Abstract
Interferon-γ (IFN-γ) and perforin (pfp) are important effector mechanisms used by CD8 T cells to clear virus-infected cells. In this study, we used IFN-γ/pfp double knockout mice to address if these two effector molecules play redundant roles in the control of acute infection with murine gammaherpesvirus-68 (MHV-68) in BALB/C mice. Perforin knockout (KO) mice and wild-type mice cleared infectious virus from the lungs, even following high-dose infection. However, the IFN-γ KO and IFN-γ/pfp double knockout (DKO) groups had higher virus titers in the lungs at day 10 post-infection, and both groups had higher mortality rates. In IFN-γ/pfp DKO mice, the virus titer and mortality rate were significant higher than in IFN-γ KO mice, indicating a role for perforin in protection from disease. WT mice given IFN-γ blocking antibody also showed significantly higher viral titers. In contrast, IFN-γ KO mice on a C57BL/6 background controlled respiratory infection comparably to wild-type mice. These data show that perforin plays a redundant role in the control of virus replication, but IFN-γ plays an essential role in BALB/C mice infected with MHV-68. We conclude that there is a marked strain-dependent difference in the effector mechanisms needed to control acute MHV-68 infection between C57BL/6 and BALB/C mice. In addition we show that immune therapy that re-establishes viral control after spontaneous reactivation in CD4-deficient mice depends upon perforin in C57BL/6 mice but IFN-γ in BALB/C mice.
Introduction
The human gammaherpesviruses, Epstein-Barr virus, and human herpesvirus-8, cause significant disease in immune suppressed patients, indicating that immune surveillance is critical for long-term control of these infections. However, we do not know exactly which immune effector mechanisms are necessary to control the gammaherpesviruses. Murine gammaherpesvirus-68 (MHV-68) is a natural pathogen of small rodents and is thought to spread via the respiratory route. Mice inoculated intranasally with the virus develop an acute infection in alveolar epithelial cells, then the virus remains in the host in a latent form mostly within B lymphocytes (8,33,38). The virus has also been observed to persist in lung epithelial cells, dendritic cells, and macrophages (7,30,39). Infection with MHV-68 in laboratory mice shares important biological features with gammaherpesvirus infection in humans, such as a tropism for B cells and associations with lymphoproliferative disease (33,34). MHV-68 has proven to be a valuable experimental small animal model for studying the pathophysiology and immunology of gammaherpesvirus infection in the natural host (22,24).
Control of MHV-68 in the lung is known to be dependent largely on CD8 T cells (6), and depletion of these cells also results in elevated numbers of latently infected B cells in the spleen (6). Similarly, in EBV infection it is known that CTLs prevent the uncontrolled growth of immortalized B lymphocytes, and individuals lacking an adequate CTL response can develop lymphoproliferative disease (12,41). Interferon-γ (IFN-γ) and perforin (pfp) are important effector mechanisms used by CD8 T cells to clear virus-infected cells. Perforin is a cytolytic protein found in CD8 T cells and NK cells, and is critical in the control of several virus infections (13). Previous studies in C57BL/6 mice showed that control of MHV-68 infection was independent of perforin (37). Studies also have shown that MHV-68 induces high levels of IFN-γ production in the spleens and lymph nodes (26). IFN-γ contributes to protection against multiple different viral infections, including those of the herpesvirus family. Among the infections in which a protective role of IFN-γ has been demonstrated are infections with hepatitis B virus (11,19), herpes simplex virus (2), and lymphocytic choriomeningitis virus (21), but mice lacking IFN-γ are resistant to influenza virus infection (10). Previous studies in the MHV-68 system, infecting mice by the IP route, showed that IFN-γ is important for containing the latent infection (29,35). Original studies using the IN route of infection indicated that IFN-γ was dispensible for controlling the respiratory infection (26). The finding that perforin-deficient mice also controlled the lung infection (37) suggested that perforin and IFN-γ operated in a redundant fashion. However, a recent report showed that BALB/C mice lacking IFN-γ failed to control the lung infection and developed a fatal pneumonia (17). Given these discrepancies in the literature, and the fact that some studies focused on the BALB/C strain and others the C57BL/6 strain, we tested the role of IFN-γ in acute respiratory MHV-68 infection.
In the mouse, immune responses and the outcome of infection vary depending on the genetic make-up of the host. Moreover, it has become clear that in humans, the outcome of viral infection may be influenced by the expression of allelic variants of specific genes (14). There are numerous reports of mouse strain differences in controlling infection with various viruses, bacteria, and parasites (1,16,27). For example, in the murine cytomegalovirus model, infection of C57BL/6 mice results in resistance to infection, but in BALB/C mice the virus replicates to high titers in visceral organs (31). In vesicular stomatitis virus (VSV) infection by the intranasal route, studies demonstrated the difference between BALB/C and 129SvEv mice; high levels of virus were present in the lungs of BALB/C but not 129SvEv mice (4).
