pramilite infusion improves glycemic controls in type 1 diabetes better than insulin injection alone.11,14
In type 2 diabetes, when serum IAPP disappears in the blood stream with impaired glucose tolerance,16,17 soluble IAPP in β-cells disappears9 and non-soluble amyloid deposits start to build around a nidus, which develop to IAPP polymers in the islet stroma, consisting of 20 proteins including amyloid P components and heparan sulfate-type proteoglycans.4 Freshly prepared intermediate IAPP polymers (25–6,000 IAPP molecules) have a toxic effect on β islet cells.17–21 Relocation of granular IAPP-positive β-cell cytoplasm with small molecular weight to perivascular amorphous amyloid deposits without granular appearance consisting of larger IAPP polymer (>106 molecules per particle) is much debated subjects as cause or result for type 2 diabetes.4,17–21 Interstitial amyloid deposits in islets are characteristic for type 2 diabetes and do not occur in type 1 diabetic islets.
There were different islet cell components depending on the age of type 1 diabetics: in three patients younger than 40 years of age, islets were generally smaller and atrophic, consisting of 50 small non-β-cells per islet, and β-cells were markedly reduced in Case 1 and totally absent in Cases 2 and 3, most likely as a result of insulin depletion after diabetic ketoacidosis (Table 1). IAPP positive cells were present at the compatible number of remaining β-cells in Case 1, and were also identified in about 10% of insulin-negative cells, in whom some insulin-negative cells were positively stained for IAPP (Table 1, Figs. 2 and 4) whereas in Case 2 and 3, insulin staining was totally absent but IAPP staining still retained, suggesting a total insulin depletion but a partial IAPP retaining during diabetic ketoacidosis. In all three cases, glucagon cells were the major islet cells, followed by SRIF cells. In seven patients older than 40 years of age, who died of chronic diabetic complications, glucagon cells with small cytoplasm were the major cells, followed by insulin cells with abundant cytoplasm and SRIF cells with an intermediate sized cytoplasm between insulin cells and glucagon cells (Fig. 2). IAPP positive cells were about 50% that of insulin cells (Table 1). In four cases (Cases 6, 7, 9 and 10), about 10% of islet cells were extra-large and consisted of more than 100 islet cells, containing more islet cells than control islets (Table 1). These extra-large regenerating islets consisted of the major glucagon cells with smaller cytoplasm than β-cells, followed by insulin cells and SRIF cells at the same IAPP:insulin cell ratio of 1:2 as also seen in the other islets (Table 1) and the relative percentage of insulin, glucagon, SRIF and IAPP positive cells and IAPP:insulin cell ratio were about the same in the three different sizes of islets (Table 1). In two cases older than 40 years of age, the total cell numbers in large islets were 30–40 cells per islet, smaller than control large islets, but islet cell components were similar to that of large diabetic islets, with glucagon cells 10% more than insulin cells (Table 1). Thus, in all three patients younger than 40 years of age (Cases 1, 2 and 3) and two cases older than 40 years (Cases 4 and 8), islet cell numbers were less than controls (Table 1).
Despite all 10 patients presented typical clinical type 1 diabetes, islet cell components were quite variable: in younger than 40 years of age, β-cells were markedly reduced or totally absent, which resulted from total insulin depletion and caused diabetic ketoacidosis in Cases 2 and 3 without histologic evidence of islet cell regeneration, and in older than 40 years, insulin-positive β-cells were less than glucagon cells but more than SRIF cells (Table 1). IAPP positive cells were generally less in three patients younger than 40 years, but two of three patients retained IAPP-positive cells in insulin-negative islets, supporting at least some insulin-depleted β-cells and non-β-cell granules retained IAPP immunostaining. IAPP-positive cells were generally less in seven patients older than 40 years, and also IAPP: insulin ratio was less in five of seven patients than the controls (Table 1). Despite IAPP positive staining in type 1 diabetic islets, immunostaining intensity was much less than solidly granular staining in control insulin staining (Figs. 1, 2 and 4). Thus, it appears that decreasing insulin-stained and insulin-negative β-cells correspond to decreasing IAPP immunostaining in insulin-negative islets, supporting a partial presence of IAPP in insulin-depleted β-cells and non-β-cells including glucagon and SRIF cells.
