Abstract
The lethality of Mycobacterium tuberculosis remains the highest among infectious organisms and is linked to inadequate immune response of the host. Containment and cure of tuberculosis requires an effective cell-mediated immune response, and the absence, during active tuberculosis infection, of delayed-type hypersensitivity (DTH) responses to mycobacterial antigens, defined as anergy, is associated with poor clinical outcome. To investigate the biochemical events associated with this anergy, we screened 206 patients with pulmonary tuberculosis and identified anergic patients by their lack of dermal reactivity to tuberculin purified protein derivative (PPD). In vitro stimulation of T cells with PPD induced production of IL-10, IFN-γ, and proliferation in PPD+ patients, whereas cells from anergic patients produced IL-10 but not IFN-γ and failed to proliferate in response to this treatment. Moreover, in anergic patients IL-10–producing T cells were constitutively present, and T-cell receptor–mediated (TCR-mediated) stimulation resulted in defective phosphorylation of TCRζ and defective activation of ZAP-70 and MAPK. These results show that T-cell anergy can be induced by antigen in vivo in the intact human host and provide new insights into mechanisms by which M. tuberculosis escapes immune surveillance.
Introduction
Clinical conditions that induce impaired cell-mediated immunity like AIDS, malignancy, and immunosuppressive therapy result in impaired MHC class II–mediated delayed-type hypersensitivity (DTH) reaction (1, 2). Moreover, long-standing clinical observations have established that certain diseases that do not induce a generalized immunosuppressive state, also induce impaired DTH reaction to specific antigens, a state clinically defined as “anergy.” Classical paradigms of these diseases include tuberculosis (TB), sarcoidosis, and Hodgkin’s disease.
TB is the leading cause of death from infectious diseases worldwide (3), accounting for eight million new cases and three million deaths annually (6). The lethality of TB is due to both the absence of an effective vaccine and to the poor understanding of how the mycobacteria escape immune surveillance. Anergy in the setting of TB refers to the paradoxical absence of dermal reactivity to intradermal injection with tuberculin purified protein derivative (PPD) in infected persons. It occurs in about 15% of patients with active pulmonary disease and is associated with absence of granuloma formation and all other manifestations of cellular hypersensitivity (3–5). We thus chose to examine the biochemical events that regulate the induction of TB anergy since this understanding may also provide insights into the pathophysiology of this disease.
Anergy in vitro and its in vivo counterpart, tolerance, are immunologically defined as the inability of antigen-specific T cells to produce IL-2 and clonally expand on rechallenge with fully competent antigen-presenting cells (APC) (7, 8). Induction of anergy is an active signaling process induced when T-cell receptor (TCR) is ligated by antigen without costimulation. Anergy can also be induced in the presence of costimulation if the TCR is ligated by superantigen or by altered peptide ligands that bear a single amino acid substitution in the sequence of the agonistic peptide (9). Although quite distinct, these three approaches to induce anergy appear to share common biochemical events characterized by hypophosphorylation of TCRζ and defective activation of ZAP-70 and Ras (10–15), indicating the generalized significance of these findings in the anergic state. Recently, IL-10 in the presence of a TCR signal was reported to induce anergy (16). Although it was initially thought that IL-10 induces anergy by downregulating the expression of costimulatory molecules on the APC, later studies provided evidence that IL-10 mediates a direct anergizing effect on T cells in vitro and in vivo (17).
To examine whether the molecular basis of TB anergy in the intact human host displayed the biochemical events that characterize the experimentally induced anergy in in vitro cellular systems and in vivo murine models (9), we studied patients with pulmonary TB with detectable or undetectable DTH to PPD. Here we show that in vitro stimulation of T cells with PPD induced IL-10, IFN-γ, and proliferation in PPD+ patients, but only IL-10 and neither IFN-γ nor proliferation in anergic patients. Strikingly, IL-10–producing T cells were constitutively detected in anergic patients in whom TCR-mediated stimulation resulted in defective phosphorylation of TCRζ and defective activation of ZAP-70 and MAPK. These results show, we believe for the first time, that T-cell anergy can be induced by antigen in vivo in the intact human host and results in the identical biochemical findings determined in in vitro anergized T-cell clones and tolerant murine T cells that do not induce graft versus host disease.
Methods
Patients.
The study subjects were unrelated Cambodian patients recruited from a TB treatment program in eastern rural Cambodia (Svay Rieng Province) (18). The diagnosis of clinical TB was made on the basis of history, physical examination, and detection of acid-fast bacilli in sputum using light microscopy. After consent was obtained, A 5TU PPD (Pasteur-Merieux, Paris, France) was injected intradermally in the forearm of TB inpatients and was evaluated for induration 48 hours later. Blood was drawn from PPD– (no induration) or PPD+ (greater than 10 mm of induration) TB patients, and PBMCs were isolated by Ficoll-Hypaque gradient separation and cryopreserved. Before further analysis, serum samples from all individuals were screened for HIV-1 by enzyme-linked immunoadsorbent assay (Abbott Laboratories, Abbott Park, Illinois, USA).
Cell preparation and culture.