There have been no reports of strain-dependent differences in susceptibility to MHV-68 infection; however, there are reports showing differences in chemokine levels or Vβ4 T-cell expansion between C57BL/6 and BALB/C mice following infection (36,39). Although BALB/C and C57BL/6 mice both control viral replication and latency similarly after MHV-68 infection, here we show that the mechanisms used for viral control differ between these mouse strains. We use IFN-γ/pfp double knockout mice to address if IFN-γ and perforin play redundant roles in the control of acute infection with MHV-68 in BALB/C mice. Comparison of the viral titers in wild-type control and three knockout mice strains (IFN-γ KO, pfp KO, and IFN-γ/pfp DKO) showed that IFN-γ was critical for viral control. In addition, wild-type mice given anti-IFN-γ blocking antibody had significantly higher viral titers in the lung. These data showed that perforin plays a redundant role, but IFN-γ plays an essential role in BALB/C mice infected with MHV-68. We conclude that there is a marked strain-dependent difference in the effector mechanisms needed to control acute MHV-68 infection between C57BL/6 and BALB/C mice. Consistent with these data, immune therapy that re-establishes viral control after spontaneous reactivation in CD4-deficient mice, which has been shown to require perforin in C57BL/6 mice (20), operates instead via IFN-γ in BALB/C mice.
Materials and Methods
Mice, virus, and reagents
MHV-68 virus (clone G2.4) was originally obtained from Dr. A. A. Nash (University of Edinburgh, Edinburgh, U.K.). Virus was propagated and titered as previously described. For MHV-68 infections, mice were infected intranasally (IN) with 400 PFU under anesthesia with ketamine/xylazine. BALB/C mice were purchased from The National Cancer Institute (Bethesda, MD). C57BL/6 IFN-γ KO mice were purchased from the Jackson Laboratory (Bar Harbor, ME). IFN-γ KO, perforin KO, and IFN-γ/pfp DKO mice on the BALB/C background were obtained from Dr. W. Green (Dartmouth Medical School, Lebanon, NH), and bred in the Dartmouth-Hitchcock Medical Center mouse facility. The Animal Care and Use Program of Dartmouth College approved all animal experiments.
Viral plaque assay
MHV-68 titers in the lungs were determined by plaque assays as previously described (32). Lungs were homogenized in 1 mL of medium and then subjected to one freeze-thaw cycle. Samples were serially diluted and assayed on a monolayer of 3T3 cells in minimal volume. The virus was allowed to adsorb for 1 h before being overlaid with carboxymethyl cellulose. After 5 d of incubation at 37°C, the assay mixtures were fixed with a solution of 100% methanol for 15 min and stained with Giemsa stain for 4 h, and then the plaques were enumerated microscopically.
Tissue preparation
Single-cell suspensions of splenocytes were prepared by passing through cell strainers. For the preparation of lung lymphocytes, lungs were injected with 2 mL of MEM containing 2.33 mg/mL collagenase (Sigma-Aldrich, St. Louis, MO) and 200 μg/mL DNase I (Roche Diagnostics Corp., Indianapolis, IN), minced with scissors, then incubated for 30 min at 37°C, and passed through cell strainers, washed, counted, and stained for flow cytometric analysis.
Depletion of CD4+ lymphocytes
For CD4 depletion, mice were given 500 μg of GK1.5 mAb 1 d before infection, 500 μg at the time of infection, and 200 μg of GK1.5 twice per week for the duration of the experiment.
Antibody staining and flow cytometric analysis
Cells were stained with the following markers: PerCP eFluor710-conjugated anti-CD8a (53-6.7), PE-conjugated anti-CD4 (RM4-5), PE-conjugated anti-MHC class II (I-A/I-E) (M5/114.15.2), FITC-conjugated anti-CD11b (M1/70), and APC-conjugated anti-CD11c (N418). Staining was done as previously described (23). Analysis was done using Accuri C6 software.
Granzyme B ELISPOT assay
The frequency of granzyme B-secreting cells was determined after stimulation with peptides in an ELISPOT assay. The Granzyme B ELISPOT kit was purchased from R&D Systems, Inc. (Minneapolis, MN). The CD8 T-cell epitope derived from the ORF65 (131–140, LGPDKSGLGF) protein was synthesized as peptide and used in this study. Peptide was purchased from New England Peptide (Gardner, MA). In brief, the anti-granzyme B antibody-coated plates were blocked with sterile culture media and then irradiated (3000 RAD). BALB/C spleen cells (5 × 105 cells/well) were added, together with a graded number of responder spleen cells, 2 μg/mL of peptide, and 10 U/mL recombinant human IL-2 (Tecin; National Cancer Institute). The plates were then incubated for 24 h at 37°C. A biotinylated polyclonal mouse granzyme B antibody was added and then incubated at 4°C overnight, followed by streptavidin-alkaline phosphatase for 2 h at room temperature. Following addition of the BCIP/NBT Chromogen, visible spots were enumerated using a dissecting microscope. The frequency of CD4 T cells producing granzyme B was calculated, together with the total numbers per spleen.