The possible presence of IAPP in non-β-cells is supported by IAPP immunostaining in non-β-cell pancreatic endocrine tumors (PETs).21 Rindi et al. reported 20 of 21 PETs (95%) consisting of 10 insulinomas, 1 glucagonoma, 2 gastrinomas, 1 vipoma and 7 non-functioning tumors were at least partially positive for IAPP.22 Using the same IAPP antibody as used by us, Eissele et al. reported in 65 cases of PETs in which 13 of 15 insulinomas (85%), 3 of 21 gastrinomas (14%) and 2 of 20 non-functioning tumors (7%) were positive for IAPP with a total of 18 of 65 cases (28%) being positive for IAPP.23 PETs are derived from pleuripotential gastropancreatic endocrine cells and are not the same cell lines as seen in the residual degenerating cells in medium-sized and large islets and regenerating cells in extra large islets from type 1 diabetics.
Materials and Methods
All cases of pancreatic tissues from type 1 diabetes and non-diabetic controls were collected at autopsy in the University of Kansas Medical Center between 1980 and 2000. A total of ten cases of type 1 diabetic pancreata were studied together with eight cases of age-matched non-diabetic controls. All tissues were routinely fixed in buffered formalin and were embedded in paraffin. Deparaffinized sections were treated with antigen retrieval procedure using citrate buffer pH 6.2. All staining procedures were same as previously reported except for IAPP immunostaining, in which rabbit antihuman IAPP 1–13 was used at 1:400 and 1:800 dilutions (Peninsula Laboratory, San Carlos, CA). All the continuous sections were systematically immunostained for insulin, glucagon, SRIF and IAPP. The counting immunopositive cytoplasm for insulin, glucagon, SRIF and IAPP was performed by counting positively stained cytoplasms at 10 × 20 = ×200 to cumulatively count the total islet cell numbers by adding all hormone positive cells per islet for both diabetic and control islets, with which relative percentage of insulin, glucagon, SRIF and IASPP positive cells were calculated by dividing the each hormone positive cells by the total islet cell numbers. The islets were divided into large islets containing more than 34 islet cells and medium sized islets containing 14–33 islet cells excluding small islets of less than 14 islet cells as these small islets or parts of large and medium sized islets provided large variations of islet cell compositions and IAPP cells. Type 1 diabetic islets were calculated for the total islet cells including large, medium sized and extra-large islets and in four diabetic cases (Cases 6, 7, 9 and 10), who presented extra-large regenerating islets with more than 100 islet cells, which were calculated for extra-large islets and were listed separately from large and medium sized islets.
Acknowledgments
I sincerely thank Dr Ov Slayden for kindly allowing me to use his research laboratory to perform immunocytochemical staining at Reproductive Science Division, Oregon National Primate Center, Beaverton, Oregon. This study was supported in part by ONRRC Core Grant: NIH RR 000163.
References
- 1.Westermark P, Westermark C, Wilander E, Stetten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem Biophys Res Commun. 1986;140:827–831. doi: 10.1016/0006-291x(86)90708-4. [DOI] [PubMed] [Google Scholar]
- 2.Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreas of type 2 diabetic pancreas. Proc Natl Acad Sci USA. 1987;84:8627–8631. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn S, Andrilopuis S, Verchere CB. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–253. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- 4.Hooper JWM, Ahren B, Lips CM. Islet amyloid and type 2 diabetes mellitus. N Eng J Med. 2000;343:411–419. doi: 10.1056/NEJM200008103430607. [DOI] [PubMed] [Google Scholar]
- 5.Portala-Gomes GFM, Johansson H, Olding L, Grimelius L. Co-localization of neuroendocrine hormones in the fetal pancreas. Eur J Endocrinol. 1999;141:526–533. doi: 10.1530/eje.0.1410526. [DOI] [PubMed] [Google Scholar]
- 6.Iki K, Pour PM. Distribution of pancreatic endocrine cells including IAPP-expressing cells in non-diabetic and type-2 diabetic cases. J Histochem Cytochem. 2007;55:111–118. doi: 10.1369/jhc.6A7024.2006. [DOI] [PubMed] [Google Scholar]
- 7.Verchere CB, D'Alessio DA, Pringeon R, Hill RL, Kahn SE. The constitutive pathway is a major route for islet amyloid polypeptide secretion in neonatal but not adult rat islet cells. Diabetes. 2000;49:1477–1484. doi: 10.2337/diabetes.49.9.1477. [DOI] [PubMed] [Google Scholar]
- 8.Tingstedt JE, Edlund H, Madsen OD, Karssib LI. Gastric amylin expression: Cellular identity and lack of requirement for the hemeobox protein PDX-1 deficient animals with a cautionary note on antiserum evaluation. J Histochem Cytochem. 1999;47:973–980. doi: 10.1177/002215549904700801. [DOI] [PubMed] [Google Scholar]
- 9.Kruger DF, Gatcomb PM, Owen SK. Clinical implications of amylin and amylin deficiency. Diabetes Educ. 1999;25:389–397. doi: 10.1177/014572179902500310. [DOI] [PubMed] [Google Scholar]
- 10.Kolterman OG, Gottlieb A, Moyses C, Colburn W. Reduction of postprandial hyperglycemia in subjects with IDDM by intravenous infusion of AC 137, a human amylin analogue. Diabetes Care. 1995;18:1179–1182. doi: 10.2337/diacare.18.8.1179. [DOI] [PubMed] [Google Scholar]
- 11.Weyer C, Maggs DG, Young AA, Kolterman OG. Amylin replacement with Pramlintide as an adjuvant to insulin therapy in type 1 and type 2 diabetes mellitus: a physiological approach toward improved metabolic control. Curr Pharm Des. 2001;7:1353–1373. doi: 10.2174/1381612013397357. [DOI] [PubMed] [Google Scholar]
- 12.Woods SC, Lutz TA, Geary N, Langhans W. Pancreatic signals controlling food intake, insulin, glucagon and amylin. Phil Trans R Soc. 2006;361:1219–1235. doi: 10.1098/rstb.2006.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labovitz HE. Adjunct therapy for type 1 diabetes mellitus. Nat Rev Endocrinol. 1010;6:326–334. doi: 10.1038/nrendo.2010.49. [DOI] [PubMed] [Google Scholar]
- 14.Buse JB, Weyer C, Maggs DG. Amylin replacement with Pramlintide in type 1 and type 2 diabetes: a physiological approach to overcome barriers with insulin therapy. Clin Diabetes. 2002;20:137–144. [Google Scholar]
- 15.Fineman M, Weyer C, Maggs DG, Stobel S, Kolterman OG. The human amylin analog, Pramlintide reduces postprandial hyperglucagonemia in patients with type 2 diabetes mellitus. Horm Metab Res. 2002;34:504–508. doi: 10.1055/s-2002-34790. [DOI] [PubMed] [Google Scholar]
- 16.Makimattilla S, Finemanm MS, Yki-Jarrivinen H. Deficiency of total and non-glycosylated amylin in plasm characterizes subjects with impaired glucose tolerance and type 2 diabetes. J Clini Endocrinol Metab. 2000;85:2822–2827. doi: 10.1210/jcem.85.8.6721. [DOI] [PubMed] [Google Scholar]
- 17.Hull RL, Westrmark GT, Westermark P, Kahn SE. Islet amyloid: a critical entity in pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3629–3643. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 18.Lorenzo A, Razzabony B, Weir GC, Yankner BA. Pancreatic islet toxicity of amylin associated with type 2 diabetes mellitus. Nature. 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 19.Jaikaran E, Clark A. Islet amyloid and type 2 diabetes: from molecular, missfolding to islet pathophysiology. Biochem Biophy Acta. 2001;1537:179–203. doi: 10.1016/s0925-4439(01)00078-3. [DOI] [PubMed] [Google Scholar]
- 20.Marzbn A, Park K, Verchere CB. Islet amyloid polypeptide and type 2 diabetes. Exper Gerontol. 2003;38:347–351. doi: 10.1016/s0531-5565(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 21.Kloppel G, Perren A, Heitz P. The gastrointestinal neuroendocrine cell system and its tumors. The WHO classification. Ann NY Acad Sci. 2004;1014:13–27. doi: 10.1196/annals.1294.002. [DOI] [PubMed] [Google Scholar]
- 22.Rindi G, Terenghi G, Westermark G, Wstermark P, Moscoso G, Polak J. Islet amyloid polypeptide in proliferating pancreatic B cells during develoment. Am J Pathol. 1991;138:1321–1331. [PMC free article] [PubMed] [Google Scholar]
- 23.Eissele R, Neuhaus C, Trautmann ME, Funk A, Arnold R, Hofler H. Immunoreacting and expression of amylin in gastropancreatic endocrin tumors. Am J Pathol. 1993;143:283–291. [PMC free article] [PubMed] [Google Scholar]