For the isolation of APC and the T-cell fractions, total PBMC were incubated in tissue culture plates at 37°C for 2 hours. The nonadherent population was collected, and the CD4+ T-cell population was further enriched by separation from residual monocytes, B cells, CD8+ T cells, and NK cells by mAb and magnetic bead depletion using the mAb’s anti-CD14, anti-CD11b, anti-CD8, anti-CD20, and anti-CD16, which have been described previously, produced in our laboratory (19). The adherent fraction (APC), was recovered from the tissue culture plate by washing with cold PBS and incubation with 0.05 mM EDTA. The purity of each population was analyzed in each case by flow cytometry (Epics Elite; Coulter Electronics, Hialeah, Florida, USA). The CD4+ T-cell fraction was consistently greater than 95% CD3 positive and the adherent fraction was greater than 85% CD14 positive. Where indicated, APC were loaded with PPD (25 ng/mL) by incubating at 37°C for 3 hours during constant end-to-end mixing. Loaded APC were then irradiated at 2,500 rad, excess peptide was removed, and cells (105/well) were cultured with autologous T cells at 1:1 ratio in 96-well plates in complete medium consisting of RPMI-1640 supplemented with 10% heat-inactivated human serum, 2% glutamine, and 1% penicillin/streptomycin at 37°C with 5% CO2. Optimal concentrations of peptide, responder/stimulator ratio, and time length of culture were established in pilot experiments with samples from healthy volunteer donors (data not shown). Culture was continued for 5 days, and 3H-thymidine incorporation was assessed during the last 18 hours of culture. For stimulation with nonspecific mitogens, PMA was used at 5 ng/mL and phytohemagglutinin (PHA) at 5 μg/mL. Culture was continued for 3 days and 3H-thymidine incorporation was assessed during the last 16 hours of culture. For allogeneic mixed lymphocyte reactions (MLRs), T cells (105/well) were used as responders and APC as stimulators at 1:1 ratio in a 7-day culture, and 3H-thymidine incorporation was assessed during the last 24 hours of culture.
In vitro T-cell stimulation and Western blot.
T cells were resuspended at 107 cells/mL and incubated with anti-CD3 (OKT3, IgG1; American Type Culture Collection, Rockville, Maryland, USA) (1 μg/mL) and anti-CD28 (3D10, IgG1) (2 μg/mL) at 4°C for 20 minutes. Cells were washed and incubated with rabbit anti-mouse IgG (20 μg/mL) at 37°C for various time intervals. After the indicated time intervals of culture, cell lysates were prepared and equal amounts of protein (30 μg/sample) were analyzed by 10% SDS-PAGE, transferred onto nitrocellulose membranes, and immunoblotted with anti-phosphotyrosine mAb (4G10) (Upstate Biotechnology, Lake Placid, New York, USA), ZAP-70–specific antiserum (Santa Cruz Biotechnology, Santa Cruz, California, USA), p-ERK1/2 antibody (Santa Cruz Biotechnology), or ERK1/2 antibody (Upstate Biotechnology). Immunodetection was performed by incubation with horseradish peroxidase-conjugated anti-mouse IgG (1:5000) or anti-rabbit IgG (1:10000) (Promega Corp., Madison, Wisconsin, USA) as indicated by the host origin of the primary antibody and developed by chemiluminescence (NEN Life Science Products Inc., Boston, Massachusetts, USA). Stripping and reprobing of the immunoblots were performed as described (14).
Flow-cytometric analysis of intracellular cytokines.
T cells from anergic and PPD+ patients were cultured with PPD-loaded autologous APC for 16 hours. Brefeldin A (Sigma Chemical Co., St Louis, Missouri, USA) was added (10 μg/mL), and 2 hours later cells were collected, washed, and stained with FITC-labeled anti-CD3 mAb (Coulter Electronics Ltd.), followed by goat anti-mouse IgG, then washed and fixed with formaldehyde. Subsequently, cells were incubated with the following mAb’s: anti–IFN-γ/FITC (PharMingen, San Diego, California, USA), isotype-matched Ig/FITC control, anti–IL-10/PE (PharMingen), or isotype-matched Ig/PE control. At the same time, freshly prepared T cells without in vitro culture, from the same patients, were also stained as above. Samples were analyzed immediately by flow cytometry (Coulter Electronics Ltd.).
Cytokine analysis.
Thirty-six hours after stimulation of PBMC with PPD (10 μg/mL) or an aqueous sonicate of irradiated M. tuberculosis (H37RV strain, 10 μg/mL), supernatants were collected and secretion of cytokines was assessed by ELISA, according to the manufacturer’s instructions (IFN-γ, TNF-α, IL-12, IL-2, IL-4, and IL-10: Endogen Inc., Woburn, Massachusetts, USA; TGF-β2: R & D Systems, Minneapolis, Minnesota, USA). We chose to examine TGF-β2 expression since previous studies in our laboratory have shown that human T cells express receptors for TGF-β2 (Cardoso et al., unpublished data).
Results
Normal APC function but defective response of CD4+ T cells from anergic TB patients to PPD.
