Abstract
In late gestation, enhanced myometrial contractility is mediated in part through increased Rho/Rho kinase. Since leptin, which is elevated in pregnancy and obesity, can directly depress myometrial function, we hypothesized that in leptin receptor-deficient mice, myometrial contractility would be greater in late pregnancy due to increased Rho/Rho kinase activity. To test this, we correlated RhoA and Rho kinase expression to contractility in myometrium from nonpregnant (NP) and late-pregnant (P18) heterozygous leptin receptor-deficient mice (db/+) vs. wild-type (WT) mice. In NP mice, KCl-induced contractions were similar between WT and db/+ myometrium. However, the Rho kinase-dependent component of the contractions was greater in db/+ mice, along with an increased expression of Rho kinase. KCl-induced contractions increased in strength in myometrium from P18 WT and db/+ compared with NP. Although the contribution of Rho kinase to contractions was unchanged in P18 WT mice, it was decreased in P18 db/+ mice. The decrease in Rho kinase-dependent contractions in P18 db/+ mice coincided with reduced RhoA and Rho kinase expression relative to NP db/+. Addition of high-fat-induced abnormal glucose utilization prevented changes in Rho kinase function. We conclude that abnormal leptin signaling increases expression and function of Rho kinase to maintain contractile function in NP myometrium and that during pregnancy the contribution of RhoA and Rho kinase expression to myometrial function is reduced despite an increase in myometrial contractility. Thus, other signaling mechanisms appear to compensate when leptin signaling is reduced to maintain contractile function during pregnancy.
Keywords: pregnancy, obesity, gestational diabetes
currently in the us, one in five pregnant women is obese, which is associated with increased perinatal mortality and morbidity and a fivefold increase in the average cost of prenatal care and hospital stay (12, 20, 28). Maternal complications in both overweight and obese women include development of gestational diabetes (GDM), preeclampsia, increased miscarriages and preterm births, poor labor progression, and failed spontaneous term deliveries (15, 28, 44). Because of the failure to deliver spontaneously in obese mothers, the number of postterm labor inductions and cesarean deliveries is high. The increased number of cesarean sections cannot be explained by an increased infant birth weight (44). These pregnancy-associated complications observed in obese women suggest that maternal obesity has a significant impact on myometrium, which is due possibly to abnormal uterine smooth muscle function. This notion is supported by the finding that both the force and frequency of spontaneous contractions are markedly decreased in myometrial strips (taken in the process of cesarean section at term) from overweight and obese women vs. underweight or normal-weight women (44). However, the mechanisms underlying the reduced myometrial contractile function in obese pregnant women are not fully understood.
Initiation and completion of delivery require activation of multiple signaling pathways that promote myometrial contractility. One pathway that contributes significantly to contractility of myometrial smooth muscle is RhoA and/or Rho kinase signaling (3, 24, 35). In smooth muscle, RhoA and Rho kinase regulate Ca2+ sensitivity of contractile proteins by inhibiting myosin light chain phosphatase and dephosphorylation of myosin light chain. RhoA and both Rho kinase isoforms, ROCK1 and ROCK2, are expressed in myometrium, and their expression and/or activation are higher in uteri from late-term pregnant compared with nonpregnant (NP) human and animal models (3, 11, 24, 29, 34, 35). An increase in Rho kinase activity at term is thought to underlie the increase in myometrial contractions at this time, since inhibitors of Rho kinase activity dampen contractile responses (3). These findings suggest that upregulation of RhoA and Rho kinase expression and/or activity accounts for the enhanced myometrial activity needed for successful delivery.
Pregnancy is known to affect multiple signaling pathways that could interface with the RhoA/ROCK signaling cascade to regulate myometrial contractility. Levels of leptin, a circulating adipocyte-derived hormone that regulates energy metabolism and appetite, increase during pregnancy due to both expansion of adipose tissue and release from the developing placenta (7, 10, 15, 21). Leptin contributes to reproduction by regulating ovarian function, oocyte maturation, embryo development, and implantation (4). Despite these contributions to reproduction, little attention has been given to the potential direct effect of the leptin regulation of myometrial function. Pregnant women who are overweight or obese or develop GDM all have high leptin levels and experience complications with parturition, frequently requiring a cesarean section (32, 41). Polymorphisms in the leptin promoter, which is responsible for the expression of leptin specifically in adipose tissue, are associated with GDM (40). Interestingly, mice heterozygous for a leptin receptor mutation that is expected to reduce expression of the long isoform develop GDM despite an elevation in leptin levels (19, 42, 43). Although acute administration of leptin to isolated myometrial strips from mice decreases both spontaneous and oxytocin-induced contractions (31), the long-term effects of physiological concentrations of leptin on myometrial function and the mechanisms involved are unknown.
To explore the relationship between leptin and myometrial function during pregnancy, we tested the hypothesis that, in leptin receptor-deficient mice, myometrial contractility would be greater in late pregnancy due to increased Rho/Rho kinase activity. To test this hypothesis, we characterized expression of RhoA and Rho kinase, as well as Rho kinase-mediated contractile function, in myometrium from both NP and pregnant mice. These characterizations were done in mice with a mutation in the gene for the leptin receptor and wild-type (WT) mice. Maintaining these mice on normal or high-fat diets allowed us to study the effects of altered leptin expression separately from effects of hyperglycemia.
METHODS
Experimental model.
All procedures complied with the Guide for the Care and Use of Laboratory Animals of the American Physiological Society. The protocol was reviewed and approved by both the Iowa City Veterans Affairs Health Care System and the University of Iowa Animal Care and Use Committees. Female C57BLKS/J (WT) and heterozygous leptin receptor-deficient mice (BKS.Cg-Dock7m +/+ Leprdb/J, db/+) were obtained from Jackson Laboratories. WT mice were fed a normal mouse diet (ND; 4% kcal fat, 7001 Teklab Mouse/Rat Diet; Harlan Laboratories), whereas db/+ mice were fed either normal (ND; 4% kcal fat above or 6% kcal fat; 7013 NIH-31 Modified Open Formula Mouse Diet) or high-fat diet (HF; 45% kcal fat; Research Diets, D12451). In preliminary studies, db/+ mice fed the 4% fat diet did not demonstrate altered glucose utilization in late gestation (17, 42, 43). Mice fed the 6% fat diet, which is considered to be within a normal range and used in the studies describing the gestational diabetes phenotype (43), also did not demonstrate altered glucose utilization; thus data from mice on the 4 and 6% fat diets were combined. Mice fed the HF diet displayed abnormal glucose utilization and were analyzed separately. Mice were mated at 3–12 mo of age. The presence of a copulatory plug was used to identify day 0 of pregnancy. Prior to euthanasia with pentobarbital sodium (150 mg/kg ip), mice were weighed, and a glucose tolerance test was performed. A portion of the uterine tissue isolated from each mouse was used for tension recordings, or a separate sample was flash-frozen in liquid nitrogen for Western immunoblotting.
Glucose tolerance test.
All mice were subjected to a glucose tolerance test following an overnight fast. A drop of blood was collected from the tail vein prior to glucose injection (2 mg/kg ip) and also at 15, 30, and 60 min postinjection. Glucose levels in each sample were measured with a hand-held glucometer.
Measurement of myometrial contractile function.
For measurement of uterine contractile activity, uterine tissue was isolated and cut into 4 × 8 mm strips in cold Krebs solution containing (in mM) 118.3 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 2.5 CaCl2, and 11 glucose. Strips were mounted to force transducers in organ baths filled with oxygenated Krebs (95% O2, 5% CO2) solution at 37°C, and tension was recorded with a Powerlab data acquisition system (ADInstruments, Castle Hill, NSW, Australia). Basal tension (1 g) was applied to strips, and tissue was equilibrated for 30–45 min prior to study. Maximal and steady-state levels of uterine contractions in response to KCl (80 mM) were measured before and 30 min after addition of the Rho kinase inhibitor H1152 (1 μM). H1152 is a specific inhibitor of Rho kinase with minimal effects on serine/threonine kinases, protein kinase A and C, and myosin light-chain kinase (2, 16, 36).
Assessment of RhoA and Rho kinase expression by immunoblotting.
Uteri were isolated and flash-frozen in liquid nitrogen, minced, and sonicated in buffer containing sucrose (25 mM), MOPS (50 mM), EDTA (2 mM), EGTA (2 mM), a Complete Protease Inhibitor tablet (Roche Molecular Biochemicals), NaF (50 mM), Na-pyrophosphate (20 mM), p-nitrophenyl phosphate (1 mM), and microcystin LR (1 μM), pH 7.4. The homogenate was centrifuged at 14,000 g for 15 min at 4°C, and the supernatant was collected. Protein concentrations of whole cell lysate were determined by the bicinchoninic acid method, and equal amounts of protein (30 or 50 μg) were separated by SDS-PAGE gel electrophoresis. After blocking in milk, immunoblotting was performed using anti-RhoA (1:100; Santa Cruz Biotechnology or BD Biosciences) or anti-ROCK1 and ROCK2 (1:500; BD Biosciences) in diluent followed by secondary antibodies conjugated with horseradish peroxidase. Immunoreactivity was visualized with enhanced chemiluminescence. Blots were digitized and normalized to β-actin (Sigma-Aldrich) for comparison (NIH Image).
Materials.
Unless otherwise noted, all chemicals were purchased from Fisher Scientific Research Products and Sigma-Aldrich, with the exception of H1152 (Alexis Chemical).
Statistical analysis.
Data are presented as means ± SE. Responses of multiple muscle strips from a given animal treated similarly were averaged, and n represents numbers of mice per group. Both spontaneous phasic and tonic contractions were measured. To account for both frequency and tension, the phasic contractions were measured as area under the curve in grams tension × time (s). For tonic contractions to KCl, maximal peak and steady-state contractions were measured in grams tension. Functional responses of myometrium were compared by analysis of variance, followed by the Student t-test. To determine the percentage of the contraction mediated by activation of Rho kinase, the contraction in the presence of the Rho kinase inhibitor was subtracted from and then divided by the baseline contraction. Other data were compared using ANOVA with Tukey's honestly significantly different or unpaired Student's t-test. Significance was defined as P < 0.05.
RESULTS
Body weight and glucose tolerance in NP and P18 mice.
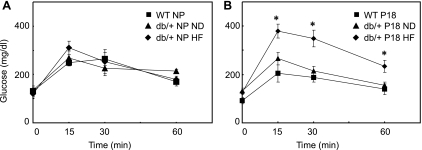
In NP mice, there were no differences in fasting basal glucose levels between WT and db/+ mice, although db/+ mice weighed more (Table 1). The difference in weight was likely due to age, since the NP db/+ mice were slightly older than the NP WT mice (Table 1). There were no differences in glucose tolerance tests in NP mice regardless of the diet fat content (Fig. 1A). All P18 mice weighed significantly more than their corresponding NP controls, but there were no significant differences in weight among the P18 groups (Table 1). All P18 mice on the normal fat diets had similar basal glucose levels and glucose tolerance tests (Table 1 and Fig. 1B).
Table 1.
Weight and basal glucose levels in NP and P18 WT and db/+ mice
| Group | Age, wk | Weight, g | Glucose, mg/dl |
|---|---|---|---|
| WT NP (n = 5) | 22 ± 3 | 18.4 ± 0.5 | 133 ± 36 |
| WT P18 (n = 5) | 23 ± 7 | 33.8 ± 2.7* | 92 ± 13 |
| db/+ NP normal diet (n = 5) | 31 ± 4 | 25.8 ± 1.2† | 134 ± 10 |
| db/+ P18 normal diet (n = 11) | 26 ± 3 | 37.0 ± 0.5* | 131 ± 12 |
| db/+ NP high-fat diet (n = 5) | 27 ± 6 | 24.1 ± 1.5 | 119 ± 17 |
| db/+ P18 high-fat diet (n = 6) | 24 ± 2 | 34.4 ± 1.4* | 119 ± 11 |
Values are means ± SE.
NP, nonpregnant; P18, pregnancy day 18; WT, wild type.
P < 0.05 vs. respective NP;
P < 0.05 vs. WT NP.
Fig. 1.
Glucose tolerance tests in nonpregnant (NP; A) and pregnancy day 18 (P18; B) wild-type (WT) and db/+ mice maintained on normal (ND) and high-fat (HF) diets (means ± SE; WT NP n = 5, db/+ NP ND n = 5, db/+ NP HF n = 5, WT P18 n = 5, db/+ P18 ND n = 11, db/+ P18 HF n = 6; *P < 0.05 vs. WT and db/+ on ND).
Since GDM has been associated with multiparity and increased age, we attempted to unmask a GDM phenotype in db/+ mice on ND by repeatedly breeding (2–3 times) or using aged db/+ mice. Neither of these interventions altered glucose tolerance tests in P18 db/+ mice, and thus data from all db/+ mice on normal fat diets were combined.
Although placing db/+ mice on a HF diet did not affect fasting glucose levels or weights (Table 1), it markedly altered glucose utilization in P18 (but not NP) db/+ compared with WT and db/+ on ND (Fig. 1B). In P18 db/+ mice on HF, glucose levels were higher than those in WT and db/+ on ND at every time point. Thus, HF diet alone was not sufficient to interfere with glucose utilization in NP mice, even when leptin receptor expression was reduced. It was only when HF was combined with reduced leptin receptor expression and pregnancy that glucose utilization was abnormal.
Spontaneous phasic contractions in NP and P18 WT and db/+ mice.
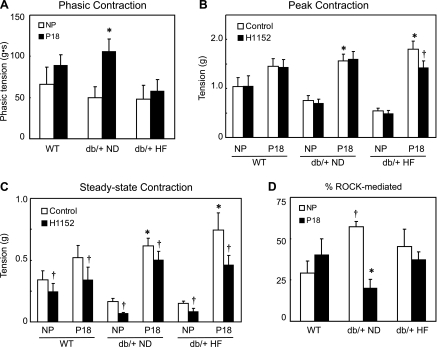
Since myometrium exhibits spontaneous phasic contractions, we compared these contractions in WT and db/+ mice. In NP mice, the spontaneous phasic contractions generated by myometrium were similar regardless of genotype or diet (Figs. 2A and 3A). In P18 mice, the only significant elevation in these contractions occurred in myometrium of db/+ mice maintained on the ND (Figs. 2A and 3A). A comparison of the spontaneous phasic contractions in myometrium from NP and P18 mice revealed that there were no differences between the WT mice and the db/+ mice on HF diet.
Fig. 2.
Representative spontaneous phasic (A) and KCl-induced (80 mM; B) contractions of myometrial strips from NP and P18 WT and db/+ mice, with black lines representing untreated muscle and gray lines representing samples treated with the Rho kinase inhibitor H1152.
Fig. 3.
Phasic and tonic myometrial contractions in NP and P18 mice. Spontaneous phasic contractions (phasic tension in g/s; A) of NP (open bars) and P18 (closed bars) WT and db/+ mice. Peak (B) and steady-state (C) tension (g) developed in response to KCl (80 mM) in NP and P18 WT and db/+ mice on ND and HF mouse diets. Contractions were measured under control conditions (open bars) and following inhibition of Rho kinase with H1152 (1 μM, solid bars). Data are presented as means ± SE (*P < 0.05 vs. respective NP; †P < 0.05 vs. control). D: %steady-state contraction in response to KCl mediated by activation of Rho kinase in NP (open bars) and P18 (closed bars) mice. The percentage of KCl-induced contraction modulated by Rho kinase (ROCK) was assessed using the following formula: control contraction (g) minus contraction (g) in the presence of H1152 divided by control contraction (%ROCK-mediated contraction; means ± SE). *P < 0.05 vs. NP; †P < 0.05 vs. WT group.
Tonic myometrial contractions in WT mice.
To determine whether the forceful tonic contractions that are necessary for parturition are affected by leptin receptor deficiency, we compared responses to depolarization by KCl. Myometrial strips isolated from NP WT mice rapidly contracted in response to KCl, but the peak contraction was not sustained (Fig. 2B, black lines). The steady-state tonic contraction to KCl was ∼35% of the peak contraction and was maintained for ≥15 min (peak 1.04 ± 0.14 g vs. steady state 0.34 ± 0.07 g; Fig. 2B, black lines, and Fig. 3, B and C). To assess the contribution of Rho kinase to peak and steady-state KCl-induced tonic contractions, we measured contractions in the presence of a Rho kinase-specific inhibitor H1152 (1 μM; Fig. 2B, gray lines). Although H1152 had no effect on peak KCl-induced contractions, it significantly reduced steady-state tonic contractions (Fig. 2B, gray lines, and Fig. 3, B and C). In NP WT mice, the contribution of Rho kinase to steady-state contractions of myometrium was 29 ± 7% (Fig. 3D). Thus, in NP WT mice, Rho kinase activation contributes to maintenance of tonic myometrial contractions but not the initial KCl-induced contraction. There were no differences in either peak or steady-state contractions with repeated KCl administration in any group (steady-state contractions in WT NP: 1st KCl, 0.136 ± 0.020 g; 2nd KCl, 0.163 ± 0.036 g).
In myometrium from P18 WT mice, both peak and steady-state KCl-induced tonic contractions tended to increase relative to NP WT (∼60%; Fig. 2B, black lines, and Fig. 3, B and C). As in the case of NP mice, the contribution of Rho kinase in peak vs. steady-state tonic contractions to KCl differed. Inhibiting Rho kinase with H1152 reduced steady-state contractions without affecting peak contractions (Fig. 2B, gray lines, and Fig. 3, B and C). The Rho kinase-dependent component of the steady-state contraction was not significantly increased in P18 vs. NP WT mice (40 ± 10%; Fig. 3D).
Tonic myometrial contractions in db/+ mice fed ND.
Although contractions in response to KCl tended to be reduced in NP db/+ mice on ND, there were no significant differences in either peak or steady-state contractions compared with WT (P < 0.10; Fig. 2B, black lines, and Fig. 3, B and C). However, assessment of the Rho kinase-dependent component of the tonic contractions in these mice yielded surprising results; inhibition of Rho kinase had a greater effect on the steady-state contractions (Fig. 3C). The contribution of Rho kinase to the steady-state contractions was almost double that in the NP WT tissue (57 ± 3 vs. 29 ± 7%; Fig. 3D).
In P18 db/+ mice on ND, both peak and steady-state KCl-induced tonic contractions of myometrium were increased relative to those observed in NP db/+ mice, but neither peak nor steady-state contractions differed significantly from P18 WT mice (Figs. 2B and 3, B and C). In contrast to WT mice, where the Rho kinase component was similar in NP and P18 mice, in db/+ mice Rho kinase inhibition had a very small effect on contractions of myometrium from P18 compared with NP mice (Fig. 3, B and C). In myometrium from P18 db/+ mice, the Rho kinase-mediated contribution to KCl-induced steady-state tonic contractions was only 20 ± 5%, in contrast to NP db/+ tissue, where the contribution was 57 ± 3% (Fig. 3D). Thus, in db/+ mice on a normal-fat diet, Rho kinase plays a smaller role in maintaining tonic contractions in late gestation compared with the nonpregnant condition.
Tonic myometrial contractions in P18 db/+ mice fed a diet high in fat.
To examine effects of abnormal glucose utilization and leptin receptor deficiency, we measured contractile function in myometrium from db/+ mice maintained on HF diet (Fig. 1). In NP db/+ mice on a HF diet, both peak and steady-state tonic contractions to KCl were not different from db/+ on ND (Figs. 2B and 3, B and C). Thus, HF diet had no effect on myometrial function of NP db/+ mice. In P18 db/+ mice on a HF diet, contractions to KCl were also similar to those in P18 WT and db/+ mice on ND (Figs. 2B and 3, B and C). Both peak and steady-state KCl-induced myometrial tonic contractions were greater than those in NP db/+ mice on a HF diet but did not differ significantly from those in P18 WT or db/+ mice on ND (Figs. 2B and 3, B and C). Inhibition of Rho kinase decreased steady-state contractions to KCl, but the Rho kinase component of contractions of myometrium from db/+ mice on a HF diet differed from db/+ mice on a ND. The Rho kinase component of the steady-state tonic contractions to KCl was no longer different from WT mice and did not change in late gestation (Fig. 3D). Surprisingly, Rho kinase inhibition also significantly reduced the peak KCl-induced contraction in these mice (Fig. 3A). Thus, the HF diet partially restored the Rho kinase contribution to myometrial contractions during pregnancy in db/+ mice.
Expression of RhoA and Rho kinase in myometrium.
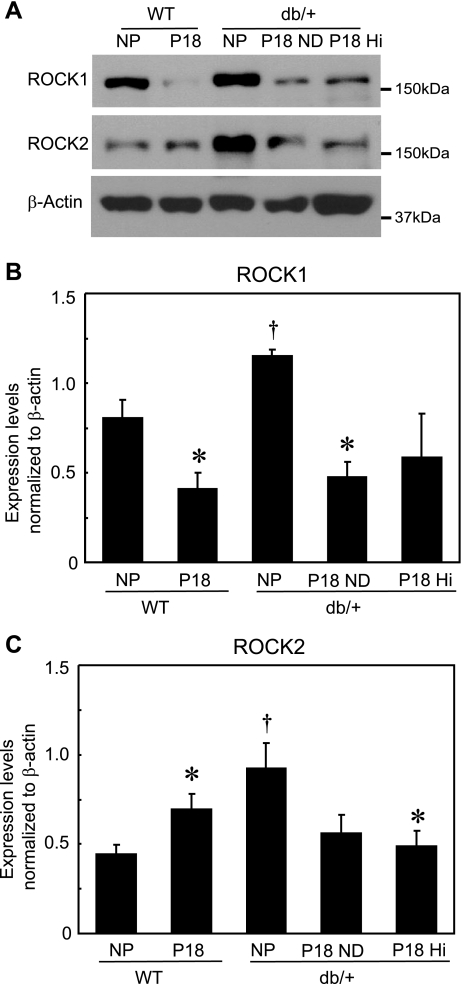
To determine whether altered expression of the two Rho kinase isoforms present in the myometrium accounts for the observed differences in Rho kinase-mediated myometrial function in mice with leptin deficiency, we compared expression of these proteins in myometrial tissue. Consistent with an increase in the contribution of Rho kinase in myometrial contractions in NP db/+ mice (Fig. 3C), both ROCK1 and ROCK2 were expressed at higher levels in myometrium from NP db/+ mice compared with NP WT mice (Fig. 4). In myometrium from NP db/+ mice, ROCK1 expression was ∼1.5-fold greater and ROCK2 expression twofold greater than in WT mice. In WT mice, the effects of pregnancy on expression of the two ROCK isoforms differed; at P18, myometrial ROCK2 expression was ∼1.5-fold higher, and ROCK1 was one-half of the level in NP myometrium (Fig. 4). These changes in Rho kinase expression in WT mice late in pregnancy are consistent with the lack of a significant change in the contribution of Rho kinase to contractile function at P18 (Fig. 3D). In contrast to WT mice, expression of both ROCK1 and -2 decreased in P18 db/+ (on both ND and HF diet) compared with NP, although the decrease in ROCK1 in P18 mice with HF diet and ROCK2 in P18 mice with ND did not attain significance (P < 0.07 each vs. NP db/+; Fig. 4). The changes in expression of ROCK1 and ROCK2 in myometrium from P18 db/+ mice are consistent with the observed reduction in the contribution of Rho kinase to myometrial function in P18 db/+ mice on either diet. These data would suggest that leptin receptor deficiency is the primary cause of the changes in both the expression and function of myometrial Rho kinase.
Fig. 4.
Representative Western immunoblots of Rho kinase (ROCK1 and -2) and β-actin (loading control) (A) and mean expression levels of ROCK1 and -2 (B) normalized to β-actin in myometrium from NP and P18 WT and db/+ mice on ND or HF diet (means ± SE; n = 4–5/group). *P < 0.05 vs. respective NP group, †P < 0.05 vs. NP WT group.
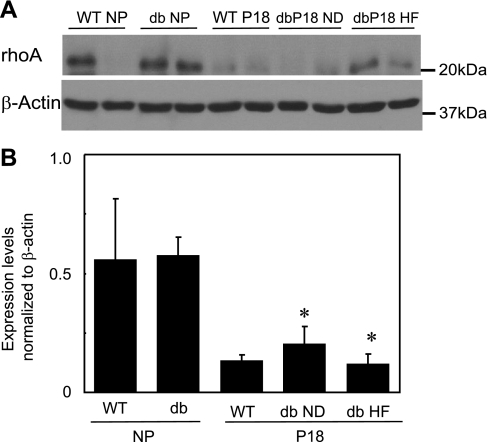
To determine whether the observed changes in ROCK protein expression in mice with leptin receptor deficiency and in pregnant WT mice were specific for Rho kinase, we compared expression of the upstream regulator of Rho kinase activity, RhoA, in myometrium from NP and P18 WT and db/+ mice. In contrast to the expression of Rho kinase, RhoA did not differ in myometrium from NP db/+ and NP WT mice or P18 WT and db/+ mice regardless of diet (Fig. 5). However, as in the case of ROCK expression, RhoA expression was significantly decreased in myometrium isolated from P18 db/+ mice independent of leptin receptor or diet (Fig. 5). Because of the variability in expression in the NP WT myometrium, pregnancy had no effect on the expression of RhoA in these mice (Fig. 5). Thus, whereas RhoA expression is attenuated by pregnancy, leptin receptor deficiency primarily affects expression of Rho kinase.
Fig. 5.
Representative Western immunoblots of RhoA and β-actin (2 lanes/group; A) and mean expression levels of RhoA normalized to β-actin (B) in myometrium from NP and P18 WT and db/+ mice on normal (db ND) and high-fat (db HF) diets (means ± SE; n = 5–6/group). *P < 0.05 vs. respective NP group.
DISCUSSION
Both our functional results and expression data suggest that the role of Rho kinase signaling in maintaining myometrial function is most pronounced in NP mice with reduced leptin signaling. We did not observe a difference in spontaneous phasic contractions in myometrium from NP mice of the WT vs. db/+ genotypes. Given this finding and the fact that Rho kinase does not to contribute significantly to phasic myometrial contractions (22), we did not examine the contribution of Rho kinase to spontaneous contractions. Although the present study showed that expression of both Rho kinase isoforms was markedly elevated in mice heterozygous for the leptin receptor gene, the increased expression did not result in enhanced tonic contractions. In fact, tonic contractions of myometrium from db/+ mice tended to be lower than WT, suggesting that other mechanisms regulating contractile function are downregulated in the leptin receptor-deficient animal. Nevertheless, when leptin receptor-deficient mice were near term, the tonic contractions and the Rho kinase component of the contractions were similar to WT mice. Hyperglycemia in late pregnancy had no effects on protein expression but appeared to restore Rho kinase function to levels found in wild-type states.
Contribution of leptin vs. glucose in regulating Rho kinase function.
Our data suggest that leptin signaling plays a greater role than hyperglycemia in regulating myometrial Rho kinase activity. This conclusion is based on several findings. First, expression and function of Rho kinase were increased in myometrium from NP db/+ mice with reduced leptin signaling, but glucose utilization was similar compared with NP WT. This suggests that leptin normally suppresses Rho kinase expression and function. Second, the expression of RhoA and Rho kinase and their contributions to contractile function were reduced late in gestation in myometrium from P18 db/+ mice on ND compared with NP mice in the absence of hyperglycemia or altered glucose utilization. However, when db/+ mice were placed on HF diet to induce abnormal glucose utilization, ROCK function was markedly reduced. These findings differ from those for WT mice and suggest that abnormal leptin signaling in the absence of a change in glucose levels results in changes in the expression and function of Rho kinase in these mice. In WT and db/+ mice, leptin levels increase throughout pregnancy, but the increase is greater in heterozygous leptin receptor-deficient mice, perhaps to compensate for reduced leptin signaling (8, 17, 23). The importance of the observed shift in the contribution of RhoA/Rho kinase to myometrial contractile function is unclear but may in part underlie abnormal contractile function that results in overweight and obese women who have higher than normal leptin levels, experience difficulties in implantation, and are unable to successfully deliver at term. It is possible that the elevated leptin levels suppress Rho kinase expression late in gestation, resulting in an inability to generate tonic contractions needed for delivery.
Role of leptin signaling in myometrial function.
Our results suggest that reduced leptin signaling in the context of hyperglycemia positively influences Rho kinase expression. Given that leptin receptor expression should be reduced in the heterozygous mouse, leptin signaling should also be reduced despite an increase in circulating levels of leptin. Normal pregnancy is characterized not only by a rise in leptin levels but also by a shift in the expression of leptin receptor isoforms (1). In pregnant rodents, hypothalamic levels of the long isoform of the receptor (which is completely absent in homozygous db/db mice) decrease, whereas those of the short isoforms of the receptor increase (1). In type 1 diabetes, levels of the short isoform are higher than normal, whereas levels of the long isoform are normal (1, 14). Although there is little evidence for leptin receptor deficiency in humans, polymorphisms in the long receptor isoform are associated with GDM (40) and may contribute to the complications associated with pregnancy in GDM. Our mouse model represents a similar scenario, since it is heterozygous for the long receptor. The functional consequence of a shift in expression of leptin receptor subtypes during pregnancy is unknown but could potentially contribute to the onset of labor. Currently, attempts to measure the levels of specific leptin receptor isoforms at the protein level are hampered by the lack of antibodies with the required specificity, although message for long and short isoforms of the leptin receptor is present in myometrium of humans and mice (4, 14). Initially, the short isoforms of the leptin receptor were believed to be inactive; however, studies show that they serve as transporters for leptin and are coupled to downstream signaling pathways distinct from those coupled to the long isoform (15, 18). Also, the circulating soluble form of the short receptors acts as a sink to sequester leptin and to prevent it from activating other receptors. Although the NP db/+ mice used in this study likely express reduced levels of the long isoform, the increase in levels of circulating leptin could potentially activate the other receptor subtypes that are present (8, 27, 42). Advances in the availability of tools to dissect the molecular regulation of leptin and its receptors should ultimately make their physiological roles clearer.
Experimental model: heterozygous leptin receptor-deficient mouse.
The present study took advantage of the heterozygous leptin receptor-deficient mouse Leprdb/+ (db/+), which is thought to represent a model of GDM (17, 19, 42, 43). This mouse has a spontaneous mutation that results in deletion of the long form of the leptin receptor gene, rendering homozygotes completely deficient for the leptin receptor (33). Heterozygotes are thought to develop a form of GDM that closely mimics the human condition; the db/+ mothers gain excess weight, are glucose intolerant, and have elevated levels of Hb A1c, and the offspring have increased levels of insulin and are macrosomic (17, 19, 26). Pregnant db/+ mice also express higher levels of leptin than their WT counterparts, most likely because of the increase in fat mass (8, 17).
The GDM phenotype in db/+ mice is dependent on several factors, including genetic background and diet (13). Genetic background has a marked influence on development of obesity-induced diabetes in the homozygous mouse (5, 6, 13). It also influences the phenotype of WT mice used for comparisons. Because the db locus is closely linked to the misty (m) mutation for coat color that lies on the same chromosome and in repulsion to the db allele, it is used as a marker for predicting the db phenotype (6, 39). Although the original publications suggest that the Dock7m (or m) mutation had no independent effects on the phenotype of db/+ or db/db mice, subsequent studies and our own preliminary data from mice homozygous for the m mutation (Dock7m/Dock7m) demonstrated that these mice weigh less and have less brown adipose mass than other “WT” mice (39). Differences in development of glucose intolerance, hyperinsulinemia, body weight, mortality, and islet cell morphology have also been reported between db/db mice with and without the m mutation (5, 6). In our preliminary studies, Dock7m/Dock7m mice were poor breeders (data not shown), suggesting that the m mutation may also affect the ability to conceive or support implantation. Thus, in the current study we used C57BLKS/J mice, the background strain for the db/+ mouse, as WT controls. Because the onset of GDM is linked with multiparity and increased age (9, 30, 37, 38), we carried out multiple breedings (2–3 litters) and also bred older mice; however, we were unable to reliably reproduce GDM in mice heterozygous for the leptin receptor. Last, we tested the effects of a diet high in fat since diet composition plays a substantial role in the phenotype of the db/db mouse (13) and could potentially contribute to abnormal glucose utilization in pregnant db/+ mice. However, the nonpregnant heterozygous db/+ mice maintained on ND and HF diets and also P18 db/+ mice on ND did not exhibit abnormal glucose utilization. Only db/+ mice maintained on the HF diet during breeding and pregnancy developed glucose intolerance. The db/+ mice were not on the HF diet long enough to become obese. Surprisingly, it was in P18 db/+ mice on the ND and not the HF diet that the effects of RhoA/Rho kinases differed from those in WT mice. Thus, we conclude that the primary factor that influences the contribution of RhoA/Rho kinase pathway signaling on myometrial function in pregnancy is leptin signaling. Further study of the roles of specific leptin receptor subtypes in regulating leptin's effect on the myometrium may provide important clues to therapeutic options for controlling delivery in overweight or obese women.
GRANTS
This work was supported by resources and the use of facilities at the Department of Veterans Affairs Iowa City Health Care System, Iowa City, IA. This work was also supported by grants from the Carver College of Medicine, the March of Dimes (21-FY08-566), and the National Institute of Child Health and Human Development (HD-037831) awarded to S. K. England. S. L. Pierce was supported by an American Heart Association Predoctoral Fellowship (09PRE2280322). The contents of this article are the sole responsibility of the authors and do not necessarily represent the official views of the granting agencies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Christine Blaumueller for editorial contributions and Benjamin Evans for technical support.
REFERENCES
- 1.Bajoria R, Sooranna S, Ward B, Chatterjee R. Prospective function of placental leptin at maternal-fetal interface. Placenta 23: 103–115, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Breitenlechner C, Gassel M, Hidaka H, Kinzel V, Huber R, Engh RA, Bossemeyer D. Protein kinase A in complex with Rho-kinase inhibitors Y-27632, Fasudil, and H-1152P: structural basis of selectivity. Structure 11: 1595–1607, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Cario-Toumaniantz C, Reillaudoux G, Sauzeau V, Heutte F, Vaillant N, Finet M, Chardin P, Loirand G, Pacaud P. Modulation of RhoA-Rho kinase-mediated Ca2+ sensitization of rabbit myometrium during pregnancy—role of Rnd3. J Physiol 552: 403–413, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cervero A, Domínguez F, Horcajadas JA, Quiñonero A, Pellicer A, Simón C. The role of the leptin in reproduction. Curr Opin Obstet Gynecol 18: 297–303, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Chick WL, Lavine RL, Like AA. Studies in the diabetic mutant mouse. V. Glucose tolerance in mice homozygous and heterozygous for the diabetes (db) gene. Diabetologia 6: 257–262, 1970 [DOI] [PubMed] [Google Scholar]
- 6.Chick WL, Like AA. Studies in the diabetic mutant mouse. IV. DBM, a modified diabetic mutant produced by outcrossing of the original strain. Diabetologia 6: 252–256, 1970 [DOI] [PubMed] [Google Scholar]
- 7.Chien E, Hara M, Rouard M, Yaro H, Phillippe M, Polonsky KS, Bell GI. Increase in serum leptin and uterine leptin receptor mRNA during pregnancy in rats. Biochem Biophys Res Commun 237: 476–480, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Chung WK, Belfi K, Chua M, Wiley J, Mackintosh R, Nicolson M, Boozer CN, Leibel RL. Heterozygosity for Lepob or Leprdb affects body composition and leptin homeostasis in adult mice. Am J Physiol Regul Integr Comp Physiol 274: R985–R990, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Cleary-Goldman J, Malone FD, Vidaver J, Ball RH, Nyberg DA, Comstock CH, Saade GR, Eddleman KA, Klugman S, Dugoff L, Timor-Tritsch IE, Craigo SD, Carr SR, Wolfe HM, Bianchi DW, D'Alton M; FASTER Consortium Impact of maternal age on obstetric outcome. Obstet Gynecol 105: 983–990, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Fattah C, Barry S, O'Connor N, Farah N, Stuart B, Turner MJ. Maternal leptin and body composition in the first trimester of pregnancy. Gynecol Endocrinol 27: 263–266, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Friel A, Curley M, Ravikumar N, Smith T, Morrison J. Rho A/Rho kinase mRNA and protein levels in human myometrium during pregnancy and labor. J Soc Gynecol Invest 12: 20–27, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Galtier-Dereure F, Boegner C, Bringer J. Obesity and pregnancy: complications and cost. Am J Clin Nutr 71: 1242S–1248S, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Gunnarsson R. Function of the pancreatic B-cell during the development of hyperglycaemia in mice homozygous for the mutations “diabetes” (db) and “misty” (m). Diabetologia 11: 431–438, 1975 [DOI] [PubMed] [Google Scholar]
- 14.Hauguel-de Mouzon S, Lepercq J, Catalano P. The known and unknown of leptin in pregnancy. Am J Obstet Gynecol 194: 1537–1545, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Henson M, Castracane V. Leptin in pregnancy: an update. Biol Reprod 74: 218–229, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Ikenoya M, Hidaka H, Hosoya T, Suzuki M, Yamamoto N, Sasaki Y. Inhibition of rho-kinase-induced myristoylated alanine-rich C kinase substrate (MARCKS) phosphorylation in human neuronal cells by H-1152, a novel and specific Rho-kinase inhibitor. J Neurochem 81: 9–16, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Ishizuka T, Klepcyk P, LIu S, Panko L, Liu S, Gibbs EM, Friedman JE. Effects of overexpression of human GLUT4 gene on maternal diabetes and fetal growth in spontaneous gestational diabetic C57BLKS/J Lepr(db/+) mice. Diabetes 48: 1061–1069, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Kastin AJ, Pan W, Maness LM, Koletsky RJ, Ernsberger P. Decreased transport of leptin across the blood-brain barrier in rats lacking the short form of the leptin receptor. Peptides 20: 1449–1453, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann RC, Amankwah KS, Dunaway G, Maroun L, Arbuthnot J, Roddick JW., Jr An animal model of gestational diabetes. Am J Obstet Gynecol 141: 479–482, 1981 [DOI] [PubMed] [Google Scholar]
- 20.Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in pre-pregnancy obesity in nine states, 1993–2003. Obesity 15: 986–993, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Krizova J, Eretova V, Haluzikova D, Anderlova K, Housova J, Kotrlikova E, Haluzik M. Soluble leptin receptor and leptin levels in pregnant women before and after delivery. Endocr Res 30: 379–385, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Kupittayanant S, Burdyga T, Wray S. The effects of inhibiting rho-associated kinase with Y27632 on force and intracellular calcium in human myometrium. Pflugers Arch Eur J Physiol 443: 112–114, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Lammert A, Brockmann G, Renne U, Kiess W, Bottner A, Thiery J, Kratzsch J. Different isoforms of the soluble leptin receptor in non-pregnant and pregnant mice. Biochem Biophys Res Commun 298: 798–804, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Lartey J, Smith M, Pawade J, Strachan B, Mellor H, Bernal AL. Up-regulation of myometrial Rho effector proteins (PKN1 and DIAPH1) and CPI-17 (PPP1R14A) phosphorylation in human pregnancy is associated with increased GTP-RhoA in spontaneous preterm labor. Biol Reprod 76: 971–982, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence S, Warshaw J, Nielsen HC. Delayed lung maturation in the macrosomic offspring of genetically determined diabetic (db/+) mice. Pediatr Res 25: 173–179, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379: 632–635, 1996 [DOI] [PubMed] [Google Scholar]
- 28.McDonald SD, Han Z, Mulla S, Beyene J; Knowledge Synthesis Group Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ 341: c3428, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moran CJ, Friel AM, Smith TJ, Cairns M, Morrison JJ. Expression and modulation of rho kinase in human pregnant myometrium. Mol Hum Reprod 8: 196–200, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Moses RG. The recurrence rate of gestational diabetes in subsequent pregnancies. Diabetes Care 19: 1348–1350, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Moynihan A, Hehir M, Glavey S, Smith T, Morrison J. Inhibitory effect of leptin on human uterine contractility in vitro. Am J Obstet Gynecol 195: 504–509, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Naylor CD, Sermer M, Chen E, Sykora K. Cesarean delivery in relation to birth weight and gestational glucose tolerance: pathophysiology or practice style? Toronto Trihospital Gestational Diabetes Investigators. JAMA 275: 1165–1170, 1996 [PubMed] [Google Scholar]
- 33.Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med 22: 359–370, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Riley M, Baker PN, Tribe RM, Taggart MJ. Expression of scaffolding, signalling and contractile-filament proteins in human myometria: effects of pregnancy and labour. J Cell Mol Med 9: 122–134, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riley M, Wu X, Baker PH, Taggart MJ. Gestational-dependent changes in the expression of signal transduction and contractile filament-associated proteins in mouse myometrium. J Soc Gynecol Investig 2005: e33–e43, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasaki Y, Suzuki M, Hidaka H. The novel and specific Rho-kinase inhibitor (S)-(+)-2-methyl-1-[(4-methyl-5-isoquinoline)sulfonyl]-homopiperazine as a probing molecule for Rho-kinase-involved pathway. Pharmacol Ther 93: 225–232, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Savvidou M, Nelson SM, Makgoba M, Messow CM, Sattar N, Nicolaides K. First-trimester prediction of gestational diabetes mellitus: examining the potential of combining maternal characteristics and laboratory measures. Diabetes 59: 3017–3022, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seghieri G, De Bellis A, Anichini R, Alviggi L, Franconi F, Breschi MC. Does parity increase insulin resistance during pregnancy? Diabet Med 22: 1574–1580, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Truett GE, Tempelman RJ, Walker JA, Wilson JK. Misty (m) affects growth traits. Am J Physiol Regul Integr Comp Physiol 275: R29–R32, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Vaskú JA, Vaskú A, Dostálová Z, Bienert P. Association of leptin genetic polymorphism −2548 G/A with gestational diabetes mellitus. Genes Nutr 1: 117–123, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH, Saade G, Eddleman K, Carter SM, Craigo SD, Carr SR, D'Alton ME; Faster Research Consortium Obesity, obstetric complications and cesarean delivery rate—a population-based screening study. Am J Obstet Gynecol 190: 1091–1097, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Yamashita H, Shao J, Ishizuka T, Klepcyk PJ, Muhlenkamp P, Qiao L, Hoggard N, Friedman JE. Leptin administration prevents spontaneous gestational diabetes in heterozygous Leprdb/+ mice: effects on placental leptin and fetal growth. Endocrinology 142: 2888–2897, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Yamashita H, Shao J, Qiao L, Pagliassotti M, Friedman JE. Effect of spontaneous gestational diabetes on fetal and postnatal hepatic insulin resistance in Lepr(db/+) mice. Pediatr Res 53: 411–418, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Bricker L, Wray S, Quenby S. Poor uterine contractility in obese women. BJOG 114: 343–348, 2007 [DOI] [PubMed] [Google Scholar]