IL-2 immune complex treatment
MHV-68-infected intact or CD4-depleted mice were injected with either 50 μg of anti-IL-2 mAb (clone S4B6) mixed with 1.5 μg of murine IL-2 (mIL-2; eBioscience, San Diego, CA), or 50 μg of control rat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) IP daily for 4 d. Treatment began 42 d after IN infection for all experiments.
IFN-γ blockade
Mice were given IP injections of the IFN-γ blocking antibody R4-6A2 (1 mg/mouse) on day −1 and 0 of challenge, and then on days 2, 4, 6, and 8 post-infection.
Statistical analysis
p Values were calculated using Student's t-test or the Mann-Whitney U test.
Results
Viral control and survival of MHV-68-infected BALB/C mice depends upon IFN-γ
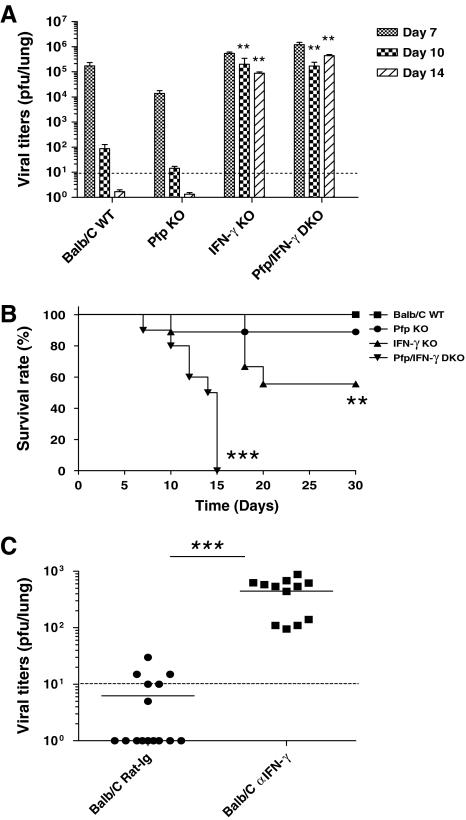
In order to study whether IFN-γ and perforin play redundant roles in controlling MHV-68 infection, BALB/C WT, pfp KO, IFN-γ KO, and pfp/IFN-γ DKO mice were infected with 400 PFU MHV-68 IN. Lungs were taken at day 7, 10, or 14 after infection, homogenized, and assayed for virus titer. The mice were also monitored for survival. In all four independent experiments, virus was controlled at day 14 after infection in WT and pfp KO mice (Fig. 1A). Virus could not be controlled in IFN-γ KO and pfp/IFN-γ DKO mice, as the viral titers were significantly higher in IFN-γ KO and pfp/IFN-γ DKO than BALB/C WT and pfp KO mice. IFN-γ was also important in survival following infection. BALB/C WT mice all survived the infection, the survival rate of pfp KO mice was 80%, and the survivors were healthy at 3 mo post-infection (Fig. 1B). IFN-γ KO mice were sick at day 10 and the survival rate was approximately 50%; however, the survivors remained healthy at 3 months post-infection. Pfp/IFN-γ DKO mice were very sick at day 7, and by day 15 all were moribund and were euthanized according to the Dartmouth College IACUC guidelines. These results suggested that IFN-γ was the critical mechanism to control virus replication, but both IFN-γ and perforin are necessary for optimal protection from disease caused by this virus. However, it was possible that the results obtained in the knockout strains were an artifact caused by the lack of key effector mechanisms in these strains. To test for this, wild-type BALB/C mice were given anti-IFN-γ blocking antibody or control rat-IgG antibody at days –1, 0, 2, 4, 6, and 8 relative to intranasal infection. Lungs were taken at day 10 and plaque assays performed. Viral titers in mice given anti-IFN-γ antibody were significantly higher than the control group (p < 0.001) (Fig. 1C). These data showed that IFN-γ was important for the clearance of virus from the lung in BALB/C mice, and the data were not due to artifacts from the use of transgenic knockout strains.
FIG. 1.
Control of respiratory MHV-68 infection depends upon IFN-γ in BALB/c mice. (A) Mice were infected with 400 PFU of MHV-68 intranasally. Lungs were harvested at days 7, 10, and 14 after infection, homogenized, and assayed for virus titer. The limit of detection was 10 PFU per lung (dashed line). (B) Survival of infected mice was observed over a period of 30 d. The Kaplan-Meier survival curve is shown for groups of 10 to 15 mice. (C) BALB/C mice were given IFN-γ-blocking antibody at days −1, 0, 2, 4, 6, and 8 relative to infection, and infected with 400 PFU MHV-68 intranasally at day 0. Lungs were removed at day 10 and plaque assays performed. Each dot represents data from an individual animal and horizontal bars indicate the mean. Data are representative of 3–4 experiments (**p < 0.01, ***p < 0.001).
A mouse strain-dependent role for IFN-γ in the control of MHV-68
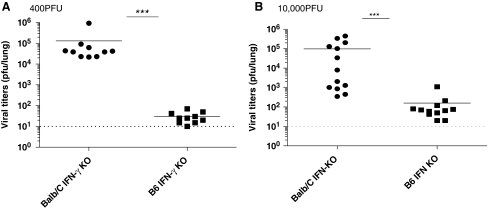
Previous data indicated that IFN-γ played no role in lung infection with MHV-68; however, most studies were performed with C57BL/6 mice. To determine if the role of IFN-γ was strain-dependent, C57BL/6 IFN-γ KO and BALB/C IFN-γ KO mice were infected with a low dose (400 PFU) or high dose (10,000 PFU) of MHV-68 intranasally. Lungs were taken at day 10 post-infection and virus titers measured using plaque assay. Viral titers in C57BL/6 IFN-γ KO mice infected with either dose of MHV-68 were very low or undetectable at day 10 post-infection. However BALB/C IFN-γ KO mice were sick and viral titers were significantly higher than in C57BL/6 IFN-γ KO mice (p < 0.001) (Fig. 2A and B). These results showed that IFN-γ plays a strain-dependent role in controlling MHV-68 replication. IFN-γ was not essential to control virus on the C57BL/6 genetic background during the acute respiratory phase of infection, but it was critical on the BALB/C background.
FIG. 2.
C57BL/6 mice do not require IFN-γ to control MHV-68 in the lung. Mice were infected with 400 PFU (A) or 10,000 PFU (B) of MHV-68 intranasally and lungs were removed at day 10 post-infection. The viral titers were measured using a standard plaque assay. Each dot represents data from individual animals and horizontal bars indicate the mean. Data are representative of three experiments. The limit of detection was 10 PFU per lung (***p < 0.001).
Examination of inflammatory cell populations and effector function in WT and IFN-γ KO mice
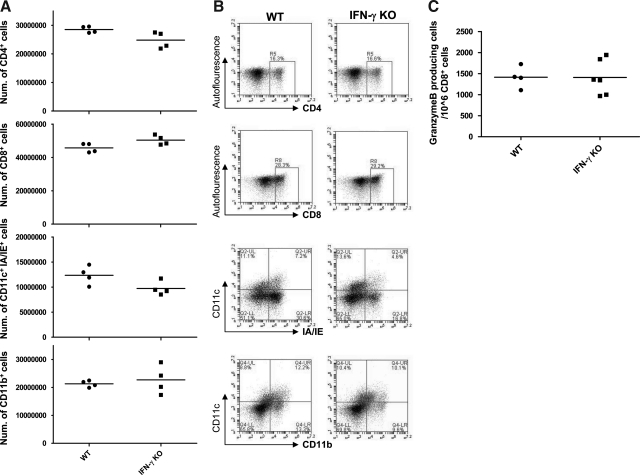
It was important to examine if the absence of the IFN-γ gene would affect the cell populations recruited to the lungs after infection. We therefore isolated whole lung inflammatory infiltrates from the lungs of MHV-68-infected WT and IFN-γ KO mice, and then measured the frequencies of CD4 T cells, CD8 T cells, and antigen-presenting cell populations (defined by the markers CD11c, CD11b, and MHC class II). Total numbers of these cell populations in the lungs at day 10 post-infection were not significantly different between the WT and IFN-γ KO mice (Fig. 3A, representative plots shown in Fig. 3B). Similar data were obtained from the spleens (data not shown). The frequency of NK cells in the lungs was also equivalent between the two groups (data not shown). Furthermore, to test if other effector functions of CD8 T cells were affected by the lack of IFN-γ, we used ELISPOT analysis to measure the frequency of granzyme B-producing CD8 T cells in WT and IFN-γ KO mice (Fig. 3C). No difference was detected between the two groups, indicating that the absence of IFN-γ does not affect other effector mechanisms in CD8 T cells. We conclude that the absence of IFN-γ does not have indirect effects on other cell populations or CD8 T-cell effector functions that would account for the lack of viral control in IFN-γ KO mice.
FIG. 3.
Absence of IFN-γ does not affect recruitment of other cell populations or other CD8 T-cell effector functions. (A) Total cell numbers of various cell populations in the lungs at day 10 post-infection. (B) Representative FACS plots showing the distribution of cells of various phenotypes in the lungs at day 10 post-infection. Numbers indicate percentages of the relevant populations in the gates shown. (A and B) Data are representative of three experiments. (C) Frequencies of granzyme B-producing CD8 T cells. Granzyme B ELISPOT was performed at day 11 post-infection. Numbers indicate the frequency of granzyme B-producing cells among the CD8 T-cell population. Each dot represents data from an individual animal and horizontal bars indicate the mean. Data are representative of two experiments.
Immune therapy for virus reactivation depends upon IFN-γ in BALB/C mice
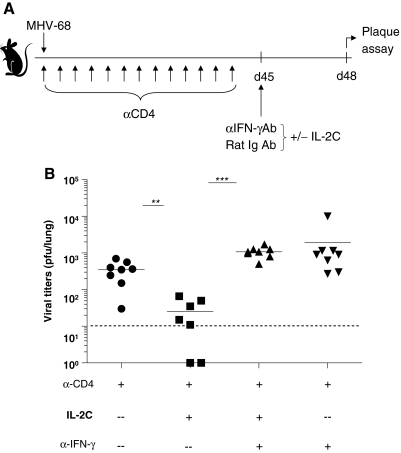
Previous studies reported that mice lacking CD4 T cells controlled acute infection with MHV-68, and no virus was detected in the lungs ∼2 wk post-infection. However, the virus spontaneously reactivated in the lungs around day 40 post-infection (3). Administering IL-2 immune complexes (a combination of IL-2 and anti-IL-2 mAb [S4B6]) to CD4-deficient mice improved immune surveillance and dramatically reduced the viral burden (20). This effect was observed in C57BL/6 mice, and was mediated by enhancement of the perforin-granzyme pathway; however, blockade of IFN-γ had no effect on the immune therapy (20). As control of virus replication in BALB/C mice was much less dependent upon perforin/granzyme, we tested whether supplying IL-2 immune complexes could decrease chronic viral replication, and whether the antiviral effect was mediated by IFN-γ in this strain. Mice were depleted of CD4 T cells using a depleting antibody at day −1 day prior to infection with MHV-68, and was continued by administering antibody twice per week. After 45 d, mice were given IL-2 complex, anti-IFN-γ antibody, or rat IgG for 4 d IP, as shown in Fig. 4A. CD4-depleted mice had high levels of reactivating virus as previously reported for C57BL/6 mice. Mice treated with IL-2 complex had significantly lower levels of virus in the lungs, demonstrating that this immune therapy also worked in the BALB/C strain (Fig. 4B). However, when IFN-γ was blocked with a neutralizing antibody, the effect of the IL-2 complex was negated. This demonstrates that IL-2 complex immune therapy depends upon IFN-γ in the BALB/C strain, in contrast to C57BL/6 mice, in which the perforin/granzyme system is required.
FIG. 4.
IL-2C immune therapy reduces the viral load and depends upon IFN-γ in CD4-depleted BALB/C mice. (A) Mice were depleted of CD4 T cells starting at day −1 relative to infection, and continuing for the remainder of the experiment. After 45 d, mice were given 4 daily injections of IL-2C plus either rat-Ig or anti-IFN-γ-blocking Ab. Viral titers were determined by plaque assay at day 48. (B) Each dot represents data from an individual animal and horizontal bars indicate the mean. The limit of detection was 10 PFU per lung (α-CD4, anti-CD4; α-CD8, anti-CD8; α-IFN-γ, anti-IFN-γ). Data are representative of three experiments (**p < 0.01, ***p < 0.001).
Discussion
Interferon-γ (IFN-γ) and perforin are both important effector mechanisms used by CD8 T cells to combat virus infections. Previous studies have shown roles for both these effector mechanisms after IP infection with MHV-68, with IFN-γ playing the dominant role in the peritoneum, whereas perforin was dominant in the spleen (35). However, the mechanism underlying the control of the virus in the lung has remained obscure, as mice deficient in either of these effector pathways have been reported to control the respiratory infection (5,26,37). The majority of previous studies used the C57BL/6 mouse strain; however, in this report we show in BALB/C mice a very clear role for IFN-γ in clearance of virus from the lungs, with perforin playing an auxiliary role, particularly in protection from mortality.
Variations between the effector mechanisms employed by different mouse strains are important, as the genetic diversity in the human population will likely result in variations in the effector mechanisms used by individual patients in controlling virus infections. Genetic studies have revealed that the host response is not only influenced by individual genes, but also by gene combinations and polymorphic variants of susceptibility genes. Mouse model systems have revealed that hundreds of genes are involved in the host defense against infections, and that there is a complex interaction between these different host factors (1,16,27). Several studies in diverse infection models, such as murine cytomegalovirus (MCMV), VSV, and influenza A virus, demonstrated differences according to the genetic background of the host (4,27,31). Pertinent to our study, IFN-γ has been shown to prevent EBV-induced B-cell transformation (18), and inhibit HHV-8 production (25), indicating that it likely plays an important role in human gammaherpesvirus infections. Another study reported differences in the immune response between C57BL/6 and BALB/C mice infected with MHV-68, with several differences seen in chemokine production (40). IFN-γ was higher in BALB/C mice than C57BL/6 mice, but viral titers were also higher, indicating no correlation with increased resistance to infection (40). Also, MHV-68-induced lymphomas in the absence of MHC class I-mediated immune surveillance preferentially occur in the BALB/C background (34). Lastly, we have noted a higher level of spontaneous reactivation that occurs in CD4-depeleted mice on the BALB/C background compared with the C57BL/6 background (Molloy et al., unpublished observations), which adds to the evidence that these two strains make distinctly different responses.
In earlier studies authors reported that BALB/C mice deficient in IFN-γ resist MHV-68 infection (26). While the reasons why our data differ from this study are not clear, our findings are supported by a more recent report from different investigators showing that BALB/C mice depend upon IFN-γ to control the acute infection (17). Our studies extend these observations by showing that dependency upon IFN-γ is not an artifact produced by the use of transgenic mice, as IFN-γ blocked by a neutralizing antibody in wild-type mice also prevented control of the virus. We also show that IFN-γ is redundant in controlling the virus in C57BL/6 mice, and that an immune therapy that relies upon perforin/granzyme B in the C57BL/6 strain instead requires IFN-γ in BALB/C mice. Previous studies have shown that IFN-γ inhibited reactivation of MHV-68 from latency in a cell type-specific manner. IFN-γ inhibits reactivation of MHV-68 in peritoneal cells but not splenocytes. These studies indicated that macrophages are responsive to IFN-γ-mediated suppression of MHV-68 reactivation to a greater extent than B cells (28,29). In the lung the majority of virus replication occurs in lung epithelial cells (33), although further investigation is required to determine whether IFN-γ acts directly or indirectly on these cells during acute respiratory infection. Control of the acute stage of MHV-68 infection in the lung has been shown to depend upon T cells (6), and both CD4 and CD8 T cells produce IFN-γ in this infection, so these are the likely sources of IFN-γ.
One obvious question raised by these studies is whether IFN-γ continues to be the dominant factor controlling MHV-68 during persistent infection in BALB/C mice. In preliminary studies we neutralized IFN-γ after the establishment of a latent infection in BALB/C CD28−/− mice. This strain was chosen because the lack of a neutralizing antibody response allows virus reactivation in the lungs to be measured, and virus reactivation is contained by T cells alone (9,15). We observed no virus reactivation upon IFN-γ neutralization, which may indicate that, unlike during the acute infection, during virus persistence other mechanisms may also be critical for viral control. Despite the importance of IFN-γ in BALB/C mice, perforin does appear to have a role in disease pathology, as pfp/IFN-γ DKO mice became sicker and exhibited higher mortality than IFN-γ KO mice. The reason for this requires further investigation, but is likely due to a reduction in pneumonia or lung pathology in the presence of perforin.
In conclusion, this report illustrates the central role of IFN-γ in controlling MHV-68 infection in the lung. IFN-γ is critical in the BALB/C strain; however, it is of less importance during the acute respiratory infection in the C57BL/6 strain. Importantly, this dependence upon IFN-γ was also seen when using an IL-2-based immunotherapy, a therapy that depends upon the perforin/granzyme system in the C57BL/6 strain (20). This demonstrates that immune-based therapies that control virus infections will likely operate through different mechanisms depending on the genetics of the host.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Buer J. Balling R. Mice, microbes and models of infection. Nat Rev Genet. 2003;4:195–205. doi: 10.1038/nrg1019. [DOI] [PubMed] [Google Scholar]
- 2.Cantin E. Tanamachi B. Openshaw H. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J Virol. 1999;73:3418–3423. doi: 10.1128/jvi.73.4.3418-3423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardin RD. Brooks JW. Sarawar SR. Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durbin RK. Mertz SE. Koromilas AE. Durbin JE. PKR protection against intranasal vesicular stomatitis virus infection is mouse strain dependent. Viral Immunol. 2002;15:41–51. doi: 10.1089/088282402317340224. [DOI] [PubMed] [Google Scholar]
- 5.Dutia BM. Clarke CJ. Allen DJ. Nash AA. Pathological changes in the spleens of gamma interferon receptor-deficient mice infected with murine gammaherpesvirus: a role for CD8 T cells. J Virol. 1997;71:4278–4283. doi: 10.1128/jvi.71.6.4278-4283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehtisham S. Sunil-Chandra NP. Nash AA. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J Virol. 1993;67:5247–5252. doi: 10.1128/jvi.67.9.5247-5252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flano E. Husain SM. Sample JT. Woodland DL. Blackman MA. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J Immunol. 2000;165:1074–1081. doi: 10.4049/jimmunol.165.2.1074. [DOI] [PubMed] [Google Scholar]
- 8.Flano E. Kim IJ. Woodland DL. Blackman MA. Gamma-herpesvirus latency is preferentially maintained in splenic germinal center and memory B cells. J Exp Med. 2002;196:1363–1372. doi: 10.1084/jem.20020890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuse S. Obar JJ. Bellfy S. Leung EK. Zhang W. Usherwood EJ. CD80 and CD86 control antiviral CD8+ T-cell function and immune surveillance of murine gammaherpesvirus 68. J Virol. 2006;80:9159–9170. doi: 10.1128/JVI.00422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Sastre A. Durbin RK. Zheng H. Palese P. Gertner R. Levy DE. Durbin JE. The role of interferon in influenza virus tissue tropism. J Virol. 1998;72:8550–8558. doi: 10.1128/jvi.72.11.8550-8558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidotti LG. Morris A. Mendez H. Koch R. Silverman RH. Williams BR. Chisari FV. Interferon-regulated pathways that control hepatitis B virus replication in transgenic mice. J Virol. 2002;76:2617–2621. doi: 10.1128/JVI.76.6.2617-2621.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johannessen I. Asghar M. Crawford DH. Essential role for T cells in human B-cell lymphoproliferative disease development in severe combined immunodeficient mice. Br J Haematol. 2000;109:600–610. doi: 10.1046/j.1365-2141.2000.02066.x. [DOI] [PubMed] [Google Scholar]
- 13.Kagi D. Seiler P. Pavlovic J. Ledermann B. Burki K. Zinkernagel RM. Hengartner H. The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur J Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- 14.Khakoo SI. Thio CL. Martin MP, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 15.Kim IJ. Flano E. Woodland DL. Blackman MA. Antibody-mediated control of persistent gamma-herpesvirus infection. J Immunol. 2002;168:3958–3964. doi: 10.4049/jimmunol.168.8.3958. [DOI] [PubMed] [Google Scholar]
- 16.Lam-Yuk-Tseung S. Gros P. Genetic control of susceptibility to bacterial infections in mouse models. Cell Microbiol. 2003;5:299–313. doi: 10.1046/j.1462-5822.2003.00276.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee KS. Cool CD. van Dyk LF. Murine gammaherpesvirus 68 infection of gamma interferon-deficient mice on a BALB/c background results in acute lethal pneumonia that is dependent on specific viral genes. J Virol. 2009;83:11397–11401. doi: 10.1128/JVI.00989-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lotz M. Tsoukas CD. Fong S. Carson DA. Vaughan JH. Regulation of Epstein-Barr virus infection by recombinant interferons. Selected sensitivity to interferon-gamma. Eur J Immunol. 1985;15:520–525. doi: 10.1002/eji.1830150518. [DOI] [PubMed] [Google Scholar]
- 19.McClary H. Koch R. Chisari FV. Guidotti LG. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J Virol. 2000;74:2255–2264. doi: 10.1128/jvi.74.5.2255-2264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molloy MJ. Zhang W. Usherwood EJ. Cutting edge: IL-2 immune complexes as a therapy for persistent virus infection. J Immunol. 2009;182:4512–4515. doi: 10.4049/jimmunol.0804175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nansen A. Jensen T. Christensen JP. Andreasen SO. Ropke C. Marker O. Thomsen AR. Compromised virus control and augmented perforin-mediated immunopathology in IFN-gamma-deficient mice infected with lymphocytic choriomeningitis virus. J Immunol. 1999;163:6114–6122. [PubMed] [Google Scholar]
- 22.Nash AA. Dutia BM. Stewart JP. Davison AJ. Natural history of murine gamma-herpesvirus infection. Philos Trans R Soc Lond B Biol Sci. 2001;356:569–579. doi: 10.1098/rstb.2000.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obar JJ. Crist SG. Leung EK. Usherwood EJ. IL-15-independent proliferative renewal of memory CD8+ T cells in latent gammaherpesvirus infection. J Immunol. 2004;173:2705–2714. doi: 10.4049/jimmunol.173.4.2705. [DOI] [PubMed] [Google Scholar]
- 24.Olivadoti M. Toth LA. Weinberg J. Opp MR. Murine gammaherpesvirus 68: a model for the study of Epstein-Barr virus infections and related diseases. Comp Med. 2007;57:44–50. [PubMed] [Google Scholar]
- 25.Pozharskaya VP. Weakland LL. Offermann MK. Inhibition of infectious human herpesvirus 8 production by gamma interferon and alpha interferon in BCBL-1 cells. J Gen Virol. 2004;85:2779–2787. doi: 10.1099/vir.0.80214-0. [DOI] [PubMed] [Google Scholar]
- 26.Sarawar SR. Cardin RD. Brooks JW. Mehrpooya M. Hamilton-Easton AM. Mo XY. Doherty PC. Gamma interferon is not essential for recovery from acute infection with murine gammaherpesvirus 68. J Virol. 1997;71:3916–3921. doi: 10.1128/jvi.71.5.3916-3921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava B. Blazejewska P. Hessmann M, et al. Host genetic background strongly influences the response to influenza a virus infections. PLoS One. 2009;4:e4857. doi: 10.1371/journal.pone.0004857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steed A. Buch T. Waisman A. Virgin HWT. Gamma interferon blocks gammaherpesvirus reactivation from latency in a cell type-specific manner. J Virol. 2007;81:6134–6140. doi: 10.1128/JVI.00108-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steed AL. Barton ES. Tibbetts SA. Popkin DL. Lutzke ML. Rochford R. Virgin HWT. Gamma interferon blocks gammaherpesvirus reactivation from latency. J Virol. 2006;80:192–200. doi: 10.1128/JVI.80.1.192-200.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart JP. Usherwood EJ. Ross A. Dyson H. Nash T. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J Exp Med. 1998;187:1941–1951. doi: 10.1084/jem.187.12.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sumaria N. van Dommelen SL. Andoniou CE. Smyth MJ. Scalzo AA. Degli-Esposti MA. The roles of interferon-gamma and perforin in antiviral immunity in mice that differ in genetically determined NK-cell-mediated antiviral activity. Immunol Cell Biol. 2009;87:559–566. doi: 10.1038/icb.2009.41. [DOI] [PubMed] [Google Scholar]
- 32.Sunil-Chandra NP. Efstathiou S. Arno J. Nash AA. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J Gen Virol. 1992;73:2347–2356. doi: 10.1099/0022-1317-73-9-2347. [DOI] [PubMed] [Google Scholar]
- 33.Sunil-Chandra NP. Efstathiou S. Nash AA. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J Gen Virol. 1992;73:3275–3279. doi: 10.1099/0022-1317-73-12-3275. [DOI] [PubMed] [Google Scholar]
- 34.Tarakanova VL. Suarez F. Tibbetts SA, et al. Murine gammaherpesvirus 68 infection is associated with lymphoproliferative disease and lymphoma in BALB beta2 microglobulin-deficient mice. J Virol. 2005;79:14668–14679. doi: 10.1128/JVI.79.23.14668-14679.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tibbetts SA. van Dyk LF. Speck SH. Virgin HWT. Immune control of the number and reactivation phenotype of cells latently infected with a gammaherpesvirus. J Virol. 2002;76:7125–7132. doi: 10.1128/JVI.76.14.7125-7132.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tripp RA. Hamilton-Easton AM. Cardin RD, et al. Pathogenesis of an infectious mononucleosis-like disease induced by a murine gamma-herpesvirus: role for a viral superantigen? J Exp Med. 1997;185:1641–1650. doi: 10.1084/jem.185.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Usherwood EJ. Brooks JW. Sarawar SR, et al. Immunological control of murine gammaherpesvirus infection is independent of perforin. J Gen Virol. 1997;78:2025–2030. doi: 10.1099/0022-1317-78-8-2025. [DOI] [PubMed] [Google Scholar]
- 38.Usherwood EJ. Stewart JP. Robertson K. Allen DJ. Nash AA. Absence of splenic latency in murine gammaherpesvirus 68-infected B cell-deficient mice. J Gen Virol. 1996;77:2819–2825. doi: 10.1099/0022-1317-77-11-2819. [DOI] [PubMed] [Google Scholar]
- 39.Weck KE. Kim SS. Virgin HI. Speck SH. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J Virol. 1999;73:3273–3283. doi: 10.1128/jvi.73.4.3273-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinberg JB. Lutzke ML. Alfinito R. Rochford R. Mouse strain differences in the chemokine response to acute lung infection with a murine gammaherpesvirus. Viral Immunol. 2004;17:69–77. doi: 10.1089/088282404322875467. [DOI] [PubMed] [Google Scholar]
- 41.Yao QY. Tierney RJ. Croom-Carter D, et al. Frequency of multiple Epstein-Barr virus infections in T-cell-immunocompromised individuals. J Virol. 1996;70:4884–4894. doi: 10.1128/jvi.70.8.4884-4894.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]