We screened a total of 206 patients with pulmonary TB and acid-fast bacilli–positive (AFB-positive) sputum undergoing anti-tuberculous chemotherapy in Svay Rieng Province, Cambodia, for response to PPD in July 1996, February 1998, and January 1999. We identified 24 individuals who developed no induration 48 hours after intradermal injection with 5 TU PPD, who we defined as anergic (Table 1). Serologic testing to determine whether absence of DTH response to PPD was due to HIV infection revealed that one patient was HIV+, and he was excluded from the study. Of the 18 patients identified in 1999, three became PPD+ upon rescreening 8 months later and were also excluded from the study (Table 1). Follow-up of the surviving anergic individuals revealed that although they had all been successfully treated and had no AFB+ sputum, they remained anergic in 1999 (Table 1). Thus, lack of DTH to PPD was not a transient phenomenon associated with active pulmonary TB, but was a persistent finding in the majority of the anergic patients.
Table 1.
Persistent anergy in TB patients
Previous studies have shown that both CD4+ and CD8+ T lymphocytes play an active role in the induction of the immune response to M. tuberculosis (Mtb) antigens (20–22). Moreover, HIV+ individuals with aberrant CD4/CD8 ratio and AIDS patients have an impaired response to PPD on skin testing (1). Therefore, we first examined whether the impaired response of anergic TB patients to PPD was secondary to altered subsets of T cells. No difference in CD4/CD8 ratio was observed among normal control individuals, and PPD+ and PPD– TB patients (data not shown).
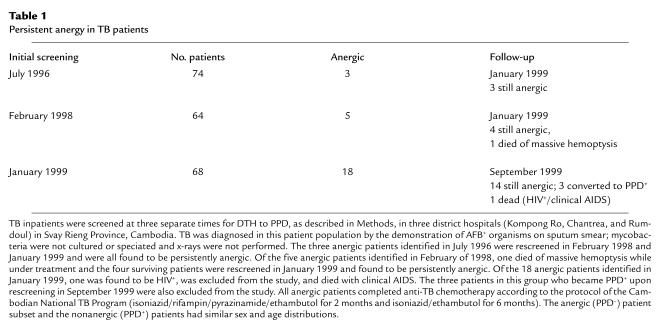
DTH is generated by class II–mediated presentation of antigens to CD4+ T cells. Therefore, we next examined whether the absence of response to PPD in anergic TB patients was due to the inability of their CD4+ T cells to recognize PPD antigen presented by autologous APC. APC were isolated from anergic and PPD+ patients and loaded with PPD. CD4+ T cells were also isolated and stimulated with the PPD-loaded autologous APC. As shown in Figure 1a, the proliferative response of T cells from anergic TB patients to PPD was dramatically reduced as compared with the response of T cells from PPD+ patients. Thus, stimulation of T cells derived from anergic TB patients, with PPD presented by autologous APC, initiates an inefficient immune response of antigen-specific T cells that does not lead to clonal expansion and effector function in vivo and in vitro.
Figure 1.
APC from anergic TB patients do not generate a PPD-specific proliferative response of autologous T cells but can efficiently generate allo-MLR. (a) CD4+ T cells from PPD+ (responding) patients (RP) and anergic patients (AP) were stimulated with autologous APC loaded with PPD, and 3H-thymidine incorporation was determined. Results of four representative patients among 10 studied in each group are shown. (b) T cells from the same healthy volunteer donor were stimulated with APC from RP, AP, and healthy control individuals (Ctl) for 7 days, and DNA synthesis was determined by 3H-thymidine incorporation. Results of three representative individuals among eight studied in each group are shown. T cells from anergic TB patients have diminished responses to alloantigen and nonspecific mitogens. (c) CD4+ T cells from anergic and PPD+ patients were used as responders, and APC from the same healthy volunteer donor were used as stimulators. Cultures were continued for 7 days and response was assessed by 3H-thymidine incorporation. (d) CD4+ T cells from TB patients were cultured with PMA and PHA and response was examined by 3H-thimindine incorporation. Results of four representative patients among eight studied in each group are shown in c and d.
These results suggested that PPD unresponsiveness in anergic TB patients might be due to the inability of their APC to present antigens or, conversely, due to the inability of their T cells to respond to antigen-specific stimulation. To dissect these possibilities, we isolated APC from PPD+ patients, anergic TB patients, and healthy control individuals and examined their ability to induce allogeneic MLR. Stimulation of T cells from healthy volunteer donors with APC isolated from anergic or PPD+ TB patients induced allogeneic responses comparable to those induced by APC isolated from healthy control individuals (Figure 1b). Therefore, APC from anergic TB patients do not have a global inability to present antigens since they can function efficiently as alloantigen-presenting cells and present their own HLA antigens to allogeneic T cells.
Antigen- nonspecific immunosuppressive activity is present in the peripheral blood of anergic TB patients.
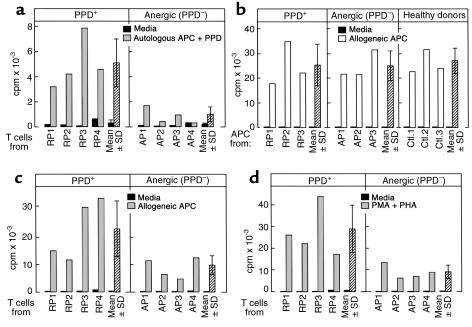
To determine the ability of CD4+ T cells from anergic TB patients to respond to alloantigens and mitogens, CD4+ T cells from anergic and control patients were cultured with allogeneic APC or nonspecific mitogens. Stimulation of T cells from anergic patients with allogeneic APC (Figure 1c), or PMA and PHA (Figure 1d) resulted in a proliferative response, albeit the magnitude of these responses was diminished as compared with the response of T cells from PPD+ TB patients (Figure 1c and d). These findings led us to hypothesize that the unresponsive PPD-specific cells in anergic patients may have immunosuppressive properties that diminish the responses of other T-cell subsets with different specificity in these bulk T-cell cultures. Importantly, it has been reported that anergic antigen-specific T cells may mediate antigen-nonspecific immunosuppressive effects and inhibit the response of T cells with different antigenic specificity (17, 23), a phenomenon previously termed “infectious tolerance” (24). The inhibitory effect of such cells has been shown to be mediated by cell-to-cell contact and by soluble factors (17, 23). These observations were also true in our experimental system because CD4+ T cells from anergic TB patients and supernatants of T-cell cultures from these patients stimulated with PPD could suppress responses of healthy control T cells in allogeneic MLR (Figure 2) and stimulation with nonspecific mitogens (data not shown).
Figure 2.
Culture supernatants after PPD stimulation and CD4+ T cells from anergic TB patients inhibit allogeneic MLR. CD4+ T cells and APC from HLA-disparate healthy individuals were used as responders and stimulators, respectively. Incubation was continued for 7 days with either media alone or in the presence of the indicated concentrations of culture supernatants (Cs) collected at 36 hours after PPD stimulation of CD4+ T cells from PPD+ TB patients, anergic (PPD–) TB patients, and control healthy individuals, or with CD4+ T cells (Tc) from the same individuals. Response was examined by 3H-thymidine incorporation. Results from experiments with one representative patient among three tested in each group are shown.
IL-10+ T cells with immunosuppressive properties are present in anergic TB patients.
It has been shown that both Th1 and Th2 cells recognize Mtb (25–27) and that IFN-γ–producing antigen-specific T cells have a significant role in establishing a TB-specific cellular immune response (28). Similarly, IL-12 increases resistance to Mtb infection and promotes development of protective immunity against TB (29–31). In contrast, although IL-4 and IL-10 production are detected during TB infection, they do not appear to contribute to the protection or to the clearance of the infection (32, 33). In addition to these cytokines, TNF-α has been suggested to have an important role in granuloma formation and containment of disease (34, 35).
Since cytokines play a significant role in the generation of Mtb-mediated immunity and some of the Mtb-induced cytokines such as IL-10 and IL-4 have immunosuppressive properties, we next examined the expression of various cytokines in response to stimulation with Mtb antigens in three representative anergic and PPD+ patients. In vitro stimulation of PBMC from PPD+ patients and anergic patients with PPD or an aqueous sonicate of irradiated Mtb (H37RV), did not result in detectable production of IL-2, IL-4, IL-12, TGF-β2, or TNF-α (data not shown). By contrast, IFN-γ that was constitutively low in both patient groups (mean value: < 10 pg/mL) was strongly induced in PPD+ individuals by mycobacterial antigens (mean value: 153 pg /mL) and PPD (mean value: 98.7 pg/mL). However, IFN-γ was only minimally induced in anergic patients by mycobacterial antigens (mean value: 41.5 pg/mL) or PPD stimulation (mean value: 23.5 pg/mL). Interestingly, constitutive IL-10 production was detected in PBMC from anergic patients (mean value: 29.5 pg/mL) that was further augmented by stimulation with mycobacterial antigens (mean value: > 350 pg/mL) and PPD (mean value: 155 pg/mL). By contrast, IL-10 was not detectable before stimulation, but was induced by mycobacterial antigens (mean value: 225 pg/mL) and PPD (mean value: 140 pg/mL) in PBMC from PPD+ patients. Thus, differential patterns of IFN-γ and IL-10 production were detected in anergic and PPD+ patients.
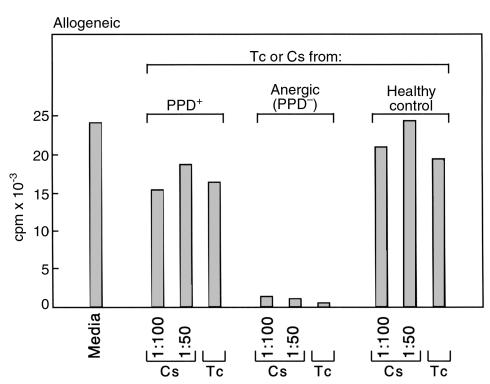
Recent studies have shown that prolonged and repetitive stimulation of CD4+ T cells in the presence of IL-10 induces the differentiation of a unique subset of antigen-specific T cells, termed T regulatory cells 1 (Tr1) (17). These cells have antigen-nonspecific immunosuppressive properties, produce IL-10 but not IL-2, IL-4, or IFN-γ, and proliferate poorly in response to antigenic stimulation. Therefore, the constitutive IL-10 detection, the high induction of IL-10, and the defective induction of IFN-γ after Mtb stimulation of PBMC from anergic TB patients, and the fact that T cells from these patients have defective proliferative response to PPD and immunosuppressive properties (Figure 1a and Figure 2), suggested that PPD-specific Tr1-type cells may be present in the peripheral blood of these patients. To examine this possibility, we purified CD4+ T cells from anergic and PPD+ TB patients and determined the expression of intracellular IFN-γ and IL-10. As shown in Figure 3, in anergic TB patients IL-10–producing T cells were constitutively detected before and after stimulation, whereas PPD stimulation did not result in detection of IFN-γ–producing T cells. By contrast, in PPD+ TB patients, both IL-10 and IFN-γ–producing T cells were detected after PPD stimulation.
