Abstract
Several processes contribute to variation in fasting insulin concentration, including fasting glucose, insulin resistance, insulin secretion, and insulin clearance. Our goal was to determine the relative contribution of each of these insulin-related traits, plus anthropometric parameters, to fasting insulin among 470 Mexican Americans. The euglycemic hyperinsulinemic clamp yielded insulin sensitivity (M value) and metabolic clearance rate of insulin (MCRI). Acute insulin secretion was estimated by the insulinogenic index (IGI30) from the oral glucose tolerance test. Regression (univariate) and generalized estimating equations (multivariate) were used to describe the relationship of insulin-related traits to fasting insulin. Univarate analyses were used to select which traits to include in the multivariate model. In multivariate analysis, MCRI, M, BMI, waist circumference, and fasting glucose were independently associated with fasting insulin. Decreasing M and MCRI were associated with increasing fasting insulin, whereas increasing BMI, waist circumference, and fasting glucose were associated with increasing fasting insulin. Standardized coefficients allowed determination of the relative strength of each trait's association with fasting insulin in the entire cohort (strongest to weakest): MCRI (−0.35, P < 0.0001), M (−0.24, P < 0.0001), BMI (0.20, P = 0.0011), waist circumference (0.16, P = 0.021), and fasting glucose (0.11, P = 0.014). Fasting insulin is a complex phenotype influenced by several independent processes, each of which might have its own environmental and genetic determinants. One of the most associated traits was insulin clearance, which has implications for studies that have used fasting insulin as a surrogate for insulin resistance.
Keywords: insulin sensitivity, insulin secretion, Mexican American
abnormalities of insulin action and/or hyperinsulinemia are central to several conditions such as diabetes mellitus, the metabolic syndrome, and polycystic ovary syndrome. Insulin may also affect the development of atherosclerosis, as elevated fasting insulin is a risk factor for subsequent atherosclerosis in epidemiologic studies (4, 17). Ambient insulin concentrations, which vary widely between individuals, are influenced by several distinct physiological processes, including insulin sensitivity, insulin secretion, and insulin clearance.
Because of ease of measurement, fasting insulin [or the closely related homeostatic model assessment of insulin resistance (HOMA-IR)] has been frequently used as a surrogate marker of insulin resistance in large studies. The correlation of fasting insulin-derived measures with gold-standard measures of insulin resistance (e.g., euglycemic clamp or frequently sampled intravenous glucose tolerance test) is imperfect (r values ranging from 0.2 to 0.7) (1, 23), suggesting the influence of other physiological factors. The relative contribution of insulin sensitivity, secretion, and clearance to the variability in fasting insulin concentrations is not known, particularly on a population scale.
Mexican Americans have a high prevalence of hyperinsulinemia/insulin resistance and the metabolic syndrome (15, 27, 28). In the Mexican-American Coronary Artery Disease (MACAD) study, insulin phenotypes were evaluated using the hyperinsulinemic-euglycemic clamp study (11), regarded as a gold-standard technique for direct physiological measurement of insulin sensitivity (3, 32). The clamp also allows calculation of the metabolic clearance rate of insulin (MCRI). In the MACAD study, insulin secretion was calculated from the oral glucose tolerance test (OGTT). Given that all these aspects of insulin action and dynamics were measured in the same subjects, we assessed the relationship of these three parameters, as well as fasting glucose and anthropometric markers, with fasting insulin concentration and identified MCRI, insulin sensitivity (M value), and BMI as quantitatively the most important correlates in this population.
EXPERIMENTAL PROCEDURES
Study subjects.
Associations with fasting insulin were assessed in participants of the Cedars-Sinai/UCLA Mexican-American Coronary Artery Disease (MACAD) study, a study of Mexican American families from Los Angeles (11, 12). In the present report, 128 families were included, comprising 470 subjects from the offspring generation (adult offspring of probands with CAD and the spouses of those offspring) who underwent phenotyping. By design, the offspring were free of diabetes and clinically manifested cardiovascular disease, thus avoiding secondary changes in phenotype caused by overt disease. All studies were approved by Human Subjects Protection Institutional Review Boards at UCLA and Cedars-Sinai Medical Center. All subjects gave informed consent prior to participation.
Phenotyping procedures.
Four hundred seventy adult offspring and their spouses underwent a phenotyping protocol that included an OGTT on one day, and a hyperinsulinemic-euglycemic clamp study on a separate day.
The fasting insulin measurements reported in this study were obtained after a 12-h fast, immediately prior to the hyperinsulinemic-euglycemic clamp. Fasting insulin was measured four times within 30 min prior to the clamp; the average of these four measurements was used as our fasting insulin value. Insulin was measured using the Human Insulin Specific RIA Kit (LINCO Research, St. Charles, MO; <0.2% cross-reactivity with proinsulin; manufacturer's inter- and intra-assay coefficients are 2.9–6.0 and 2.2–4.4%, respectively).
During the hyperinsulinemic-euglycemic clamp (3), a priming dose of human insulin (Novolin, Clayton, NC) was given and followed by infusion for 120 min at a constant rate (60 mU·m−2·min−1) with the goal of achieving a plasma insulin concentration of 100 μIU/ml or greater. Blood was sampled every 5 min, and the rate of 20% dextrose coinfused was adjusted to maintain plasma glucose concentrations at 95 to 100 mg/dl. The glucose infusion rate (M value, mg·m−2·min−1) over the last 30 min of steady-state insulin and glucose concentrations reflects glucose uptake by all tissues of the body (primarily insulin-mediated glucose uptake in muscle) and is therefore directly correlated with tissue insulin sensitivity (3). Often, an insulin sensitivity index (SI, mg·m−2·min−1·μIU−1·ml) is calculated as M/I, where I is the steady-state insulin level. In this study, to clearly distinguish between insulin sensitivity and insulin clearance in multivariate analyses, we relied on M as an approximation for insulin sensitivity in our primary analyses, because the calculations of SI and insulin clearance both use steady-state insulin in the denominator. The metabolic clearance rate of insulin (MCRI, ml·m−2·min−1) was calculated as the insulin infusion rate divided by the steady-state plasma insulin level of the euglycemic clamp, as previously described (3, 13). To test the effect of the definitions of insulin sensitivity and clearance, we conducted secondary analyses that used SI as the insulin sensitivity measure.
The OGTT consisted of baseline glucose and insulin measurements followed by administration of 75 g of oral glucose with blood draws at 30, 60, 90, 120, and 180 min. Insulin secretion was obtained from the OGTT fasting and 30-min time point glucose and insulin measurements to estimate early insulin secretion, as the insulinogenic index [(insulin at 30 min − fasting insulin)/(glucose at 30 min − fasting glucose), μIU·dl·mg−1·ml−1] (18). The IGI30 is a measure of β-cell insulin response to oral glucose and therefore represents a dynamic measurement rather than a steady-state or basal assessment.
Data analysis.
Log-transformed (BMI, IGI30, fasting insulin) or square-root-transformed (M, SI, MCRI) trait values were used to normalize the distribution for all statistical analyses. Unpaired, two-sided t-tests were used to compare trait values between men and women. Simple regression was utilized for univariate analyses of fasting insulin vs. the traits of interest. Multivariate analyses were generated using generalized estimating equations (GEE) to assess the joint effects of BMI, waist circumference, fasting glucose, M (or SI), MCRI, and IGI30 on fasting insulin, adjusting for familial relationships. The weighted GEE1 (34) was computed assuming an exchangeable correlation structure and using the sandwich estimator of the variance to account for familial correlation present in family data. GEE was used to derive standardized regression coefficients, which in any one regression equation are measured on the same scale, with a mean of 0 and a standard deviation of 1. They are then directly comparable to one another, with the largest coefficient indicating which independent variable has the greatest influence on the dependent variable (26). We also generated multivariate models for fasting insulin in men and women separately; these analyses were followed by a repeat analysis in the entire cohort, including sex-by-trait interaction terms to evaluate the significance of any observed sex differences.
By use of procedures similar to those described above, additional exploratory analyses were conducted examining correlates of insulin secretion and insulin clearance separately.
RESULTS
The clinical characteristics of the 470 subjects (197 men, 273 women) who had quantitative assessment of insulin resistance are shown in Table 1. Insulin secretion (IGI30) was lower and insulin sensitivity (M and SI) and waist circumference significantly higher in men than in women. Fasting glucose was slightly but statistically significantly higher in the men. The other insulin-related traits did not differ significantly by sex.
Table 1.
Clinical characteristics of the study cohort
| Men (n = 197) | Women (n = 273) | P Value | |
|---|---|---|---|
| Age, yr | 33.0 (14.0) | 34.0 (12.0) | 0.58 |
| BMI, kg/m2 | 28.2 (5.2) | 28.2 (7.0) | 0.98 |
| Waist circumference, cm | 95.5 (13.0) | 88.5 (17.1) | <0.0001 |
| Fasting glucose, mg/dl | 94.9 (12.1) | 92.0 (11.6) | 0.0001 |
| Fasting insulin, μIU/ml | 11.3 (8.1) | 12.0 (7.4) | 0.95 |
| M, mg·m−2·min−1 | 254.3 (155.9) | 205.7 (119.7) | 0.008 |
| SI, mg·m−2·min−1·μIU−1·ml | 1.92 (1.62) | 1.70 (1.18) | 0.039 |
| MCRI, ml·m−2·min−1 | 479.2 (157.5) | 483.9 (141.8) | 0.46 |
| IGI30, μIU·dl·mg −1·ml−1 | 1.10 (1.05) | 1.43 (1.53) | 0.0004 |
Data are medians (interquartile range). M, glucose infusion rate; SI, insulin sensitivity index; MCRI, metabolic clearance rate of insulin; IGI30, insulinogenic index.
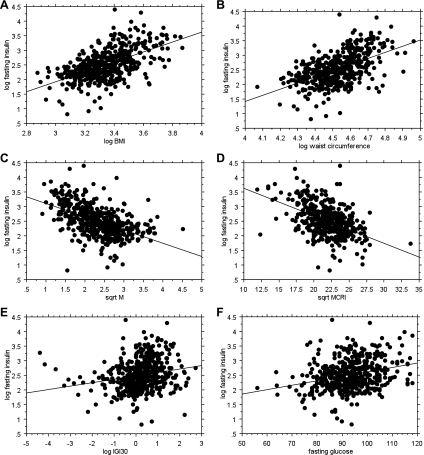
Univariate analyses (Table 2) found significant relationships of fasting insulin with BMI, waist circumference, fasting glucose, M, SI, MCRI, and IGI30. Regression plots are displayed in Fig. 1. Joint analysis of BMI, waist circumference, fasting glucose, M, MCRI, and IGI30 on fasting insulin revealed that all except IGI30 were independently significantly associated with fasting insulin (Table 3). Increasing MCRI and M were associated with decreasing fasting insulin. Increasing BMI, waist circumference, and fasting glucose were associated with increasing fasting insulin. Similar results were obtained when SI was used as the insulin sensitivity measure [Suppl. Table S1 (supplementary materials are found with the online version of this paper at the Journal website)].
Table 2.
Univariate correlations of insulin-related and anthropometric traits with fasting insulin
| Entire Cohort |
Men |
Women |
||||
|---|---|---|---|---|---|---|
| R value | P value | R value | P value | R value | P value | |
| Age | –0.035 | 0.45 | –0.005 | 0.94 | –0.068 | 0.26 |
| BMI | 0.56 | <0.0001 | 0.60 | <0.0001 | 0.54 | <0.0001 |
| Waist circumference | 0.54 | <0.0001 | 0.60 | <0.0001 | 0.54 | <0.0001 |
| Fasting glucose | 0.28 | <0.0001 | 0.23 | <0.0001 | 0.33 | <0.0001 |
| M | –0.47 | <0.0001 | –0.49 | <0.0001 | –0.45 | <0.0001 |
| SI | –0.60 | <0.0001 | –0.58 | <0.0001 | –0.63 | <0.0001 |
| MCRI | –0.48 | <0.0001 | –0.43 | <0.0001 | –0.52 | <0.0001 |
| IGI30 | 0.21 | <0.0001 | 0.23 | 0.0012 | 0.20 | 0.0009 |
Fig. 1.
Regression plots on fasting insulin of the traits significantly associated with it in univariate analyses. The r values and P values are listed in Table 2. A: BMI. B: waist circumference. C: M value. D: MCRI. E: IGI30. F: fasting glucose.
Table 3.
Multivariate correlations of insulin-related and anthropometric traits with fasting insulin
| Standardized Coefficient | Standard Error | 95% Confidence Limits | P Value | ||
|---|---|---|---|---|---|
| Entire cohort (51.7% fasting insulin variance) | |||||
| BMI | 0.20 | 0.062 | 0.081 | 0.32 | 0.0011 |
| Waist circumference | 0.16 | 0.071 | 0.025 | 0.30 | 0.021 |
| Fasting glucose | 0.11 | 0.034 | 0.042 | 0.18 | 0.0014 |
| M | −0.24 | 0.045 | −0.33 | −0.16 | <0.0001 |
| MCRI | −0.35 | 0.037 | −0.42 | −0.27 | <0.0001 |
| IGI30 | 0.034 | 0.030 | −0.024 | 0.093 | 0.25 |
| Men (51.2% of variance explained) | |||||
| BMI | 0.31 | 0.11 | 0.10 | 0.53 | 0.0037 |
| Waist circumference | 0.19 | 0.11 | −0.022 | 0.40 | 0.079 |
| Fasting glucose | 0.063 | 0.054 | −0.043 | 0.17 | 0.24 |
| M | −0.22 | 0.067 | −0.35 | −0.086 | 0.0012 |
| MCRI | −0.33 | 0.066 | −0.46 | −0.20 | <0.0001 |
| IGI30 | 0.036 | 0.042 | −0.046 | 0.118 | 0.39 |
| Women (53.5% of variance explained) | |||||
| BMI | 0.14 | 0.075 | −0.007 | 0.29 | 0.063 |
| Waist circumference | 0.15 | 0.081 | −0.014 | 0.31 | 0.073 |
| Fasting glucose | 0.13 | 0.044 | 0.043 | 0.22 | 0.0032 |
| M | −0.26 | 0.047 | −0.35 | −0.17 | <0.0001 |
| MCRI | −0.36 | 0.038 | −0.44 | −0.29 | <0.0001 |
| IGI30 | 0.037 | 0.037 | −0.035 | 0.11 | 0.31 |
Significant values are indicated in boldface. P values were derived using generalized estimating equations to account for familial relationships.
Comparison of standardized coefficients allowed determination of the relative strength of each trait's association with fasting insulin (listed strongest to weakest): MCRI (−0.35, P < 0.0001), M (−0.24, P < 0.0001), BMI (0.20, P = 0.0011), waist circumference (0.16, P = 0.021), and fasting glucose (0.11, P = 0.014). These factors explained 52% of the variance in fasting insulin concentrations. Similar standardized coefficients were obtained from the model utilizing SI rather than M for insulin sensitivity: SI (−0.28, P < 0.0001), MCRI (−0.22, P < 0.0001), BMI (0.22, P = 0.0004), waist circumference (0.15, P = 0.015), fasting glucose (0.10, P = 0.0022) (Suppl. Table S1).
To determine whether these relationships differed by sex, we conducted multivariate analyses separately in the men and women. While MCRI exhibited the highest standardized coefficient within each sex group, the contribution of BMI to fasting insulin was relatively more important in men. The statistical significance of the differential contribution of BMI was confirmed by running the GEE model with interaction terms of sex with the other independent variables; only the sex-by-BMI interaction term was significant (P = 0.016).
Because insulin secretion and insulin clearance are the physical determinants of circulating insulin levels, we also conducted exploratory analyses characterizing the traits associated with each of these factors. Univariate analyses (Suppl. Table S2) identified BMI, SI, and waist circumference as associated with IGI30; these traits, as well as sex (Table 1) were analyzed jointly for their relationship with IGI30, revealing standardized coefficients of 0.19 for BMI (P = 0.011), 0.16 for SI (P = 0.0026), and 0.13 for sex (P = 0.0045) (Suppl. Table S3). Univariate analyses (Suppl. Table S4) identified BMI, waist circumference, and M for inclusion in multivariate analyses for MCRI, in which only waist circumference was significant (standardized coefficient −0.16, P = 0.012) (Suppl. Table S5).
DISCUSSION
We found that insulin clearance and insulin sensitivity, as well as BMI, waist circumference, and fasting glucose, are independently associated with variation in fasting insulin concentrations, together accounting for 52% of the variation in fasting insulin. Insulin clearance, insulin sensitivity, and BMI were the most strongly associated traits on a population level. To our knowledge, this is the first study reporting the joint effect of these insulin-related and anthropometric phenotypes on fasting insulin. Most prior studies examining trait relationships with fasting insulin were focused on anthropometric, ethnic, dietary, and lifestyle effects on insulinemia (10, 22, 25, 30). A twin study found nongenetic variation in BMI to be associated with fasting insulin; however, that study did not quantify insulin resistance, clearance, or secretion (24). The European Group for the Study of Insulin Resistance (EGIR) study, in which a large number of subjects underwent the euglycemic clamp, determined that the main associations with fasting insulin were, in descending order, BMI, insulin clearance, insulin sensitivity, and plasma glucose; these four factors accounted for 30% of the variation in fasting insulin (9). In that work, insulin secretion and body fat distribution were not included in the model; the authors assumed that the remaining variation was explained by insulin secretion. Another study utilizing the clamp found that MCRI and C-peptide (index of insulin secretion), but not hepatic insulin sensitivity, were significantly associated with HOMA-IR (which tracks closely with fasting insulin); that study did not evaluate anthropometric traits (21).
In our study, insulin sensitivity was strongly associated with fasting insulin, highlighting the importance of compensatory hyperinsulinemia in response to insulin resistance. This close relationship between fasting insulin and insulin sensitivity has led to the use of fasting insulin as a surrogate measure of insulin sensitivity by many investigators; however, given the contributions of insulin clearance, BMI, waist circumference, and fasting glucose to fasting insulin, it is clear that fasting insulin also reflects other aspects of insulin dynamics. This may contribute to the paucity of associations found with fasting insulin in large genome-wide association studies (6).
Of the dynamic factors, secretion and clearance, that physically determine insulin concentration, insulin clearance exhibited a stronger association with population variation in fasting insulin than acute insulin secretion. In support of this result, a clamp study of 10 men with intentional weight gain (BMI 21.8 to 23.8 kg/m2) found that reduction in insulin clearance was the primary contributor to the resulting increased basal and stimulated insulin levels (7). A second study, characterizing increased postprandial insulin levels going from normal weight to overweight to obese, found that insulin secretion plateaued in the overweight group, whereas reductions in insulin clearance progressed in obese and morbidly obese groups (8).
Insulin binding to its receptor triggers internalization of the insulin-insulin receptor complex. Within the cell, insulin is cleaved by insulin-degrading enzyme and other enzymes (5). Clearance of insulin occurs mainly in the liver and kidneys; however, given the above mechanism, any insulin-responsive tissue cell also clears insulin (5). Insulin clearance is the least studied aspect of insulin metabolism. Our results point to the need for further study of insulin clearance in hyperinsulinemic disorders. Insulin clearance is a highly heritable trait (13), raising the possibility that genetic determinants of insulin clearance may affect risk for hyperinsulinemic disorders such as diabetes mellitus or polycystic ovary syndrome.
The relatively weak contribution of our measurement of insulin secretion to fasting insulin might indicate that insulin secretion does not contribute strongly to interindividual differences in fasting insulin, at least in this population. However, IGI30 is an index of acute insulin secretion in response to a glucose load. Although a relationship between stimulated and basal insulin secretion is likely, it is probable that basal insulin secretion is a more important contributor to variation in fasting insulin concentrations. Fasting C-peptide, a reflection of basal insulin secretion, was not obtained in the MACAD study herein. Acute insulin secretion may be influenced by incretin hormones. Also, our estimation of insulin secretion by 30-min OGTT values may represent a less precise measurement of this trait than values obtained by procedures such as the hyperglycemic clamp or frequently sampled intravenous glucose tolerance test (FSIGT). A study utilizing the FSIGT found that insulin hypersecretion was more important than reduced hepatic insulin extraction in the hyperinsulinemia present in obese adolescents (2).
Fasting glucose exhibited a significant and independent association with fasting insulin levels. Fasting glucose likely reflects both basal insulin secretion and hepatic glucose output (31). As found in previous studies (25, 30), BMI and waist circumference were also independently associated with fasting insulin, even when included in the same regression model, suggesting that total adiposity as well as central fat distribution influence fasting insulin. This may be due to adipokines or similar factors produced by adipose tissue that may directly or indirectly regulate insulin production, clearance, and/or resistance. When the sex groups were analyzed separately, the association of BMI with fasting insulin remained significant in the men only. We speculate that this might be related to sexual dimorphism in adipokine levels [e.g., higher adiponectin in women (29)] and/or differences in the genetic regulation of adiposity (20).
The factors that we considered together explained 52% of the overall variation in fasting insulin levels, suggesting the existence of other physiological regulators of fasting insulin, such as diet and physical activity (10, 22, 25, 30) and/or basal insulin secretion. Our study was carried out in Mexican Americans, a group known for its high incidence of insulin resistance (15, 27, 28). Whether our results on the greater importance of MCRI and M on fasting insulin can be generalized to other populations is unknown; however, prior studies in European cohorts yielded partially consistent findings (9, 21). Caution in generalizing these findings is warranted, as Mexican Americans have been found to have lower insulin sensitivity and insulin clearance than non-Hispanic whites (16), but higher insulin clearance than African Americans (14, 19, 33).
Blood levels of insulin are directly controlled by insulin secretion (input) and insulin clearance (output). In separate multivariate models of these two traits, we found that insulin sensitivity, BMI, and waist circumference were all associated with insulin secretion, whereas only waist circumference was associated with insulin clearance. That insulin sensitivity, BMI, and waist circumference remained significantly associated with fasting insulin when analyzed jointly with insulin secretion and clearance suggests that there may be aspects of insulin secretion and insulin clearance that are not fully captured by the IGI30 and MCRI variables available to us.
Our measurement of insulin clearance was not perfect. The euglycemic clamp induces a hyperinsulinemic state, during which the physiological machinery of insulin clearance is likely to be saturated; therefore, the MCRI we calculated may be lower than expected at physiological insulin levels. The clamps performed in the MACAD study did not utilize radiolabeled glucose or insulin, which may have affected the accuracy of our insulin sensitivity and insulin clearance measurements, respectively.
In conclusion, insulin clearance, insulin sensitivity, and body mass index were the most important correlates of variability in fasting insulin in the population under study. Fasting insulin is thus a complex phenotype influenced by several independent processes, each of which might have its own environmental and genetic determinants. The convenience of fasting insulin in epidemiological and genetic studies must therefore be tempered by its heterogeneous underpinnings.
GRANTS
This study was supported, in part, by National Institutes of Health Grants R01-HL-088457, R01-DK-079888, P30-DK-063491, and M01-RR-00425 (General Clinical Research Center Grant from the NCRR), the Cedars-Sinai Winnick Clinical Scholars Award (to M. O. Goodarzi), and the Cedars-Sinai Board of Governor's Chair in Medical Genetics (J. I. Rotter).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1. Cagnacci A, Arangino S, Renzi A, Cagnacci P, Volpe A. Insulin sensitivity in women: a comparison among values derived from intravenous glucose tolerance tests with different sampling frequency, oral glucose tolerance test or fasting. Eur J Endocrinol 145: 281–287, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Cerutti F, Sacchetti C, Bessone A, Rabbone I, Cavallo-Perin P, Pacini G. Insulin secretion and hepatic insulin clearance as determinants of hyperinsulinaemia in normotolerant grossly obese adolescents. Acta Paediatr 87: 1045–1050, 1998 [DOI] [PubMed] [Google Scholar]
- 3. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E214–E223, 1979 [DOI] [PubMed] [Google Scholar]
- 4. Despres JP, Lamarche B, Mauriege P, Cantin B, