Abstract
The primary player that induces insulin resistance has not been established. Here, we studied whether or not fat can cause insulin resistance in the presence of insulin deficiency. Our results showed that high-fat diet (HFD) induced insulin resistance in C57BL/6 (B6) mice. The HFD-induced insulin resistance was prevented largely by the streptozotocin (STZ)-induced moderate insulin deficiency. The STZ-induced insulin deficiency prevented the HFD-induced ectopic fat accumulation and oxidative stress in liver and gastrocnemius. The STZ-induced insulin deficiency prevented the HFD- or insulin-induced increase in hepatic expression of long-chain acyl-CoA synthetases (ACSL), which are necessary for fatty acid activation. HFD increased mitochondrial contents of long-chain acyl-CoAs, whereas it decreased mitochondrial ADP/ATP ratio, and these HFD-induced changes were prevented by the STZ-induced insulin deficiency. In cultured hepatocytes, we observed that expressions of ACSL1 and -5 were stimulated by insulin signaling. Results in cultured cells also showed that blunting insulin signaling by the PI3K inhibitor LY-294002 prevented fat accumulation, oxidative stress, and insulin resistance induced by the prolonged exposure to either insulin or oleate plus sera that normally contain insulin. Finally, knockdown of the insulin receptor prevented the oxidative stress and insulin resistance induced by the prolonged exposure to insulin or oleate plus sera. Together, our results show that insulin and insulin signaling are required for fat induction of insulin resistance in mice and cultured mouse hepatocytes.
Keywords: fatty acid, long-chain acyl-CoA synthetase, obesity, diabetes
insulin resistance is associated with numerous modern health problems such as obesity, metabolic syndrome, type 2 diabetes mellitus, cardiovascular disorders, nonalcoholic fatty liver diseases, Alzheimer's disease, depression, asthma, some cancers, and aging (1, 2, 4, 6, 7, 30, 32, 34, 42). With ∼75% of Americans overweight, ∼33% of Americans obese, and nearly 8% of Americans diabetic, the impact of insulin resistance on the general health and economy could not be more obvious now. Establishment of the primary player that mediates the development of insulin resistance has become an urgent priority in fighting these modern health problems.
There is no doubt that different individuals are very different in their vulnerability to development of obesity and insulin resistance. Different species of animals such as C57BL/6 (B6), 129, and A/J mice may also be very different in terms of development of insulin resistance. For example, obesity and insulin resistance can be easily induced by using high-fat diet (HFD) in B6 mice but can't be induced in A/J or other mice by any diet, including HFD (44, 45). Thus, there is no doubt that genetic background plays an important role in the development of obesity and insulin resistance. However, obesity and insulin resistance may not occur in any genetic background if no excess calories are ingested. For example, it is impossible to induce obesity and insulin resistance in B6 mice with normal chow diet because they simply do not overingest calories on the chow diet, although B6 mice take in excess calories and readily develop obesity and insulin resistance when they are on HFD (44, 45). Other species of mice such as A/J mice do not normally overingest calories on any diet and do not easily develop obesity or insulin resistance even on HFD (44). That is why B6 mice instead of other species of mice are commonly used in metabolic studies. Thus, it is equally true that obesity and associated insulin resistance appear to be caused primarily by the positive energy imbalance due to overeating and/or lack of physical activity.
Consumption of HFD has generally been considered a significant contributor to the development of obesity and insulin resistance. It is well conceived that some humans and some animals, such as B6 mice, tend to take in excess calories on HFD. Thus, it is very clear that excess calories and fat are the direct cause of obesity and insulin resistance. Nevertheless, the primary player that converts the excess calories and fat into insulin resistance remains to be established. The general perception is that fat plays a primary role in the development of insulin resistance (56). However, the extremely high levels (>1.5 mM) of fatty acids together with extremely low levels of insulin in the blood of subjects with prolonged starvation are actually associated with increased insulin sensitivity instead of insulin resistance (57). It has previously been shown that insulin itself may contribute to development of insulin resistance (39). Blockade of insulin secretion with diazoxide has been shown to prevent the HFD-induced obesity and insulin resistance (43). It has also been shown that hyperinsulinemia precedes insulin resistance (11). We have shown recently that the basal level of the Akt-dependent classical insulin signaling is increased in B6 mice with obesity and insulin resistance, and this increase is responsible for the development of insulin resistance induced by HFD (21). However, the necessity of insulin signaling in the development of insulin resistance and the associated mechanisms have not been established or recognized yet. In this study, we attempted to further determine whether insulin and insulin signaling were necessary for fat to induce insulin resistance in mice and cultured hepatocytes and revealed some new novel mechanisms.
MATERIALS AND METHODS
Reagents.
Streptozotocin (STZ; cat. no. 572201) and LY-294002 were from Calbiochem. The triglyceride (TG) assay kit (cat. no. TR0100), the recombinant human insulin, and antisera against β-actin were from Sigma. Blood glucose concentrations were measured by using Breese 2 glucose meter (Bayer HealthCare). Plasma insulin levels were measured with a Linco insulin ELISA kit. Antibodies against total/phospho-Akt were from Cell Signaling Technology (Danvers, MA). Antibodies against glucose transporter 4 (Glut4) (cat. no. sc-7938) and insulin receptor (cat. no. sc-31366) were from Santa Cruz Biotechnology. Antibody against phospho-IRS-1 at serine 636 was from Abocam (cat. no. ab47764). GSH/GSSG-412 assay kit was from Bioxytech (Foster City, CA). The free fatty acid (FFA) measuring kit was from BioVision (K6K-1.0). Other materials were all obtained commercially and are of analytical quality.
Animal experiments.
Animals were housed under the usual day (12 h of day light) and night (12 h of darkness) circadian and fed ad libitum. To examine the effect of the HFD on weight gain, ectopic fat accumulation, and oxidative stress, C57BL/6 (B6) mice were fed with either normal rodent chow diet (ND) or HFD (Research Diets no. D12330; 58.0 kcal% fat, 16.0 kcal% protein, and 26 kcal% carbohydrate) for 42 days as described (5, 46, 55). To study the effect of insulin, B6 mice were treated with STZ (50 mg/kg body wt ip injection once daily for 3 days) before HFD for the same period as HFD alone. All animal studies were approved by the Institutional Animal Care and Use Committee of the Hamner Institutes for Health Sciences and fully complied with the guidelines from the National Institutes for Health.
Insulin tolerance test.
Insulin tolerance test (ITT) was performed after an overnight fast as described (22). Initial blood glucose levels were determined, followed by injection (ip) of human insulin (0.75 U/kg, cat. no. I9278; Sigma). Blood glucose levels were measured via tail vein blood at 15, 30, 60, 90, and 120 min after the injection. The glucose level at the time point 0 min was presented as 100%, and glucose level of the rest time points were calculated as the percentage of the time point 0 min.
Cells.
Mouse Hepa1c1c7 hepatoma cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum.
Measurements of mitochondrial contents of long-chain acyl CoAs, ADP, and ATP.
Liver mitochondria were isolated by using a standard method. Mitochondrial palmitoyl-CoA, stearoyl-CoA, oleoyl-CoA, ADP, and ATP were separated and purified by using an Agilent HPLC 1200 system equipped with a binary solvent manager and a sample manager (Agilent, Santa Clara, CA). Purified mitochondrial contents of palmitoyl-CoA, stearoyl-CoA, oleoyl-CoA, ADP, and ATP were quantified by positive ESI-MS mass spectrometry with an Agilent model 6220 MSD TOF mass spectrometry equipped with a dual-sprayer electrospray ionization source (Agilent). All of these procedures were performed at the University of North Carolina at Greensboro Center for Research Excellence in Bioactive Food Components located on the North Carolina Research Campus, Kannapolis, NC.
Measurement of glutathione/glutathione disulfide ratio.
Levels of glutathione (GSH) and glutathione disulfide (GSSG) in tissue lysates were determined with a kit from OXIS International (Foster City, CA) and normalized to protein levels. The GSH/GSSG ratio was calculated according to the instructions provided by the manufacturer, as described (21, 22).
RNA extraction and real-time PCR.
Total RNAs were extracted from cells or tissues with an RNeasy Mini Kit (Qiagen) and reverse transcribed into cDNAs, which were quantified by TaqMan real-time PCR, with specific probes and primers listed in Table 1, and normalized to levels of 36B4.
Table 1.
Primers used for real-time PCR
| Gene Name (Protein Name) | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| Cpt1a (liver, CPT I) | GACTCCGCTCGCTCATTC | TCTGCCATCTTGAGTGGTGA |
| Cpt1b (muscle, M-CPT I) | TGCCTTTACATCGTCTCCAA | GGCTCCAGGGTTCAGAAAGT |
| Acadm (MCAD) | TGTCGAACACAACACTCGAAA | CTGCTGTTCCGTCAACTCAA |
| Acadl (LCAD) | TGGGGACTTGCTCTCAACA | GGCCTGTGCAATTGGAGTA |
| Acadvl (VLCAD) | GGTGGTTTGGGCCTCTCTA | GGGTAACGCTAACACCAAGG |
| Srebf1 (SREBP-1) | GGTTTTGAACGACATCGAAGA | CGGGAAGTCACTGTCTTGGT |
| Fasn (FAS) | CCAAATCCAACATGGGACA | TGCTCCAGGGATAACAGCA |
| Srebf2 (SREBP2) | ACCTAGACCTCGCCAAAGGT | GCACGGATAAGCAGGTTTGT |
| Hmgcr (HMG-CoAR) | TGCGTAAGCGCAGTTCCT | TTGTAGCCTCACAGTCCTTGG |
| Cs (citrate synthase) | CCATCCATAGTGACCATGAG | CTTTGCCAACTTCCTTCTGT |
| Gpx1(glutathione reductase 1) | GTGAGCCTGGGCTCCCTGCG | ACTTGAGGGAATTCAGAATC |
| Gsr (glutathione reductase) | GGGTGGCACTTGCGTGAATG | TTCAGGCGGCTCACATAGGC |
| Hhmox1 (HO-1) | CTATGTAAAGCGTCTCCA | GTCTTTGTGTTCCTCTGTC |
| Sod1 (Cu/Zn-SOD) | CAAGCGGTGAACCAGTTGTG | TGAGGTCCTGCACTGGTAC |
| Sod2 (MnSOD) | TCATGCAGCTGCACCACAGC | CCATTGAACTTCAGTGCAGG |
| Acsl1 (ACSL1) | AAAGATGGCTGGTTACACACG | CGATAATCTTCAAGGTGCCATT |
| Acsl5 (ACSL5) | ACAACCAGTCTGTGGGGATT | GGTCATTGTTCTTCTGGAAAGC |
| 36B4 | GCAGACAACGTGGGCTCCAAGCAGAT | GGTCCTCCTTGGTGAACACGAAGCCC |
CPT I, carnitine palmitoyltransferase I; M-CPT I, muscle CPT I; MCAD, medium-chain acyl-CoA dehydrogenase; LCAD, long-chain acyl-CoA dehydrogenase; VLCAD, very long-chain acyl-CoA dehydrogenase; SREBP-1 and -2, sterol regulatory element-binding protein-1 and -2, respectively; FAS, fatty acyl synthase; HMG-CoAR, hydroxymethylglutaryl-coenzyme A reductase; Cs, catalase; Gpx1, glutiathone peroxidase 1; Gsr, glutathione reductase; ACSL, acyl-CoA synthetases.
Immunoblotting.
Immunoblotting was performed as described previously (49–55). In brief, cells were lysed in Nonidet P-40 lysis buffer [1% Nonidet P-40, 150 mM NaCl, 10% glycerol, 2 mM EDTA, 20 mM Tris (pH 8.0), 1 mM dithiothreitol, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, and 10 μg/ml aprotinin]. Cell lysates (15 μg/lane) were resolved in 4–20% Tris-glycine gels (Invitrogen) and transferred to nitrocellulose membranes (Bio-Rad). Target proteins were detected by immunoblotting with primary antibodies as indicated and alkaline phosphatase-conjugated secondary antisera. The fluorescent bands were visualized with a Typhoon 9410 variable mode imager from GE Healthcare and then quantified by densitometry using ImageQuant 5.2 software (GE Healthcare).
siRNA.
The cognate siRNA against mouse insulin receptor (siINSR1: AAGGAACCUAAUG GUCUGAUUGUGCUAUA; siINSR2: UCU UCUACAGCGAGGAGAACAAGGCUCCU) or scramble siRNA (cat. no. sc-37007; Santa Cruz Biotechnology) was introduced into 1c1c7 hepatocytes by reverse transfection with Lipofectamine 2000, as previously described (36). Briefly, the siRNA transfection mixture was preapplied to collagen-coated 12-well plates right before the plating of hepatocytes in culture medium without antibiotics. After 48 h, the cells were exposed to 10 nM insulin for an additional 24 h. After being washed extensively with warm PBS and starved in FBS-free culture medium for 1 h, the cells were acutely stimulated with 1 nM insulin for 5 min. Activation of Akt was evaluated by immunoblotting to determine the responses to insulin.
Statistical analyses.
Data are presented as means ± SE. Data were compared by Student t-test with GraphPad Prism version 4.0 for Windows (San Diego, CA). Differences at values of P < 0.05 were considered significant.
RESULTS
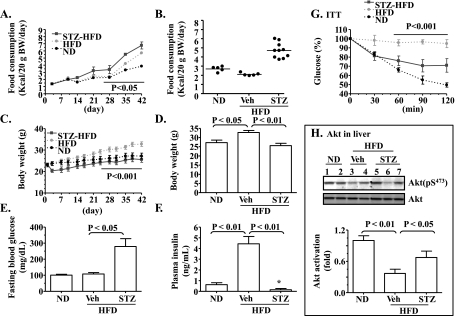
Moderate insulin deficiency induced by STZ largely prevents the HFD-mediated insulin resistance in B6 mice. We have shown recently that insulin resistance induced by HFD can be prevented by blunting insulin signaling with a phosphatidylinositol 3-kinase (PI3K) inhibitor during the time when insulin is not highly demanded, such as during sleeping (21). To determine whether or not fat can induce insulin resistance in the presence of obvious insulin deficiency, B6 mice were first treated with STZ to moderately disrupt their insulin secretion as described (53) and then fed HFD for 42 days (note: the average fasting blood glucose of mice treated with STZ was 162 ± 18 mg/dl right before the start of HFD). Subsequently, food consumption, body weights, plasma levels of fasting glucose and insulin, insulin tolerance, and insulin signaling (Akt phosphorylation) were evaluated. Compared with the mice on ND, mice on HFD ingested a similar amount of food during the first 35 days in terms of weight of food, which suggested that they were taking in more calories than the mice on ND (Fig. 1, A and B). Nevertheless, it is clear that mice on HFD gained more body weight (Fig. 1, C and D). The fasting plasma level of insulin was also increased significantly by HFD, whereas the fasting blood glucose level was not yet altered (Fig. 1, E and F). It is noteworthy that the mice with STZ-induced insulin deficiency gained much less weight, even though they consumed HFD and significantly more amounts of food than the normal mice on ND (Fig. 1, A–D). The fasting plasma insulin level in the mice treated with STZ was very low, whereas blood glucose level was high (Fig. 1, E and F). These observations demonstrate that insulin deficiency slows body weight gain in B6 mice on HFD.
Fig. 1.
Deficiency of insulin secretion caused by streptozotocin (STZ) prevents the high-fat diet (HFD)-induced insulin resistance. C57BL/6 (B6) mice (10 wk old) were fed either the normal chow diet (ND; n = 5) or HFD (n = 5) for 42 days. Another group of B6 mice was treated with STZ for 3 days before the 42-day HFD (STZ-HFD; n = 10). A–D: food consumption and body weight were monitored every 3–7 days. E: fasting blood glucose at the end of the experiment. F: fasting plasma insulin levels were measured at the end of the experiment. G: insulin tolerance test (ITT) was performed after an overnight fast at the end of the experiment. The glucose level at the time point 0 min was presented as 100%, and glucose level of the rest of the time points were calculated as %time point 0 min. H: levels of hepatic phospho- and total Akt right after ITT were measured by immunoblotting and quantified. Vehicle (Veh): saline. Results represent means ± SE. *P < 0.05 vs. ND.
Importantly, as shown in Fig. 1G, compared with the normal mice on ND, mice on HFD did not respond to insulin challenge at all, indicating the presence of insulin resistance in these mice. In contrast, mice with the STZ-induced insulin deficiency under HFD responded to insulin challenge very well, albeit not as well as the normal mice on ND. Similarly, levels of Akt phosphorylation measured in livers of the mice on HFD right after the treatment with insulin during the ITT were lower compared with the normal mice on ND (Fig. 1H). The mice with treatments with STZ and HFD responded to insulin (administered for ITT) very well in terms of Akt phosphorylation (Fig. 1H). Together, these results suggest that insulin deficiency largely prevented the HFD-induced insulin resistance.
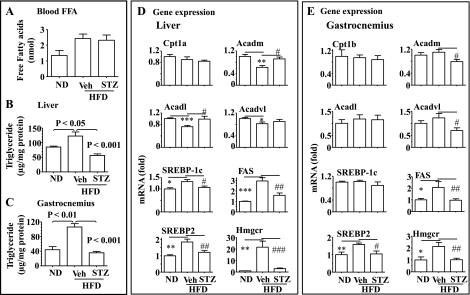
Moderate insulin deficiency induced by STZ prevents the HFD-mediated ectopic fat accumulation. Plasma levels of free fatty acids (FFA) were increased by HFD (Fig. 2A) and treatment with STZ did not alter the HFD-induced increase in the blood FFA level (Fig. 2A). As shown in Fig. 2, B and C, fat accumulation (TG content) was increased in both liver and gastrocnemius by HFD, but the increase was prevented by the STZ-induced insulin deficiency. Expression of some key lipogenic genes, including sterol regulatory element-binding protein (SREBP)-1c, SREBP-2, fatty acid synthase, and 3-hydroxy-3-methylglutaryl-CoA synthase, were all increased in liver by HFD, and the increases were prevented by the insulin deficiency (Fig. 2D). Similar results were observed in gastrocnemius (Fig. 2E). Expressions of some key genes involved in fatty acid oxidation, including mid-, long-, and very long-chain acyl-CoA dehydrogenases (Acadm, Acadl, and Acadvl) were reduced by HFD, and the decreases of some genes were prevented by the insulin deficiency. Similar but less dramatic changes in fat oxidation genes were observed in gastrocnemius. Expression of another key gene involved in fat oxidation, Cpt1, was not obviously altered (Fig. 2, D and E). Together, these results show that insulin is required for the development of ectopic fat accumulation.
Fig. 2.
Deficiency of insulin secretion caused by STZ prevents the HFD-induced ectopic fat accumulation. A: plasma levels of free fatty acids (FFA). Triglyceride contents in the liver (B) and gastrocnemius (C) of the mice described in Fig. 1 were measured and normalized to the protein level. Expression of key genes involved in fatty acid oxidation (Cpt1, Acadm, Acdal, and Acdavl) and lipogenesis were evaluated by real-time PCR in the liver (D) and gastrocnemius (E) of the mice described in Fig. 1 and normalized to 36B4. Results represent means ± SE. Veh: saline. *P < 0.05, **P < 0.01, and ***P < 0.001, Veh + HFD vs. ND. #P < 0.05, ##P < 0.01, and ###P < 0.001, STZ + HFD vs. Veh + HFD.
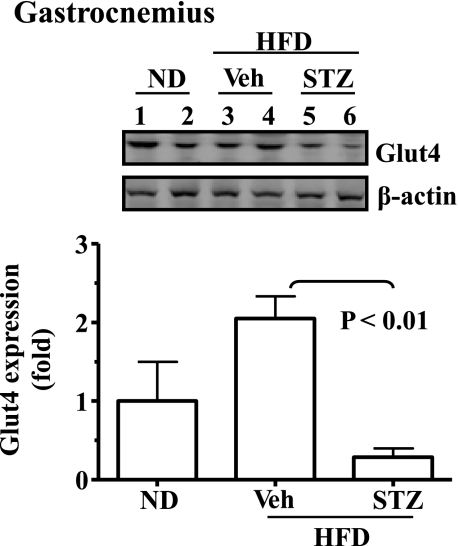
Moderate insulin deficiency induced by STZ decreases expression of the Glut4 gene in skeletal muscle. Results from Fig. 1 show that the response of STZ-treated mice under HFD to the acute insulin challenge was much better in terms of reduction of blood glucose than that of the mice under HFD alone but was not as good as that of the mice on ND during the ITT. Interestingly, it has been shown previously that expression of the Glut4 gene requires insulin (17). Thus, the expression level of Glut4 in skeletal muscles was evaluated. As shown in Fig. 3, Glut 4 mRNA level was decreased significantly in the STZ-treated mice. These results may explain why the STZ-treated mice did not respond to the acute insulin challenge as well as the normal mice on ND.
Fig. 3.
Deficiency of insulin secretion caused by STZ leads to decreased expression of the glucose transporter 4 (Glut4) gene. Expression of the Glut4 gene in the gastrocnemius of the mice described in Fig. 1 was detected by immunoblotting and quantified. Results represent means ± SE (ND, n = 5; HFD, n = 5; STZ-HFD, n = 10). Veh: saline. P < 0.05, ND compared with HFD plus STZ.
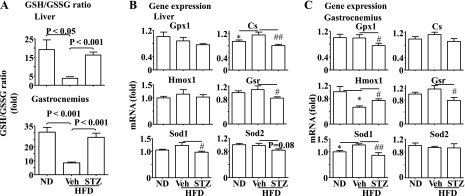
Moderate insulin deficiency induced by STZ prevents the HFD-mediated oxidative stress in liver and skeletal muscles. To study why the STZ-induced insulin deficiency prevented the development of insulin resistance induced by HFD shown in Fig. 1, levels of oxidative stress in liver and skeletal muscle were examined. As shown in Fig. 4A, the ratio of GSH/GSSG was decreased significantly in liver and gastrocnemius by HFD, suggesting the presence of oxidative stress. The HFD-induced reduction of GSH/GSSG ratio was prevented by the STZ-induced insulin deficiency (Fig. 4A).
Fig. 4.
Deficiency of insulin secretion caused by STZ prevents the HFD-induced oxidative stress in the liver and skeletal muscle. A: the ratio of reduced (GSH) and oxidized glutathione (GSSG) was evaluated in the liver and gastrocnemius of the mice described in Fig. 1. Expression of genes involved in oxidative stress was determined by real-time PCR in the liver (B) and gastrocnemius (C) of the mice described in Fig. 1 and normalized to 36B4. Results represent means ± SE. Veh: saline. *P < 0.05, Veh + HFD vs. ND. #P < 0.05 and ##P < 0.01, STZ + HFD vs. Veh + HFD. Gpx1, glutiathone peroxidase 1; Cs, catalase; Gsr, glutiathone reductase; Hmox1, heme-oxygenase-1; Sod1, and -2 superoxide dismutase-1 and -2.
Transcript levels of several oxidation-responsive genes, including antioxidant enzymes glutathione peroxidase 1, catalase, glutathione reductase, heme-oxygenase-1, and superoxide dismutase-1 and -2 (Sod1 and Sod2), were then evaluated. Expressions of some of these genes (catalase and Sod1 in liver, glutathione reductase and Sod1 in gastrocnemius) tended to increase or were enhanced by HFD (Fig. 4, B and C), further supporting the presence of oxidative stress. However, the changes were reversed by the insulin deficiency induced by STZ (Fig. 4, B and C). The only exception was expression of heme-oxygenase-1. Together, these results indicate that insulin plays a primary role in the HFD-induced oxidative stress in liver and skeletal muscles.
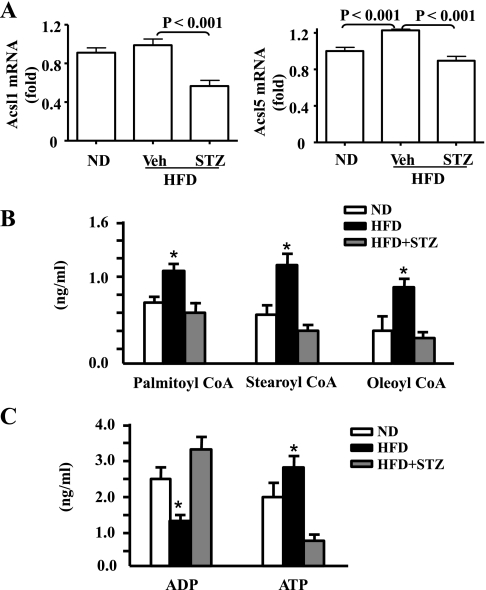
Moderate insulin deficiency induced by STZ prevents the HFD- or insulin-stimulated expression of long-chain acyl-CoA synthetases (Acsl) and altered mitochondrial contents of long-chain acyl-CoAs and ADP/ATP ratio. Activation of FFA is catalyzed by ACSL. ACSL1 and ACSL5 are the main ACSL isoforms in liver (10, 15, 31, 47). and ACSL5 is a mitochondrial isoform (27). Besides, it has been shown that expression of ACSL1 in adipocytes is insulin dependent (16, 52). Therefore, transcripts of hepatic ACSL1 and ACSL5 genes were quantified by real-time PCR. As shown in Fig. 5A, hepatic ACSL5 transcripts were significantly increased, whereas ACSL1 transcripts tended to increase in the mice under HFD. The increases were reversed by the insulin deficiency induced by STZ. These results imply that hepatic expression of ACSLs is insulin-dependent, and insulin is capable of affecting mitochondrial reactive oxygen species (mtROS) via expression of ACSL genes.
Fig. 5.
Deficiency of insulin secretion caused by STZ reverses the HFD-stimulated expression of long-chain acyl-coenzyme A (CoA) synthetases (ACSL) and changes in mitochondrial contents of long-chain acyl-CoAs, ADP, and ATP. A: expression of genes Acsl1 and Acsl5 in the livers of the mice described in Fig. 1 were determined by real-time PCR and normalized to 36B4. Mitochondrial contents of long-chain acyl CoAs (B) and ADP and ATP in liver (C) were quantified as detailed in materials and methods. Veh: saline. *P < 0.05 vs. ND and STZ + HFD.
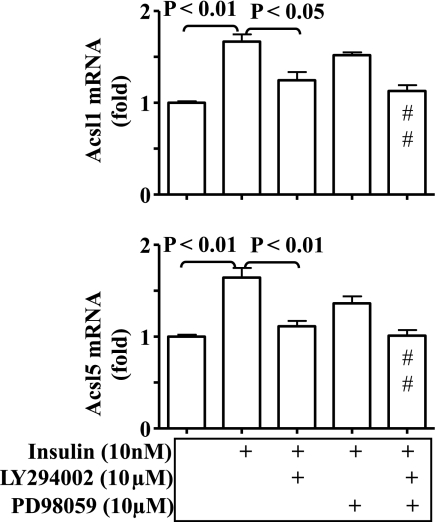
To investigate the mechanism by which insulin promotes expression of ACSLs, hep1c1c7 hepatoma cells were treated with insulin (10 nM, 24 h) in the presence or absence of the PI3K inhibitor LY-294002 or the MEK1/2 inhibitor PD-98059, followed by quantifications of ACSL1 and ACSL5 transcripts. As shown in Fig. 6, transcripts of both ACSL1 and ACSL5 were significantly increased by insulin, and the increases were blocked by LY-294002 but not by PD-98059. These results show that insulin promotes ACSL expression in hepatocytes via the classical Akt-dependent signaling pathway.
Fig. 6.
Insulin-dependent expression of ACSL in hepatocytes. Hep1c1c7 cells were treated with the phosphatidylinositol 3-kinase (PI3K) inhibitor LY-294002 (10 μM) or the MEK1 inhibitor PD-980059 (10 μM) in the presence or absence of 10 nM insulin for 24 h. Transcripts of both ACSL1 and ACSL5 genes were determined by real-time PCR and normalized to 36B4. Results represent means ± SE from 3 independent experiments, each in triplicate. Veh: saline. #P < 0.01 vs. insulin alone.
To determine the mitochondrial contents of long-chain acyl-CoAs and ADP/ATP ratio, liver mitochondria were isolated, followed by purification and quantification of palmitoyl-CoA, stearoyl-CoA, oealoyl-CoA, ADP, and ATP by HPLC and mass spectrometry. As shown in Fig. 5B, mitochondrial contents of palmitoyl-CoA, stearoyl-CoA, and oleoyl-CoA were all significantly increased by HFD, but the increases were reversed by the STZ-induced insulin deficiency. Mitochondrial ADP content was decreased whereas mitochondrial ATP content was increased by HFD (Fig. 5C). Again, the HFD-mediated changes in mitochondrial ADP and ATP were reversed by STZ-induced insulin deficiency. These results strongly indicate that the HFD-induced oxidative stress in liver was associated with changes in mitochondrial contents of long-chain acyl-CoAs, ADP, and ATP.
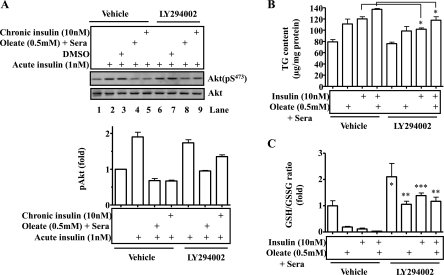
Appropriate blunting of insulin signaling by a PI3K inhibitor can prevent insulin resistance induced by either fat or insulin in hepatocytes. To recapitulate the results from animals described above, we investigated the effect of blunting insulin signaling on the development of insulin resistance induced by the prolonged exposure to either fat or insulin. To blunt insulin signaling, some hep1c1c7 cells were treated with the PI3K inhibitor LY-294002 for 24 h, followed by the addition of either insulin (10 nM) or oleate (0.5 mM) plus sera that normally contain insulin for an additional 24 h. After the extensive washing with warm PBS for 30 min, cells were then stimulated by acute treatment with 1 nM insulin for 5 min. Levels of phospho- and total Akt were then measured by immublotting. As shown in Fig. 7A, insulin stimulated Akt phosphorylation robustly (lanes 2 and 3), and the stimulation was blunted by the prolonged exposure to oleate plus sera or prolonged exposure to insulin (lanes 4 and 5). Cells with a preincubation of PI3K inhibitor LY-294002 and the subsequent washing with PBS responded to the acute insulin challenge significantly better (compare lanes 8 and 9 with lanes 4 and 5; Fig. 7A) than the cells without a preincubation with the PI3K inhibitor. These results imply that appropriate blunting of insulin signaling prevents the development of insulin resistance.
Fig. 7.
Appropriate blunting of insulin signaling with PI3K inhibitor prevents the development of insulin resistance caused by prolonged exposure to either fat or insulin in hepatocytes. Hep1c1c7 cells were treated with the PI3K inhibitor LY-294002 (10 μM) for 24 h, followed by treatment with insulin (10 nm) or oleate (0.5 mM) for additional 24 h. A: the cells were washed extensively with warm PBS for 30 min and then treated with 1 nM insulin for 5 min. Phosphorylated and total Akt were determined by immunoblotting and quantified. Triglyceride content (B) and GSH/GSSG ratio (C) were evaluated. Results represent means ± SE from 3 independent experiments, each in triplicate. Veh: saline. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. vehicle.
Since fat accumulation in hepatocytes plays a critical role in hepatic insulin resistance, TG content in the cells treated similarly as described above was quantified. As shown in Fig. 7B, prolonged exposure to insulin or oleate plus sera increased TG content, but the increase was largely prevented by LY-294002.
To determine the effect of appropriate blunting of insulin signaling on the oxidative stress induced by the prolonged exposure to insulin or oleate plus sera, GSH/GSSG ratio was evaluated in hepa1c1c7 cells. As shown in Fig. 7C, GSH/GSSG ratio was decreased significantly by the prolonged exposure to insulin and oleate plus sera. The effect of prolonged exposure to insulin or oleate plus sera was totally prevented by the preincubation with LY-294002. Together, these results indicate that appropriate blunting of insulin signaling can prevent the development of insulin resistance induced by the excess exposure to insulin or oleate plus sera.
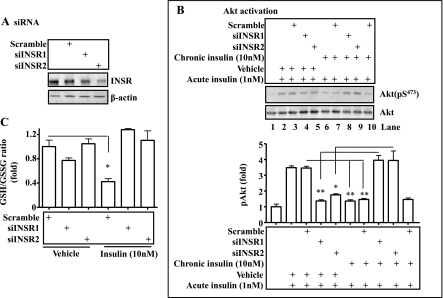
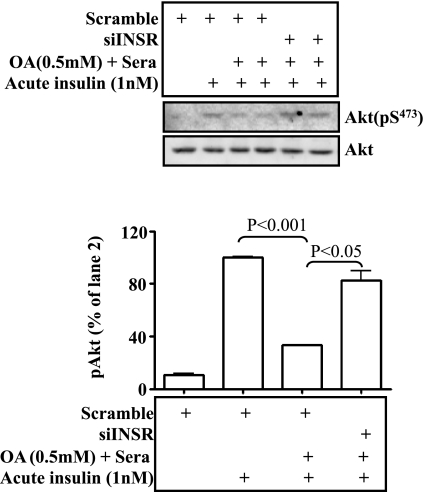
Appropriate blunting of insulin signaling by knocking down the insulin receptor can prevent insulin resistance induced by either insulin or fatty acid in hepatocytes. To more specifically blunt insulin signaling, insulin receptor was knocked down (but not knocked out) by using siRNA against the insulin receptor gene. Cells (hepa1c1c7) were then incubated with insulin for 24 h as noted, followed by the acute insulin challenge. Levels of phospho- and total Akt and GSH/GSSG ratio were then measured. As shown in Fig. 8A, insulin receptor protein level was knocked down by two different siRNAs. Acute insulin challenge stimulated robust Akt phosphorylation (Fig. 8B, lanes 2 and 3), and knockdown of the insulin receptor by either siRNA decreased the acute activation of Akt by insulin (Fig. 8B; lanes 4 and 5). Prolonged exposure to insulin blunted the response of cells to acute insulin treatment (Fig. 8B, lanes 6, 7, and 10). Surprisingly, cells with prolonged exposure to insulin plus insulin receptor knockdown responded to the acute treatment with insulin significantly better in terms of Akt phosphorylation than cells without insulin receptor knockdown (compare lanes 8 and 9 with lanes 6, 7, and 10 in Fig. 8B). As shown in Fig. 8C, prolonged exposure to insulin decreased the GSH/GSSG ratio, and the decrease was completely prevented by knocking down the insulin receptor gene by either siRNA. Together, these results demonstrate that appropriate knockdown of the insulin receptor can prevent the development of oxidative stress and insulin resistance induced by the prolonged exposure to insulin.
Fig. 8.
Reduction of insulin signaling by knocking down the insulin receptor prevents development of insulin resistance induced by the prolonged exposure to insulin. The cognate siRNA against the insulin receptor or a scramble siRNA was introduced into hep1c1c7 cells by transient transfection with Lipofectamine 2000 for 36 h, as described in materials and methods. A: Western blot of insulin receptor. Levels of insulin receptor proteins were evaluated by immunoblotting with the specific anti-insulin receptor antibody and normalized to the expression level of β-actin. B: cells after knocking down of insulin receptor for 36 h were treated with 10 nM insulin for 24 h (chronic insulin treatment). After an extensive washing with warm PBS, the cells were exposed to 1 nM insulin for 5 min (acute insulin treatment). Activation of Akt was determined by immunoblottings with specific antibodies as indicated. C: the GSH/GSSG ratio was evaluated in the cells in the presence or absence of 10 nM insulin for 24 h. Results represent means ± SE of 3 independent experiments, each in triplicate. INSR, insulin receptor. *P < 0.05, **P < 0.01 vs. vehicle.
To further determine whether or not fatty acid can induce insulin resistance in the absence of the full insulin signaling, the insulin receptor was knocked down by using siRNA as detailed in Fig. 8, followed by prolonged exposure to oleate plus sera for 16 h and acute treatment with insulin. As shown in Fig. 9, insulin treatment stimulated Akt phosphorylation, and prolonged exposure to oleate plus sera blunted the insulin-stimulated phosphorylation of Akt. Knockdown of the insulin receptor prevented the effect of oleate with sera in Akt phosphorylation. Together, these results show that knockdown of the insulin receptor can prevent fatty acid-mediated insulin resistance.
Fig. 9.
Reduction of insulin signaling by knocking down the insulin receptor prevents development of insulin resistance induced by the prolonged exposure to oleate. The cognate siRNA against the insulin receptor or a scramble siRNA was introduced into hep1c1c7 cells by transient transfection with Lipofectamine 2000 for 36 h, as described in Fig. 7. Cells after knocking down of insulin receptor for 36 h were treated with oleate with sera for 16 h. After an extensive washing with warm PBS, the cells were exposed to 1 nM insulin for 5 min. Activation of Akt was determined by immunoblottings with specific antibodies as indicated. Results represent means ± SE of 3 independent experiments, each in triplicate.
DISCUSSION
Insulin resistance is either a precursor or key component of numerous modern major health problems of industrialized societies, including obesity, metabolic syndrome, cardiovascular disorders, nonalcoholic fatty liver disease, depression, Alzheimer's disease, asthma, some cancers, and aging (1, 2, 4, 6, 7, 30, 32, 34, 42). As a result, insulin resistance itself has been blamed for all of these health problems. The primary cause of these problems is the positive energy imbalance that is due to overeating and/or lack of physical activity. Our previous reports and results from this study show that insulin and insulin signaling are necessary for fat induction of insulin resistance.
Insulin resistance frequently occurs in obese subjects. However, whether or not insulin resistance occurs in obese subjects depends on ectopic fat accumulation in liver and skeletal muscles instead of severity of obesity. Insulin resistance may not occur in the absence of ectopic fat accumulation even if obesity is severe (18). Insulin resistance will ensue whenever ectopic fat accumulation is present, no matter how slim the subjects are (50). Excessive fat accumulation will not occur anywhere in the absence of insulin because insulin is the single most dominant hormone that promotes energy storage as fat while inhibiting energy consumption via catabolism, including lipolysis and fat oxidation. Here, we disrupted insulin secretion first and then fed B6 mice with HFD. Mice did not gain much weight or ectopic fat accumulation, although they consumed significantly more food. The reason that STZ mice on HFD did not gain much weight was likely decreased anabolism (syntheses of glycogen, proteins, and fat-like TG) and increased catabolism (lipolysis and hepatic fat oxidation) when insulin was deficient. It is conceivable that the excess calories were burned via β-oxidation and released as heat in STZ mice on HFD. Importantly, these mice did not develop insulin resistance [note: this observation is contrary to some previous reports that HFD induces insulin resistance in rats by using a lower dose of STZ (25–45 mg/kg body wt) to induce type 2 diabetes mellitus in rats (14, 51), which is likely due to different levels of insulin deficiency]. That means that ectopic fat accumulation, a frequent component of insulin resistance in most cases, will not occur in the presence of insulin deficiency. The mechanism by which ectopic fat accumulation causes insulin resistance is another hotly debated matter.
Many intermediate metabolites of stored fat (TG) have been shown to induce insulin resistance via various kinases. For example, ceramide has been shown to induce insulin resistance through various kinases such as JNK, p38, and NF-κB (reviewed in Ref. 56). Diacylglyceride is known to be able to induce insulin resistance through PKCs (8). FFA may also induce insulin resistance via p38, JNK, and NF-κB (23, 56). FFA may induce insulin resistance indirectly through stimulating secretion of cytokines such as TNFα. However, it is noteworthy that animals with ectopic fat accumulation may not have insulin resistance. Specifically, when hepatic reactive oxygen species is scavenged by the overexpression of Mn-SOD (sod1), mice with diet-induced obesity and ob/ob mice with severe obesity do not have insulin resistance, even if they have ectopic fat accumulation (19, 37). Besides, athletes with increased TG content in skeletal muscles have better insulin sensitivity (38). Mice with fatty liver induced by transgenic overexpression of acyl-CoA:diacylglycerol acyltransferase 2 do not have insulin resistance (29). A common feature of these subjects is the absence of oxidative stress. Moreover, increased fat level in the blood may not cause insulin resistance either. For example, subjects with prolonged starvation have significantly increased fatty acid levels due to increased lipolysis, but they do not have insulin resistance (57). Again, one key feature of these starved subjects is decreased oxidative stress level. Our results here show that mice with both increased blood lipids and STZ-induced moderate insulin deficiency had very good insulin sensitivity, and the HFD-induced oxidative stress is insulin dependent [note: severe insulin deficiency induced by a high dosage of STZ has previously been shown to cause obvious insulin resistance, which probably due to decreased expression and activation of Glu4 in skeletal muscle and adipose tissue (48); the insulin deficiency induced by multiple low dosages in this study was moderate]. Therefore, it appears that fat in either the blood or tissue (ectopic fat) is insufficient to induce insulin resistance, and oxidative stress appears to be necessary for the induction of insulin resistance. This notion is further supported by some recent reports. For example, fat or cytokines cannot induce insulin resistance in various kinds of cultured cells when mtROS is scavenged (12, 13). It is well established that oxidative stress can activate all kinases known to be involved in insulin resistance (9). Nevertheless, how fat induces insulin resistance through oxidative stress it is still debated.
Theoretically, the increased oxidation of any nutrients such as fatty acids can increase mtROS production due to increased consumption of oxygen. That may be why some have speculated that fat causes insulin resistance through increased fat oxidation and consequently elevated mtROS production. However, this may not be correct in reality because it is established that increased oxygen consumption seen in active mitochondrial respiration promotes electron flux in the mitochondria respiration chain and leads to less mtROS production. In contrast, resting mitochondria consume less oxygen but produce more mtROS due to slowed electron flux in the mitochondrial respiration chain (reviewed in Ref. 3). It is also known that mtROS production is influenced heavily by the supply of substrates such as ADP for converting the proton gradient into ATP. Long-chain acyl-CoAs are established inhibitors of the ADP transporter Ant (20, 33, 40, 41, 54). Conversion or activation of FFA into long-chain acyl-CoAs is catalyzed by long-chain acyl-CoA synthetases. It has previously been shown that expression of long-chain acyl-CoA synthetases is insulin dependent in adipocytes. Here, we show that hepatic expression of ACSL1 and -5 is stimulated by insulin. Thus, it is conceivable that insulin can induce oxidative stress and insulin resistance through expression of ACSLs, which in turn convert FFA into long-chain acyl-CoAs and consequently limit mitochondrial ADP availability and lead to increased mitochondrial membrane potential. In support of this notion, our results show that hepatic expression of ACSL5 is increased by the HFD-induced hyperinsulinemia, but the increase was prevented by the insulin deficiency caused by STZ. Similarly, expressions of both ACSL1 and -5 were promoted by insulin in a PI3K-dependent manner in cultured hepatocytes. Furthermore, our results show that mitochondrial contents of long-chain acyl-CoAs were increased, whereas mitochondrial ADP/ATP ratio was decreased by HFD in an insulin-dependent manner. Finally, our results show that appropriate blunting of insulin signaling by either a chemical inhibitor of PI3K or knocking down the insulin receptor prevents fat accumulation, oxidative stress, and insulin resistance in cultured cells. This may be due to the fact that intracellular fat accumulation and fatty acid activation cannot occur without insulin, and activated fatty acids (long-chain acyl-CoAs) are critical components that can affect mtROS production, as detailed above. Besides, we have recently shown that prolonged exposure to insulin can suppress both production of new mitochondria via biogenesis and removal of aged/damaged mitochondria via autophagy (24, 25). The reduced production of new mitochondria and retention of aged/damaged mitochondria caused by the excess exposure to insulin will also likely contribute to the increased mtROS production. We have shown previously that increased basal level of the Akt-dependent insulin signaling is responsible for the HFD-induced ectopic fat accumulation, oxidative stress, and insulin resistance (21). Interestingly, it has previously been shown that blunting insulin secretion by using diazoxide can also prevent the HFD-induced insulin resistance, and increased insulin level precedes insulin resistance (11, 43). Previous reports about the role of insulin signaling in the development of insulin resistance are more about direct effects of insulin on insulin-signaling components, including insulin receptor itself (39). Results from this study demonstrate that insulin and insulin signaling induce insulin resistance through interactions with fat such as ectopic fat accumulation and then oxidative stress, which is in turn likely caused by the excessive accumulation of activated long-chain acyl-CoAs in mitochondria. In other words, fat may not be able to induce insulin resistance without insulin. Thus, insulin signaling itself is a legitimate target for prevention and reversion of insulin resistance and its numerous associated major health problems.
GRANTS
This work was supported by grants from the American Diabetes Association (7-09-BS-27; to W. Cao) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-076039; to W. Cao).
DISCLOSURES
We have no conflicts of interest to declare.
REFERENCES
- 1. Accili D. Lilly lecture 2003: the struggle for mastery in insulin action: from triumvirate to republic. Diabetes 53: 1633–1642, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Al-Shawwa BA, Al-Huniti NH, DeMattia L, Gershan W. Asthma and insulin resistance in morbidly obese children and adolescents. J Asthma 44: 469–473, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Barja G. Mitochondrial oxygen consumption and reactive oxygen species production are independently modulated: implications for aging studies. Rejuvenation Res 10: 215–224, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Burns JM, Donnelly JE, Anderson HS, Mayo MS, Spencer-Gardner L, Thomas G, Cronk BB, Haddad Z, Klima D, Hansen D, Brooks WM. Peripheral insulin and brain structure in early Alzheimer disease. Neurology 69: 1094–1104, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Cao W, Collins QF, Becker TC, Robidoux J, Lupo J, Xiong Y, Daniel K, Floering L, Collins S. p38 mitogen-activated protein kinase plays a stimulatory role in hepatic gluconeogenesis. J Biol Chem 280: 42731–42737, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Cole GM, Frautschy SA. The role of insulin and neurotrophic factor signaling in brain aging and Alzheimer's Disease. Exp Gerontol 42: 10–21, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Crowell JA, Steele VE, Fay JR. Targeting the AKT protein kinase for cancer chemoprevention. Mol Cancer Ther 6: 2139–2148, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med 16: 400–402, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23: 599–622, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Fujino T, Kang MJ, Suzuki H, Iijima H, Yamamoto T. Molecular characterization and expression of rat acyl-CoA synthetase 3. J Biol Chem 271: 16748–16752, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Gray SL, Donald C, Jetha A, Covey SD, Kieffer TJ. Hyperinsulinemia precedes insulin resistance in mice lacking pancreatic beta-cell leptin signaling. Endocrinology 151: 4178–4186, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Hoehn KL, Salmon AB, Hohnen-Behrens C, Turner N, Hoy AJ, Maghzal GJ, Stocker R, Van Remmen H, Kraegen EW, Cooney GJ, Richardson AR, James DE. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci USA 106: 17787–17792, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440: 944–948, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Jung JY, Lim Y, Moon MS, Kim JY, Kwon O. Onion peel extracts ameliorate hyperglycemia and insulin resistance in high fat diet/streptozotocin-induced diabetic rats. Nutr Metab (Lond) 8: 18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang MJ, Fujino T, Sasano H, Minekura H, Yabuki N, Nagura H, Iijima H, Yamamoto TT. A novel arachidonate-preferring acyl-CoA synthetase is present in steroidogenic cells of the rat adrenal, ovary, and testis. Proc Natl Acad Sci USA 94: 2880–2884, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kansara MS, Mehra AK, Von Hagen J, Kabotyansky E, Smith PJ. Physiological concentrations of insulin and T3 stimulate 3T3-L1 adipocyte acyl-CoA synthetase gene transcription. Am J Physiol Endocrinol Metab 270: E873–E881, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Karnieli E, Armoni M. Transcriptional regulation of the insulin-responsive glucose transporter GLUT4 gene: from physiology to pathology. Am J Physiol Endocrinol Metab 295: E38–E45, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117: 2621–2637, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumashiro N, Tamura Y, Uchida T, Ogihara T, Fujitani Y, Hirose T, Mochizuki H, Kawamori R, Watada H. Impact of oxidative stress and peroxisome proliferator-activated receptor gamma coactivator-1alpha in hepatic insulin resistance. Diabetes 57: 2083–2091, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lerner E, Shug AL, Elson C, Shrago E. Reversible inhibition of adenine nucleotide translocation by long chain fatty acyl coenzyme A esters in liver mitochondria of diabetic and hibernating animals. J Biol Chem 247: 1513–1519, 1972 [PubMed] [Google Scholar]
- 21. Liu H, Hong T, Wen GB, Han J, Zhuo D, Liu Z, Cao W. Increased basal level of Akt-dependent insulin signaling may be responsible for the development of insulin resistance. Am J Physiol Endocrinol Metab 297: E898–E906, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu HY, Cao SY, Hong T, Han J, Liu Z, Cao W. Insulin is a stronger inducer of insulin resistance than hyperglycemia in mice with type 1 diabetes mellitus (T1DM). J Biol Chem 284: 27090–27100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu HY, Collins QF, Xiong Y, Moukdar F, Lupo J, Liu Z, Cao W. Prolonged treatment of primary hepatocytes with oleate induces insulin resistance through p38 mitogen-activated protein kinase. J Biol Chem 282: 14205–14212, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Liu HY, Han J, Cao YS, Hong T, Zhuo D, Shi J, Liu Z, Cao W. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J Biol Chem 284: 31484–31492, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu HY, Yehuda-Shnaidman E, Hong T, Han J, Pi J, Liu Z, Cao W. Prolonged exposure to insulin suppresses mitochondrial production in primary hepatocytes. J Biol Chem 284: 14087–14095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loschen G, Flohe L, Chance B. Respiratory chain linked H(2)O(2) production in pigeon heart mitochondria. FEBS Lett 18: 261–264, 1971 [DOI] [PubMed] [Google Scholar]
- 27. Mashek DG, Li LO, Coleman RA. Rat long-chain acyl-CoA synthetase mRNA, protein, and activity vary in tissue distribution and in response to diet. J Lipid Res 47: 2004–2010, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, Ota T, Yokoyama M, Honda M, Miyamoto K, Kaneko S. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism 57: 1071–1077, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV, Sr, Hevener AL, Farese RV., Jr Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab 6: 69–78, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Moreira PI, Santos MS, Seica R, Oliveira CR. Brain mitochondrial dysfunction as a link between Alzheimer's disease and diabetes. J Neurol Sci 257: 206–214, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Oikawa E, Iijima H, Suzuki T, Sasano H, Sato H, Kamataki A, Nagura H, Kang MJ, Fujino T, Suzuki H, Yamamoto TT. A novel acyl-CoA synthetase, ACS5, expressed in intestinal epithelial cells and proliferating preadipocytes. J Biochem 124: 679–685, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Okazaki R. [Links between osteoporosis and atherosclerosis; beyond insulin resistance]. Clin Calcium 18: 638–643, 2008 [PubMed] [Google Scholar]
- 33. Pande SV, Blanchaer MC. Reversible inhibition of mitochondrial adenosine diphosphate phosphorylation by long chain acyl coenzyme A esters. J Biol Chem 246: 402–411, 1971 [PubMed] [Google Scholar]
- 34. Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res 48: 751–762, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Pospisilik JA, Knauf C, Joza N, Benit P, Orthofer M, Cani PD, Ebersberger I, Nakashima T, Sarao R, Neely G, Esterbauer H, Kozlov A, Kahn CR, Kroemer G, Rustin P, Burcelin R, Penninger JM. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell 131: 476–491, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Rasouli N, Molavi B, Elbein SC, Kern PA. Ectopic fat accumulation and metabolic syndrome. Diabetes Obes Metab 9: 1–10, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol 52: 727–736, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 117: 1690–1698, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care 31, Suppl 2: S262–S268, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Shrago E, Shu A, Elson C. Regulation of cell metabolism by mitochondrial transport systems. In: Gluconeogenesis: Its Regulation in Mammalian Species, edited by Hanson RW. New York: John Wiley & Sons, 1976, p. 221–238 [Google Scholar]
- 41. Shug AL, Shrago E, Bittar N, Folts JD, Koke JR. Acyl-CoA inhibition of adenine nucleotide translocation in ischemic myocardium. Am J Physiol 228: 689–692, 1975 [DOI] [PubMed] [Google Scholar]
- 42. Sterry W, Strober BE, Menter A, International Psoriasis Council Obesity in psoriasis: the metabolic, clinical and therapeutic implications. Report of an interdisciplinary conference and review. Br J Dermatol 157: 649–655, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Surwit RS, Dixon TM, Petro AE, Daniel KW, Collins S. Diazoxide restores beta3-adrenergic receptor function in diet-induced obesity and diabetes. Endocrinology 141: 3630–3637, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffé-Scrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism 44: 645–651, 1995 [DOI] [PubMed] [Google Scholar]
- 45. Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37: 1163–1167, 1988 [DOI] [PubMed] [Google Scholar]
- 46. Surwit RS, Wang S, Petro AE, Sanchis D, Raimbault S, Ricquier D, Collins S. Diet-induced changes in uncoupling proteins in obesity-prone and obesity-resistant strains of mice. Proc Natl Acad Sci USA 95: 4061–4065, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Suzuki H, Kawarabayasi Y, Kondo J, Abe T, Nishikawa K, Kimura S, Hashimoto T, Yamamoto T. Structure and regulation of rat long-chain acyl-CoA synthetase. J Biol Chem 265: 8681–8685, 1990 [PubMed] [Google Scholar]
- 48. Tozzo E, Gnudi L, Kahn BB. Amelioration of insulin resistance in streptozocin diabetic mice by transgenic overexpression of Glut 4 driven by an adipose-specific promoter. Endocrinology 138: 1604–1611, 1997 [DOI] [PubMed] [Google Scholar]
- 49. Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39: 359–407, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang MY, Grayburn P, Chen S, Ravazzola M, Orci L, Unger RH. Adipogenic capacity and the susceptibility to type 2 diabetes and metabolic syndrome. Proc Natl Acad Sci USA 105: 6139–6144, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang Y, Campbell T, Perry B, Beaurepaire C, Qin L. Hypoglycemic and insulin-sensitizing effects of berberine in high-fat diet- and streptozotocin-induced diabetic rats. Metabolism 60: 298–305, 2011 [DOI] [PubMed] [Google Scholar]
- 52. Weiner FR, Smith PJ, Wertheimer S, Rubin CS. Regulation of gene expression by insulin and tumor necrosis factor alpha in 3T3-L1 cells. Modulation of the transcription of genes encoding acyl-CoA synthetase and stearoyl-CoA desaturase-1. J Biol Chem 266: 23525–23528, 1991 [PubMed] [Google Scholar]
- 53. Wilson GL, Patton NJ, McCord JM, Mullins DW, Mossman BT. Mechanisms of streptozotocin- and alloxan-induced damage in rat B cells. Diabetologia 27: 587–591, 1984 [DOI] [PubMed] [Google Scholar]
- 54. Wojtczak L, Zaluska H. The inhibition of translocation of adenine nucleotides through mitochondrial membranes by oleate. Biochem Biophys Res Commun 28: 76–81, 1967 [DOI] [PubMed] [Google Scholar]
- 55. Xiong Y, Collins QF, Lupo J, Liu HY, Jie A, Liu D, Cao W. p38 mitogen-activated protein kinase plays an inhibitory role in hepatic lipogenesis. J Biol Chem 282: 4975–4982, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Ye J. Role of insulin in the pathogenesis of free fatty acid-induced insulin resistance in skeletal muscle. Endocr Metab Immune Disord Drug Targets 7: 65–74, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Youn JH, Buchanan TA. Fasting does not impair insulin-stimulated glucose uptake but alters intracellular glucose metabolism in conscious rats. Diabetes 42: 757–763, 1993 [DOI] [PubMed] [Google Scholar]