Figure 3.
IL-10+ T cells are constitutively present but IFN-γ+ T cells are not induced by PPD in anergic TB patients, whereas both IL-10+ and IFN-γ+ T cells are induced in PPD+ patients by PPD. Before stimulation and after culture with PPD, T cells were stained with anti-CD3 mAb. Cells were fixed, permeabilized, and stained for detection of intracellular cytokines using directly conjugated mAb’s specific for human IFN-γ and IL-10 (IFN-γ-FITC and IL-10-PE) or isotype-matched control as indicated. Results of one PPD+ and one anergic patient are shown and are representative of three patients examined in each group.
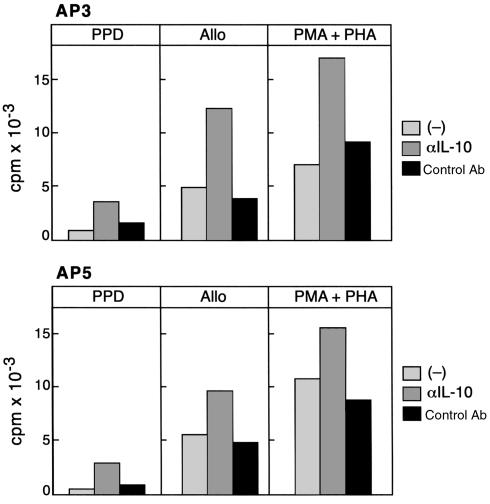
IL-10 prevents T-cell proliferation and inhibits antigen-specific T-cell responses (9, 16, 17). Therefore, we examined whether the presence of IL-10–producing cells may contribute to the diminished proliferative response of T cells from anergic TB patients to PPD, alloantigen, and nonspecific mitogens. As shown in Figure 4, proliferation of T cells from anergic TB patients in response to PPD, alloantigen, and nonspecific mitogens was augmented by the addition of a neutralizing anti–IL-10 mAb, but not by an isotype-matched control antibody.
Figure 4.
Anti–IL-10 neutralizing mAb increases proliferation of T cells from anergic TB patients in response to PPD, alloantigen, and PMA plus PHA. CD4+ T cells from two anergic patients (AP3 and AP5) were stimulated with PPD, allogeneic PBMCs, or PMA plus PHA in the presence of either media, neutralizing anti–IL-10 mAb, or isotype-matched control antibody. After the indicated time intervals of culture as described in Methods, response was determined by 3H-thymidine incorporation.
Defective phosphorylation of TCRζ and ZAP-70 and defective activation of Ras in anergic TB patients.
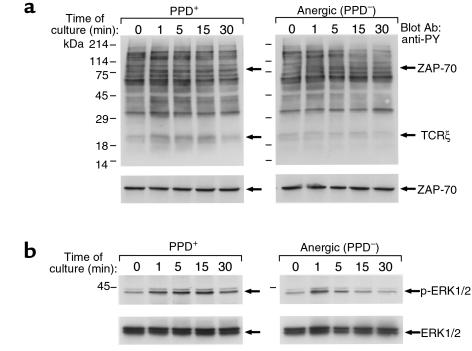
IL-10 production is associated with anergy induction in vitro (16) and, more importantly, in in vivo murine models of stem cell transplantation tolerance (36) and superantigen-mediated tolerance (15). Therefore, we examined whether anergy-specific biochemical findings could be detected in CD4+ T cells from anergic TB patients. CD4+ T cells from PPD+ and anergic TB patients were stimulated in vitro, and activation of protein tyrosine phosphorylation was determined by antiphosphotyrosine immunoblot. As shown in Figure 5a (top panel), activation of tyrosine phosphorylation of ZAP-70 and TCRζ was efficiently induced in T cells from PPD+ patients, but was defective in T cells from anergic TB patients. This was not due to quantitative differences, because equivalent amounts of ZAP-70 were present in T cells from PPD+ and anergic patients (Figure 5a, bottom panel). These findings have a striking similarity to the results obtained using in vitro–anergized human and murine T-cell clones (11–13) and tolerant alloreactive murine T cells that do not induce graft versus host disease when transferred into histoincompatible recipients (37).
Figure 5.
Defective TCR-mediated phosphorylation of TCRζ and ZAP-70 and defective activation of MAPK in anergic TB patients. (a) T cells from PPD+ and anergic TB patients (top panel) were stimulated in vitro with anti-CD3 and anti-CD28 mAb for the indicated time intervals. Cell lysates were prepared and analyzed by SDS-PAGE, transferred to nitrocellulose membrane, and blotted with anti-phosphotyrosine mAb. Blots were stripped and reblotted with ZAP-70 mAb (bottom panel). (b) Phosphorylation of ERK1 and ERK2 MAPK (top panel) was examined by immunoblotting with phospho-ERK–specific antibody, which recognizes only the activated phosphorylated form of ERK1 and ERK2. The blot was stripped and reprobed with ERK-specific antibody (bottom panel), which recognizes total ERK. The blots presented are representative of five PPD+ and five PPD– individuals.
