Cancer chemotherapy encompasses a large number of well-documented and clinically established methods for the treatment of malignant diseases. However, the efficacy of these approaches is often hampered by an insufficient therapeutic index, lack of specificity, and the emergence of drug-resistant cell subpopulations. One approach aimed at enhancing the selectivity of cancer chemotherapy for solid tumors relies on the application of gene therapy technologies.
Gene therapies are techniques for modifying the cellular genome for therapeutic benefit. In cancer gene therapy, both malignant and nonmalignant cells may be suitable targets. The possibility of rendering cancer cells more sensitive to chemotherapeutics or toxins by introducing “suicide genes” was suggested in the late 1980s. This approach has two alternatives: toxin gene therapy, in which the genes for toxic products are transfected directly into tumor cells; and enzyme-activating prodrug therapy, in which the transgenes encode enzymes that activate specific prodrugs to create toxic products. The latter approach, known as gene-directed enzyme prodrug therapy (GDEPT) (1, 2) or virus-directed enzyme prodrug therapy (VDEPT) (3), may be used in isolation or combined with other strategies, such as the biotherapies described elsewhere in this Perspective series. VDEPT using selectively replicating viruses as vectors represents a promising means to target suicide genes specifically to tumor cells, an approach that is only beginning to be explored (for examples, see Hermiston, this Perspective series, ref. 4).
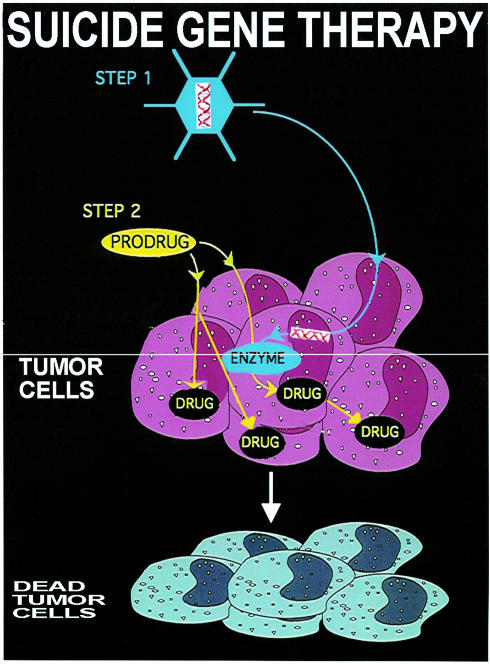
GDEPT and VDEPT are two-step treatments for solid tumors (Figure 1). In the first step, the gene for a foreign enzyme is delivered and targeted in a variety of ways to the tumor where it is to be expressed. In the second step, a prodrug is administered that is selectively activated to the drug by the foreign enzyme expressed in the tumor. Ideally, the gene for the enzyme should be expressed exclusively in the tumor cells and should reach a concentration sufficient to activate the prodrug for clinical benefit. The catalytic activity of the expressed protein must be adequate to activate the prodrug under physiological conditions. Because expression of the foreign enzymes will not occur in all cells of a targeted tumor in vivo, a bystander effect (BE) is required, whereby the prodrug is cleaved to an active drug that kills not only the tumor cells in which it is formed, but also neighboring tumor cells that do not express the foreign enzyme (5).
Figure 1.
GDEPT, a form of suicide gene therapy, aims to maximize the effect of a toxic drug and minimize its systemic effects by generating the drug in situ within the tumor. In the first step in this procedure, the gene for an exogenous enzyme is delivered and expressed in the tumor cells. Subsequently, a prodrug is administered that is converted to the active drug by the foreign enzyme expressed inside or on the surface of tumor cells.
The genes can be engineered to express their products either intracellularly or extracellularly in the recipient cells (6). There are potential advantages to each approach. When the enzyme is intracellularly expressed, the prodrug must enter the cells for activation, and subsequently the active drug must diffuse through the interstitium across the cell membrane to elicit a BE. Cells in which the enzyme is expressed (tethered to the outer surface) are able to activate the prodrug extracellularly. A more substantial BE could therefore be generated with extracellular gene product expression, but spread of the active drug into the general circulation is a possible disadvantage (1, 6).
Parameters that influence the success of GDEPT systems
Effective tumor destruction with GDEPT depends on the design of the gene-therapy vectors, the chemistry of the prodrugs and their toxic metabolites, and the means to deliver one or both components specifically to target cells. Vectors, the vehicles in which the transgenes reach the tumor cell, must be carefully tailored to specific GDEPT systems. The specificity of targeting to cancer cells and efficient transfection are essential for effective GDEPT, as are the toxicity of the vector and the uptake of prodrugs or drugs by normal and malignant cells. GDEPT systems (7–10) require the design of tailored prodrugs and the use of a foreign enzyme — one whose activity is absent from the patient’s tissues (or at least from the tissue to be treated), and that can convert the prodrug to the drug in a specific manner. Beneficial immune effects may be induced either by stimulation of the host immune system or by the use of additional cytokine gene therapy (see article on immunomodulation by Agha-Mohammadi and Lotze, this Perspective series, ref. 11). The efficiency of the BE is another key determinant of the success of these systems.
The enzymes proposed for GDEPT fall into two categories. The first comprises foreign enzymes of nonmammalian origin, with or without human homologues. Examples are viral tyrosine kinase (TK), bacterial cytosine deaminase (CD), carboxypeptidase G2 (CPG2), purine nucleoside phosphorylase (PNP), and nitroreductase (NR). The second category consists of enzymes of human origin that are absent or are expressed only at low concentrations in tumor cells, such as deoxycytidine kinase (dCK) and cytochrome P450. The human homologues of enzymes in the first category have different substrate structural requirements than the foreign enzymes have. Their main disadvantage as therapeutic agents may be the potential to elicit an immune response in humans, although this may actually provide benefits to therapy. Enzymes of the second category are unlikely to induce immune responses, but their use will in most cases lead to some prodrug activation in normal cells.
The second step is administration of the prodrug. GDEPT’s advantages over conventional prodrug therapy (its greater selectivity and higher drug concentrations) arise from the fact that the drug is generated in situ in the target tumor. Prodrugs for GDEPT must be considerably less cytotoxic than their corresponding active drugs, and must be suitable substrates for the activating enzyme under physiological conditions. In addition, they should be chemically stable under physiological conditions and diffuse readily in the tumor interstitium. They should also have good pharmacological and pharmacokinetic properties, and release an active drug with a good BE. Prodrugs must also be tailored to their site of activation: if activation occurs intracellularly, they must be able to cross the tumor cell membrane; whereas if the enzyme is expressed on the surface of cells, there is no such requirement, but the active drug must be able to cross the cell membrane.
The cytotoxicity differential between the prodrug and its corresponding drug should be as high as possible, and the active drug should be highly diffusible or be actively taken up or exported by cells. The design of a prodrug that can release a highly effective drug requires knowledge of the quantitative structure-activity relationship (QSAR). For this reason, and in order to obtain proof of principle for GDEPT, most of the prodrugs used in suicide gene therapy to date have been clinically licensed anticancer agents with known pharmacological, pharmacokinetic, dosage, and safety parameters. However, it is likely that the next generation of prodrugs will be specially tailored for GDEPT. One possibility is the use of “self-immolative” prodrugs.
A self-immolative prodrug can be defined as a compound that generates an unstable intermediate that then extrudes the active drug in subsequent steps. Although the activation process that generates the unstable species is generally enzyme-mediated, extrusion occurs spontaneously through the fragmentation of the prodrug, often at a distinct site. Self-immolative prodrugs allow their lipophilicity to be altered with minimal effect on the activation kinetics. Indeed, kinetics of activation that are unfavorable due to the chemical or steric features of the active drug can be improved by this approach. The range of drugs that can be converted to prodrugs is greatly extended, and is unrestricted by the structural substrate requirements for a given enzyme. Figure 2 shows one self-immolative prodrug activation reaction, the activation of the doxorubicin prodrug by the enzyme CPG2 (12, 13), followed by the spontaneous extrusion of the DNA-damaging agent doxorubicin.
Figure 2.
Self immolation of prodrug1 to yield the chemotherapeutic drug doxorubicin. The doxorubicin prodrug (1) is cleaved by carboxypeptidase G2 (CPG2), releasing the glutamic acid (3) and an unstable intermediate (2). The latter undergoes a spontaneous 1,6 elimination, extruding doxorubicin (4), a quinone imine (5), and carbon dioxide. This scheme can be readily modified to allow the production of structurally similar drugs, such as daunorubicin.
Table 1 summarizes pharmacokinetic data on 11 enzyme/prodrug pairs that have been designed for use in GDEPT systems. With the exception of cyclophosphamide, ifosfamide, and some prodrugs designed for CPG2 and NR, none of the prodrugs shown are self immolative. Self-immolative prodrugs derived from alkylating agents and anthracyclines have been synthesized for activation by CPG2 (12, 13), and self-immolative derivatives from secocyclopropylindolines and ene-diyne prodrugs have been synthesized for use with NR (7).
Table 1.
Quantitative data on GDEPT systems
Also shown in Table 1 are two parameters that are useful in comparing the different GDEPT systems: the potential of activation of a given system and its degree of activation. The first parameter is defined as the ratio of IC50’s of the prodrug and of the active drug in a nontransfected control tumor cell line. It represents the maximum possible efficiency of a given enzyme/prodrug system toward that cell line. The degree of activation is defined as the ratio of the IC50 of the prodrug in the nontransfected cell line to the IC50 of the prodrug in the transfected or infected derivative of the cell line that expresses the activating enzyme. Both the potential for activation and the degree of activation depend on the cell line’s sensitivity to the drug, but the degree of activation also reflects the efficiency of the prodrug system in the context of that cell line; if the enzyme in the transfected line is sufficient to convert the prodrug immediately and quantitatively to the active form of the drug, the degree of activation is identical to the potential of activation for that GDEPT system.
Improving activation kinetics
The concentration of the drug and the rate at which it is released at the activation site depend on the kinetic parameters of the enzyme/prodrug system. Unfortunately, the published Vmax and Km values — which measure the maximum velocity of the activation reaction and the concentration of substrate needed to reach half this maximum velocity — are not sufficient to allow enzyme/prodrug systems to be compared. As a rule, however, low Km and high Vmax (or kcat) would be expected to be found in relatively effective systems, and the comparison of the yeast CD with bacterial CD bears out this prediction. As shown in Table 1, the yeast enzyme, which proved to be more effective than its bacterial counterpart in a GDEPT experiment, exhibits lower Km and higher Vmax values (14). However, the literature does not supply consistent values for the Vmax of these enzymes, because Vmax has been determined under very different experimental conditions for the various systems and is expressed in different ways, making direct comparisons impossible. Despite these caveats, it appears from the data in Table 1 that prodrugs such as CMDA (a substrate of CPG2), GCV (a substrate of HSV-TK), and CPT-11 (a substrate of CA) are superior to 5-FC (a substrate of CD) or 5′-FDUR (a substrate of TP), because the latter two have high Km and low Vmax values. The turnover number, kcat, provides additional information about the reaction rate, but the implications of this measure for tumor cell killing is unclear, because it is not yet known if sudden release of the active drug is more effective than slow, constant release, or if quiescent and proliferating cells differ in their sensitivity to drugs released at different rates.
New techniques that are available to increase the efficacy of enzymes to activate prodrugs for GDEPT were recently reviewed (9). Some of these approaches build on crystallographic descriptions of the active site of the enzyme involved in the enzyme/prodrug system, which should permit the molecular modeling, and eventually the rational synthesis, of substrates that are well-suited for a GDEPT system. An alternative is to modify the active site of the enzyme by site-directed mutagenesis in order to increase its catalytic efficiency toward an existing substrate. Black et al. (15) applied these techniques to obtain mutants of HSV-TK with improved kinetic parameters for the prodrugs GCV and ACV. Similarly, Smith and colleagues (16) performed site-directed mutagenesis on carboxypeptidase A to improve the efficiency of this enzyme toward specific substrates that, by design, are less prone to interfere with other human peptidases (16).
Prodrugs may also be activated by a metabolic cascade involving the sequential action of several enzymes, for example the activation of GCV to GCV triphosphate (GCVTP) by three different kinases (HSV-TK, guanylate kinase, and nucleoside diphosphate kinase), acting in series. This approach requires the cotransfection of genes for each of the enzymes, but is expected to increase the overall yield of the desired final metabolite, the active drug. In the case of GCV, Blanche et al. recently claimed that the simultaneous transfection of these three genes allows cells to convert more than 90% of the prodrug to GCVTP (17). Likewise, the cotransfection of the genes for cytochrome P450 and P450 reductase significantly increases the conversion of CP to it toxic metabolites, and therefore improves the overall efficiency of the cytochrome P450/CP GDEPT system (18) (see Table 1).
Interpreting potential of activation and degree of activation
As discussed above, the potential of activation of a GDEPT system reflects its maximal theoretical efficiency, at least toward a specific cell type. Unfortunately, not all the systems can be defined in this way, because multiple products may be released, and the toxicity of each of these metabolites may not be available. Thus, although GCV is relatively nontoxic, its monophosphorylated derivative, GVCMP, is highly cytotoxic (19), so the potential of activation of this system cannot be calculated accurately from the known IC50 of the triphosphorylated derivative, GCVTP. Likewise, for CP activated by cytochrome P450, a maximum differential of 20- to 25-fold is theoretically achievable based on the IC50 values of CP and its corresponding phosphoramide mustard. However, the degree of activation obtained was found to be 100-fold (20), which suggests that the CP phosphoramide mustard is not the final active metabolite.
The optimal approach to improving the activation potential of a system is to design prodrugs with lower cytotoxicity, but the complementary strategies of increasing the cytotoxicity or improving the efficiency of release of the drug are also helpful. Some highly cytotoxic compounds (with IC50 in the nM range) such as ene-diynes, cyclopropylindolines, and taxoids are now available, but generally their structures are complicated and efficient ways are needed to convert them to low-cytotoxicity prodrugs. Designing self-immolative analogues of these prodrugs could be a way to move forward, as could modifications that improve the uptake of the prodrug by enzymatic modifications (21) or alter the lipophilicity of the prodrug (22). The latter capability is especially useful for tailoring the prodrug for use with an extracellular or intracellular activating enzyme.
The degree of activation, which reflects the efficiency of the system, is another parameter useful for characterizing a GDEPT system. By definition, it must be lower than or at least equal to the potential of activation for the system, as is seen for all the systems analyzed in Table 1. The interpretation of both of these parameters is complex. Their values offer some insight into the in vitro situation, where a single cell type, transfected with a gene for an activating enzyme, is challenged with the prodrug or its toxic metabolites either before or after transfection. For several reasons, these values may not accurately reflect the situation in vivo. Additional factors, such as pharmacokinetics, prodrug distribution, and immune responses complicate the overall picture. Moreover, obtained IC50 values may vary for different cell types, and not all cells in a tumor may be accurately modeled by the cell line chosen for in vitro study. Despite these concerns, these parameters provide a rational basis for comparing different GDEPT systems and should also be helpful in designing new systems.
Increasing the BE
The extent of the BE can be determined from the effect of the treatment on non–genetically modified cells that takes place after prodrug administration, when only a fraction of the tumor mass is genetically modified to express an activating enzyme (5, 8). The striking successes described in GDEPT would surely not be possible in the absence of such an effect. As described, some models require only 1–2% of cells to be genetically modified to obtain therapeutically significant results.
Toxic metabolites are formed after prodrug activation and may be released from dead and dying genetically modified cells. This mechanism is postulated for 5-FU formed from 5-FC; for the metabolites of CP and IP, aldophosphamide, phosphoramidic mustards, and acrolein; for benzoic acid mustard released from CMDA; and for 6-MeP, formed from the corresponding deoxynucleoside. Supporting this model for the BE is the observation that cell-to-cell contact is not required for the killing of untransfected cells by these agents, either in vitro or in vivo. In vitro, 30% of cells expressing CD suffices for the eradication of a whole cell population by 5-FC. This BE is dramatically greater in vivo, even with immunocompromised mice: 2% CD+ tumor cells can yield 100% tumor regression in athymic mice; with 4% CD+ cells, 66% of animals are cured of their xenografts. When similar experiments are performed in immunocompetent animals, the results are better still.
For purine or pyrimidine nucleosides, the toxic metabolites are not diffusible across cell membranes, so the HSV-TK/GCV system apparently requires cell-to-cell contact, specifically gap junction formation (23), to display a BE. Consistent with this model, one report showed that tumor cells resistant to BE did not show dye transfer from cell to cell, whereas BE-sensitive tumor cells did. Furthermore, dieldrin, a drug known to decrease gap junction communications, diminished the dye transfer and also inhibited the BE, leading to the suggestion that the BE of this system could be enhanced by pharmacological manipulation of the gap junctions in vivo. Apigenin, a flavonoid, and lovastatin, an inhibitor of HMG-CoA reductase, both upregulate gap junction function and dye transfer in tumors expressing gap junctions. Touraine et al. (24) studied the control of tumors grown from a mixture of 10% HSV-TK+ adenocarcinoma cells and 90% TK– cells using GCV. In the absence of lovastatin or apigenin, 30% of animals treated become tumor-free, but when tumor-bearing mice were administered lovastatin or apigenin during GCV treatment, their antitumor response rate doubled.
Although these results are consistent with the hypothesis that gap junctions mediate the BE after GCV treatment, other data suggest that additional mechanisms are involved. In one study of human lung tumor cell lines of different origins, significant cell killing occurred when only 10% of cells expressed HSV-TK. In this system, gap junction communication was not apparent from measuring the rapid intercellular transport of Lucifer Yellow, which detects “rapid-transfer” gap junctional communications, although it could be seen by the slow transfer of a different dye, calcein-AM, which measures the “slow-transfer” gap junctions. However, neither an inhibitor (1-octanol) nor an enhancer (all-trans retinoic acid) of gap junction communication affected the extent of the BE, suggesting either that low levels of gap junctions can produce a maximal BE or that bystander cell killing occurs by other means (25). Boucher et al. compared the efficacy of the HSV-TK/GCV system in two human carcinoma cell lines after exposure to GCV and found that the BE depended on the concentration of the enzyme, the number of cells expressing HSV-TK, and the overall confluence of the cells, but not on the activity of functional gap junctions, as assessed by the Lucifer Yellow assay (26).
Another suggestion is that the TK enzyme is transported by apoptotic vesicles or through gap junctions. Phagocytosis of material (e.g., hydrolases or other lytic enzymes) from dying TK+ cells by bystander cells has also been suggested as a mechanism for the BE. Apoptosis was detected in bystander cells and it was found that this event could be inhibited by BCL2 expression. However, during the apoptosis induction period in bystander cells cocultured with HSV-TK–expressing cells, no phagocytosis was observed. It has also been suggested that killing of tumor cells by apoptosis could heighten the immune response to wild-type tumor cells by a priming effect.
A quantitative expression of the BE was recently proposed using the NR/CB1954 system in a range of human tumor-cell types. The IC50’s of non–NR-expressing cells were measured in the presence of varying proportions of NR-expressing cells. The shift in IC50 was used to calculate a value for the BE, termed the transmission efficiency (TE), which is the decrease in IC50 caused by the BE, expressed as a percentage of the maximum measured decrease. The percentage of NR-expressing cells for which the TE was 50% (TE50) is a single data point for the BE. The TE50 in the cell lines ranged from 0.3% to approximately 2% (27).
There were early suggestions that the immune response improves the efficacy of GDEPT. Although the BE has been observed in immunocompromised animals, recent findings suggest that the BE in vivo is mediated largely through the release of cytokines. Ramesh et al. (28) report that GCV treatment of carcinomas that contained a mixture of HSV-TK+ cells resulted in almost total tumor ablation in immunocompetent mice, but not in athymic animals of the same strain. In a similar experiment, when HSV-TK was transfected into cells grown as xenografts, the tumor growth was inhibited for up to 50 days in GCV-treated, immunocompromised nude mice, but failed to eliminate all the tumor cells in these animals, and tumors regrew 40–50 days after implantation. By contrast, immunocompetent BALB/c mice developed long-lasting immunity in response to HSV-TK transduction followed by GCV treatment (29). Taken together, these studies strongly suggest that an intact immune system is important for long-term tumor suppression with TK in vivo.
IL-2 appears to be critical for immune-mediated tumor suppression in this system. In one experiment, cells grown as xenografts in syngeneic mice were injected with an adenoviral vector containing the HSV TK gene, the IL2 gene, or both, followed by treatment with GCV (30). Whereas the tumors continued to grow in the animals injected with a control vector or the vector carrying IL2, those treated with HSV TK, with or without coadministration of IL2, exhibited tumor necrosis and regression. However, only animals treated with both genes developed effective systemic antitumoral immunity against tumorigenic rechallenges. The antitumoral immunity was associated with the presence of tumor-specific cytolytic CD8+ T lymphocytes. A third vector containing the mouse GMCSF gene enhanced and prolonged this antitumoral immunity, allowing animals treated with all three genes to survive for longer than four months without recurrence. These and similar findings (30) establish the synergism between suicide gene and cytokine gene therapies.
Future perspectives
Some hurdles must be overcome before GDEPT will become a clinically efficient treatment of cancers. Major improvements are needed in vector design to enhance targeting and delivery of suicide genes. Multiple options are available, including nonviral vectors, more complex systems involving coexpression of suicide genes with immunological or tumor-suppressor genes, and selectively replicating viruses. Double suicide gene therapy, in which a combination of suicide genes is introduced simultaneously, shows promise in vitro and in vivo. The released active drugs in such an approach can act by different mechanisms, leading to a synergistic effect on tumor cells or an enhanced BE, particularly if cell-permeant and cell-impermeant active metabolites can be released together. Additionally, the occurrence of resistant populations is less likely for drugs with different mechanisms of action. Uckert et al. (31) have shown for human carcinoma cell lines grown in vivo that double suicide gene therapy (involving HSV-TK and CD) allowed the elimination of tumors, but neither gene applied individually gave this result.
Thus, GDEPT systems have already shown efficacy in vivo. Future developments in this technology should use mutagenesis to obtain more efficient activation of a given prodrug, or to adapt the active site so that it binds better to prodrugs that are not substrates for endogenous enzymes. The prodrugs, too, should be redesigned to create better substrates for the enzymes, to maximize drug release or the BE, to take advantage of self-immolative strategies of activation, or to allow the active drug to accumulate more readily in tumor cells. Finally, it will also be useful to investigate the ways in which different prodrug systems synergize with each other or with other cancer treatments. The combination of GDEPT with radiotherapy or immunotherapy has previously been suggested. Such approaches may involve a sequential treatment schedule (GDEPT/radiation therapy or GDEPT/immunotherapy). The transfection of suicide genes together with genes that are able to increase the sensitivity of the tumors to radiation or enhance the potential of the host immune system is an alternative strategy. Other combination therapies are possible, such as applying GDEPT in conjunction with replicating oncolytic adenoviruses, such as ONYX-015 (32; see also Heise and Kirn, this Perspective series, ref. 33) or ONYX-838, or the use of such viruses as carriers for the suicide genes (see Hermiston, this Perspective series, ref. 4). The combination of these replicating adenoviruses with conventional chemotherapy has proven highly effective, and replacing the chemotherapeutic arm in the ONYX-015 study with GDEPT might well provide additional benefits.
Acknowledgments
This work was funded by the Cancer Research Campaign (grants SP2330/0201 and SP2330/0102) and the Institute of Cancer Research. We would like to thank P. Workman and K. Harrap for support.
References
- 1.Marais R, Spooner RA, Light Y, Martin J, Springer CJ. Gene-directed enzyme prodrug therapy with a mustard prodrug/carboxypeptidase G2 combination. Cancer Res. 1996;56:4735–4742. [PubMed] [Google Scholar]
- 2.Bridgewater JA, et al. Expression of the bacterial nitroreductase enzyme in mammalian cells renders them selectively sensitive to killing by the prodrug CB1954. Eur J Cancer. 1995;31A:2362–2370. doi: 10.1016/0959-8049(95)00436-x. [DOI] [PubMed] [Google Scholar]
- 3.Huber BE, Richards CA, Austin EA. VDEPT: an enzyme/prodrug gene therapy approach for the treatment of metastatic colorectal cancer. Advanced Drug Delivery Reviews. 1995;17:279–292. [Google Scholar]
- 4.Hermiston T. Gene delivery from replication-selective viruses: arming guided missiles in the war against cancer. J Clin Invest. 2000;105:1169–1172. doi: 10.1172/JCI9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huber BE, Austin EA, Richards CA, Davis ST, Good SS. Metabolism of 5-fluorocytidine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci USA. 1994;91:8302–8306. doi: 10.1073/pnas.91.17.8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marais R, et al. A cell surface tethered enzyme improves efficiency in gene-directed enzyme prodrug therapy. Nat Biotechnol. 1997;15:1373–1377. doi: 10.1038/nbt1297-1373. [DOI] [PubMed] [Google Scholar]
- 7.Denny WA, Wilson WR. The design of selectively-activated anti-cancer prodrugs for use in antibody-directed and gene-directed enzyme prodrug therapies. J Pharm Pharmacol. 1998;50:387–394. doi: 10.1111/j.2042-7158.1998.tb06878.x. [DOI] [PubMed] [Google Scholar]
- 8.Niculescu-Duvaz I, Spooner R, Marais R, Springer CJ. Gene-directed enzyme prodrug therapy. Bioconjug Chem. 1998;9:4–22. doi: 10.1021/bc970116t. [DOI] [PubMed] [Google Scholar]
- 9.Encell LP, Landis DM, Loeb LA. Improving enzymes for gene therapy. Nat Biotechnol. 1999;17:143–147. doi: 10.1038/6142. [DOI] [PubMed] [Google Scholar]
- 10.Springer CJ, Niculescu-Duvaz I. Patent property of prodrug involving gene therapy (1996–1999) Expert Opinion on Therapeutical Patents. 1999;9:1381–1388. [Google Scholar]
- 11.Agha-Mohammadi S, Lotze MT. Immunomodulation of cancer: potential use of selectively replicating agents. J Clin Invest. 2000;105:1173–1176. doi: 10.1172/JCI10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niculescu-Duvaz D, et al. Self-immolative nitrogen mustard prodrugs for suicide gene therapy. J Med Chem. 1998;41:5297–5309. doi: 10.1021/jm980425k. [DOI] [PubMed] [Google Scholar]
- 13.Niculescu-Duvaz I, et al. Self-immolative anthracycline prodrugs for suicide gene therapy. J Med Chem. 1999;42:2485–2489. doi: 10.1021/jm980696v. [DOI] [PubMed] [Google Scholar]
- 14.Kievit E, et al. Superiority of yeast over bacterial cytosine deaminase for enzyme/prodrug gene therapy in colon cancer. Cancer Res. 1999;59:1417–1421. [PubMed] [Google Scholar]
- 15.Black ME, Newcomb TG, Wilson HM, Loeb LA. Creation of drug-specific herpes simplex virus type 1 thymidine kinase mutants for gene therapy. Proc Natl Acad Sci USA. 1996;93:3525–3529. doi: 10.1073/pnas.93.8.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith GK, et al. Toward antibody-directed enzyme prodrug therapy with the T268G mutant of human carboxypeptidase A1 and novel in vivo stable prodrugs of methotrexate. J Biol Chem. 1997;272:15804–15816. doi: 10.1074/jbc.272.25.15804. [DOI] [PubMed] [Google Scholar]
- 17.Blanche, F., Cameron, B., Couder, M., and Crouzet, J. 1997. Rhone-Poulenc Rorer. Enzymes combinations for destroying proliferative cells. Patent WO 9735024
- 18.Chen L, Yu LJ, Waxman DJ. Potentiation of cytochrome P450/cyclophosphamide-based cancer gene therapy by coexpression of the P450 reductase gene. Cancer Res. 1997;57:4830–4837. [PubMed] [Google Scholar]
- 19.Rubsam LZ, Davidson L, Shewach DS. Superior cytotoxicity with ganciclovir compared with acyclovir and 1-beta-D-arabinofuranosylthymine in herpes simplex virus-thymidine kinase-expressing-cells: a novel paradigm for cell killing. Cancer Res. 1998;58:3873–3882. [PubMed] [Google Scholar]
- 20.Waxman, D.J., and Goldstein, J.A. 1999. Trustee of Boston University. Methods of using cytochrome P450 reductase for the enhancement of P450-based anti-cancer gene therapy. Patent WO 9905299.
- 21.Tiraby M, et al. Concomitant expression of E. coli cytosine deaminase and uracil phosphoribosyltransferase improves the cytotoxicity of 5-fluorocytosine. FEMS Microbiol Lett. 1998;167:41–49. doi: 10.1111/j.1574-6968.1998.tb13205.x. [DOI] [PubMed] [Google Scholar]
- 22.Balzarini G, et al. Superior cytostatic activity of the ganciclovir elaidic acid ester due to the prolonged intracellular retention of ganciclovir anabolites in herpes simplex virus type 1 thymidine kinase gene-transfected tumor cells. Gene Ther. 1998;5:419–426. doi: 10.1038/sj.gt.3300586. [DOI] [PubMed] [Google Scholar]
- 23.Touraine RL, Ishii-Morita H, Ramsey WJ, Blaese RM. The bystander effect in the HSVtk/ganciclovir system and its relation to gap junctional communication. Gene Ther. 1998;5:1705–1711. doi: 10.1038/sj.gt.3300784. [DOI] [PubMed] [Google Scholar]
- 24.Touraine RL, Vahanian N, Ramsey WJ, Blaese RM. Enhancement of the herpes simplex virus thymidine kinase/ganciclovir bystander effect and its antitumor efficacy in vivo by pharmacologic manipulation of gap junctions. Hum Gene Ther. 1998;9:2385–2391. doi: 10.1089/hum.1998.9.16-2385. [DOI] [PubMed] [Google Scholar]
- 25.Imaizumi K, et al. Bystander tumoricidal effect and gap junctional communication in lung cancer cells. Am J Respir Cell Mol Biol. 1998;18:205–212. doi: 10.1165/ajrcmb.18.2.2821. [DOI] [PubMed] [Google Scholar]
- 26.Boucher PD, Ruch RJ, Shewach DS. Differential ganciclovir-mediated cytotoxicity and bystander killing in human colon carcinoma cell lines expressing herpes simplex virus thymidine kinase. Hum Gene Ther. 1998;9:801–814. doi: 10.1089/hum.1998.9.6-801. [DOI] [PubMed] [Google Scholar]
- 27.Friedlos F, Court S, Ford M, Denny WA, Springer CJ. Gene-directed enzyme prodrug therapy: quantitative bystander cytotoxicity and DNA damage induced by CB1954 in cells expressing bacterial nitroreductase. Gene Ther. 1998;5:105–112. doi: 10.1038/sj.gt.3300569. [DOI] [PubMed] [Google Scholar]
- 28.Ramesh R, Marrogi AJ, Munshi A, Abboud CN, Freeman SM. In vivo analysis of the “bystander effect”: a cytokine cascade. Exp Hematol. 1996;24:829–838. [PubMed] [Google Scholar]
- 29.Pavlovic J, Nawrath M, Tu R, Heinicke T, Moelling K. Anti-tumor immunity is involved in the thymidine kinase-mediated killing of tumors induced by activated Ki-ras (G12V) Gene Ther. 1996;3:635–643. [PubMed] [Google Scholar]
- 30.Chen SH, et al. Combination suicide and cytokine gene therapy for hepatic metastases of colon carcinoma: sustained antitumor immunity prolongs animal survival. Cancer Res. 1996;56:3758–3762. [PubMed] [Google Scholar]
- 31.Uckert W, et al. Double suicide gene (cytosine deaminase and herpes simplex virus thymidine kinase) but not single gene transfer allows reliable elimination of tumor cells in vivo. Hum Gene Ther. 1998;9:855–865. doi: 10.1089/hum.1998.9.6-855. [DOI] [PubMed] [Google Scholar]
- 33.Heise CC, Williams A, Olesch J, Kirn DH. Efficacy of a replication-competent adenovirus (ONYX-015) after intratumoral injection: intratumoral spread and distribution effects. Cancer Gene Ther. 6:499–504.32.Heise, C., and Kirn, D.H. 2000. Replication-selective adenoviruses as oncolytic agents. J Clin Invest. 1999;105:847–851. doi: 10.1038/sj.cgt.7700071. [DOI] [PubMed] [Google Scholar]