Since anergy also results in defective Ras activation in response to TCR–mediated stimulation (14, 38, 39), we examined the ability of T cells from anergic and control TB patients to activate the Ras pathway. Raf-1 serine/threonine kinase, a downstream effector of Ras (40), is recruited to the plasma membrane by activated Ras, where it becomes activated in a Ras-independent manner (41). Activated Raf-1 phosphorylates and activates MEK-1 (a member of the MEK [MAP/ERK] kinases), leading to activation of the mitogen-activated protein (MAP/ERK) kinase cascade (42). To determine whether T cells from anergic TB patients could activate the Ras pathway after stimulation, we examined whether activation of the ERK1 and ERK2 kinases of the MAP/ERK kinase family, which are downstream of Ras, was induced. Immunoblot with an antibody specific for activated, phosphorylated ERK1 and ERK2 demonstrated that activation of these MAP kinases was induced at 1 minute and was detectable up to 30 minutes after stimulation of T cells from PPD+ patients (Figure 5b, top panel). By contrast, stimulation of T cells from anergic TB patients resulted in defective activation of ERK1 and ERK2 that was transiently detected at 1 minute after stimulation and rapidly declined (Figure 5b, top panel). This was not due to quantitative differences, because equivalent amounts of total ERK1 and ERK2 were present in all samples from PPD+ and anergic TB patients (Figure 5b, bottom panel).
Taken together, these results show that biochemical characteristics established previously as the hallmark of anergic cells, experimentally generated in in vitro or in vivo models, are detected in T cells isolated from anergic TB patients, indicating for the first time that T-cell anergy can be induced by antigen presentation in vivo in the intact human host.
Discussion
It is well documented that IFN-γ plays an important role in the development of cellular immunity to Mtb. Humans with mutated IFN-γ receptor genes are highly susceptible to infection by atypical mycobacteria (43, 44). Consistent with these findings, mice with a disrupted IFN-γ gene fail to produce reactive nitrogen intermediates and restrict the growth of the bacilli although they develop granulomas (28, 45). This extreme model, in which the host is unable to produce any IFN-γ necessary for the generation of antimicrobial activity, demonstrates the requirement of IFN-γ for containment of mycobacterial infection. It is thus interesting to speculate that in anergic TB patients that have defective IFN-γ production in response to Mtb stimulation mycobacteria are not eradicated but survive in a persistent state in closed or encapsulated lesions, despite the absence of sputum AFB positivity.
Generation of IL-10–producing Tr1-type cells is a lengthy process that requires chronic antigen-specific stimulation of naïve T cells in the presence of IL-10 (17). This notion is compatible with the long natural history of TB and the development of TB anergy. 0ur present findings suggest that in anergic patients, sustained stimulation by Mtb, which results in IL-10 but not IFN-γ production, mediates the generation of anergic Mtb-specific T cells with Tr1 phenotype and antigen-nonspecific immunosuppressive properties. Subsequently, in the presence of IL-10, the Mtb-infected host becomes tolerant to the Mtb antigens, similar to IL-10–treated animals that become tolerant to allogeneic antigens of the transplanted grafts (36). Whether other cell subsets besides CD4+ T cells contribute to IL-10 secretion after Mtb-mediated stimulation and whether, in addition to IL-10, other immunoregulatory mechanisms suppress the immune response in the anergic TB patients, remains to be determined.
The development of Mtb-specific T-cell anergy due to chronic Mtb-mediated stimulation in the absence of IFN-γ and the presence of IL-10 may contribute to the establishment of Mtb persistence. Importantly, it has been suggested that although its mechanisms remain unknown, “persistence” may hold the key to the problems of defective eradication of Mtb, resistance to treatment, and relapse, through mechanisms independent of drug resistance (46). Remarkably, increased IL-10 levels appear to promote Mtb survival and correlate with a more severe clinical phenotype of the disease, since IL-10–transgenic mice are highly susceptible to progressive TB infection and IL-10–deficient mice have increased antimycobacterial immunity (47, 48). It was shown recently that polymorphic variations of Plasmodium falciparum can downregulate T-cell proliferative response by preferential induction of IL-10, potentially contributing to the low levels of responses to this pathogen in endemic areas (49). Therefore, such downregulatory mechanisms of the host immune response, directly mediated by various infectious organisms, may prevent elimination of the pathogens and contribute to the low levels of antibacterial responses, reinfection due to the lack of immunity, and reactivation and relapse of the disease.
Recent in vitro studies have started to decipher the molecular pathways that are required for the induction of anergy. In addition, reports from murine and subhuman primates in in vivo models of allogeneic solid organ and bone marrow transplantation provide evidence that induction of alloantigen-specific anergy results in long-term graft acceptance (50, 51) and loss of graft versus host disease (52, 53). Moreover, ex vivo anergization enabled successful histoincompatible bone marrow transplantation in humans (54). Our present studies extend those observations since they show the in vivo generation of anergy-specific biochemical events in the intact human host, and they provide evidence for the biologic significance of these findings in the pathophysiology of an infectious disease.
Finally, our results provide new insights into mechanisms by which Mtb escapes immune surveillance. The presence of immunosuppressive, IL-10–producing T cells and the detection of anergy-specific biochemical findings in the peripheral blood of anergic TB patients suggest that Mtb mediates active inhibition of the host immune response, resulting in sustained survival of the infectious organisms. Therefore, besides chemotherapy, methods to reverse the Mtb-induced anergy should be an integral part of novel treatment strategies attempting the cure and eradication of TB.
Acknowledgments
We are indebted to the Cambodian Health Committee TB patients for their generosity in participating in the study, and to Sun Sath and Sa Rom and other members of the Cambodian Health Committee staff for assistance in patient screening and follow-up. We are grateful to Barry Bloom for suggesting experiments with aqueous sonicate of M. tuberculosis, and to Pierre Grosjean for assistance with HIV-1 testing. We thank Lee Nadler, Fred Rosen, and Michael Brenner for research support, and Lee Nadler for critical reading of the manuscript. Supported by National Institutes of Health grants AI-43552, AI-41584, HL-54785, and HL-59838 and by an Established Investigator Award from the American Heart Association (to A.E. Goldfeld).
References
- 1.Daley CL, et al. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction-fragment-length polymorphisms. N Engl J Med. 1992;326:231–235. doi: 10.1056/NEJM199201233260404. [DOI] [PubMed] [Google Scholar]
- 2.Cisneros JR, Murray KM. Corticosteroids in tuberculosis. Ann Pharmacother. 1996;30:1298–1303. doi: 10.1177/106002809603001115. [DOI] [PubMed] [Google Scholar]
- 3.Bloom BR, Small PM. The evolving relation between humans and Mycobacterium tuberculosis. N Engl J Med. 1998;338:677–678. doi: 10.1056/NEJM199803053381008. [DOI] [PubMed] [Google Scholar]
- 4.Karim, M., and Elner, J.J. 1995. Mechanisms of anergy. In Tuberculosis. W.N. Rom and S.M. Garay, editors. Little, Brown & Co. New York, New York, USA. 343–351.
- 5.Daniel, T. 1991. Tuberculosis. In Harrison’s principles of internal medicine. J.D. Wilson et al., editors. McGraw-Hill. New York, New York, USA. 637–645.
- 6.1994. World Health Organization (WHO) Report on the TB Epidemic. Geneva, Switzerland.
- 7.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz RH. Models of T cell anergy: is there a common molecular mechanism? J Exp Med. 1996;184:1–8. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Migita K, et al. Defective TCR-mediated signaling in anergic T cells. J Immunol. 1995;155:5083–5087. [PubMed] [Google Scholar]
- 11.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-ζ and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 12.Madrenas J, et al. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science. 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- 13.Boussiotis VA, et al. Differential association of protein tyrosine kinases with T cell receptor is linked to the induction of anergy and its prevention by B7 family-mediated costimulation. J Exp Med. 1996;184:365–376. doi: 10.1084/jem.184.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boussiotis VA, Freeman GJ, Berezovskaya A, Barber DL, Nadler LM. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science. 1997;278:124–128. doi: 10.1126/science.278.5335.124. [DOI] [PubMed] [Google Scholar]
- 15.Miller C, Ragheb JA, Schwartz RH. Anergy and cytokine-mediated suppression as distinct superantigen-induced tolerance mechanism in vivo. J Exp Med. 1999;190:53–64. doi: 10.1084/jem.190.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groux H, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 18.Goldfeld AE, et al. Association of an HLA-DQ allele with clinical tuberculosis. JAMA. 1998;279:226–228. doi: 10.1001/jama.279.3.226. [DOI] [PubMed] [Google Scholar]
- 19.Boussiotis VA, Freeman GJ, Gray G, Gribben J, Nadler LM. B7 but not ICAM-1 costimulation prevents the induction of human alloantigen specific tolerance. J Exp Med. 1993;178:1753–1763. doi: 10.1084/jem.178.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caruso AM, et al. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;162:5407–5416. [PubMed] [Google Scholar]
- 21.Smith SM, et al. Characterization of human Mycobacterium bovis bacille Calmette-Guerin-reactive CD8+ T cells. Infect Immun. 1999;67:5223–5230. doi: 10.1128/iai.67.10.5223-5230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serbina NV, Flynn JL. Early emergence of CD8(+) T cells primed for production of type 1 cytokines in the lungs of Mycobacterium tuberculosis-infected mice. Infect Immun. 1999;67:3980–3988. doi: 10.1128/iai.67.8.3980-3988.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chai JG, et al. Anergic T cells act as suppressor cells in vitro and in vivo. Eur J Immunol. 1999;29:686–692. doi: 10.1002/(SICI)1521-4141(199902)29:02<686::AID-IMMU686>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 24.Qin S, et al. “Infectious” transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 25.Flesch IE, Kaufmann SH. Role of cytokines in tuberculosis. Immunobiology. 1993;189:316–339. doi: 10.1016/S0171-2985(11)80364-5. [DOI] [PubMed] [Google Scholar]
- 26.Torres M, Herrera T, Villareal H, Rich EA, Sada E. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect Immun. 1998;66:176–180. doi: 10.1128/iai.66.1.176-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verbon A, et al. Serum concentrations of cytokines in patients with active tuberculosis (TB) and after treatment. Clin Exp Immunol. 1999;115:110–113. doi: 10.1046/j.1365-2249.1999.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flynn JL, et al. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castro AG, Silva RA, Appelberg R. Endogenously produced IL-12 is required for the induction of protective T cells during Mycobacterium avium infections in mice. J Immunol. 1995;155:2013–2019. [PubMed] [Google Scholar]
- 30.Flynn JL, et al. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995;155:2515–2524. [PubMed] [Google Scholar]
- 31.de Jong R, et al. Severe mycobacterial and salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 32.Bonato VL, Lima VMF, Tascon RE, Lowrie DB, Silva CL. Identification and characterization of protective T cells in hsp65 DNA vaccinated and Mycobacterium tuberculosis infected mice. Infect Immun. 1998;66:169–175. doi: 10.1128/iai.66.1.169-175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baliko Z, Szereday L, Szekeres-Bartho J. Th2 biased immune response in cases with active Mycobacterium tuberculosis infection and tuberculin anergy. FEMS Immunol Med Microbiol. 1998;22:199–204. doi: 10.1111/j.1574-695X.1998.tb01207.x. [DOI] [PubMed] [Google Scholar]
- 34.Rojas M, Olivier M, Gros P, Barrera LF, Garcia LF. TNF-alpha and IL-10 modulate the induction of apoptosis by virulent Mycobacterium tuberculosis in murine macrophages. J Immunol. 1999;162:6122–6131. [PubMed] [Google Scholar]
- 35.Chensue SW, Warmington KS, Ruth JH, Lincoln P, Kunkel SL. Cytokine function during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation. Local and regional participation of IFN-gamma, IL-10, and TNF. J Immunol. 1995;154:5969–5976. [PubMed] [Google Scholar]
- 36.Zeller JC, et al. Induction of CD4+ T cell alloantigen-specific hyporesponsiveness by IL-10 and TGF-b. J Immunol. 1999;163:3684–3691. [PubMed] [Google Scholar]
- 37.Boussiotis VA, et al. p27kip1 functions as an anergy factor inhibiting IL-2 transcription and clonal expansion of alloreactive human and murine helper T lymphocytes. Nat Med. 2000;6:290–297. doi: 10.1038/73144. [DOI] [PubMed] [Google Scholar]
- 38.Li W, Whaley CD, Mondino A, Mueller DL. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 39.Fields PE, Gajewski TF, Fitch FW. Blocked Ras activation in anergic CD4+ T cells. Science. 1996;271:1276–1278. doi: 10.1126/science.271.5253.1276. [DOI] [PubMed] [Google Scholar]
- 40.Warne PH, Rodriguez Viciana PR, Downward J. Direct interaction of Ras and the aminoterminal region of Raf-1 in vitro. Nature. 1993;364:352–355. doi: 10.1038/364352a0. [DOI] [PubMed] [Google Scholar]
- 41.Stokoe D, Macdonald SG, Cadwallader K, Symonsa M, Hancock JF. Activation of Ras as a result of recruitment to the plasma membrane. Science. 1994;264:1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- 42.Alessi DR, et al. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74 raf-1. EMBO J. 1994;13:1610–1619. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newport M, et al. A mutation in the interferon-g-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 44.Jouanguy E, et al. Interferon-—receptor deficiency in an infant with fatal bacille Camelete-Guerin infection. N Engl J Med. 1996;335:1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 45.Cooper AM, et al. Disseminated tuberculosis in interferon γ gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bloom BR, McKinney JD. The death and resurrection of tuberculosis. Nat Med. 1999;5:872–874. doi: 10.1038/11309. [DOI] [PubMed] [Google Scholar]
- 47.Murray PJ, Wang L, Onufryk C, Tepper RI, Young RA. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J Immunol. 1997;158:315–321. [PubMed] [Google Scholar]
- 48.Murray PJ, Young RA. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect Immun. 1999;67:3087–3095. doi: 10.1128/iai.67.6.3087-3095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plebanski M, et al. Interleukin 10-mediated immunosuppression by a variant CD4 T cell epitope of Plasmodium falciparum. Immunity. 1999;10:651–660. doi: 10.1016/s1074-7613(00)80064-3. [DOI] [PubMed] [Google Scholar]
- 50.Larsen CP, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 51.Kirk AD, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blazar BR, Taylor PA, Linsley PS, Vallera PA. In vivo blockade of CD28/CTLA4: B7/BB1 interaction with CTLA4-Ig reduces lethal murine graft-versus-host disease across the major histocompatibility complex barrier in mice. Blood. 1994;83:3815–3825. [PubMed] [Google Scholar]
- 53.Blazar BR, Taylor P, Noelle RJ, Vallera DA. CD4+ T cells tolerized ex-vivo to host alloantigen by anti-CD40 ligand (CD40L: CD154) antibody lose their graft-versus-host lethality capacity but retain normal antigen responses. J Clin Invest. 1998;102:473–482. doi: 10.1172/JCI3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guinan EC, et al. Transplantation of anergic histoincompatible bone marrow allografts. N Engl J Med. 1999;340:1704–1714. doi: 10.1056/NEJM199906033402202. [DOI] [PubMed] [Google Scholar]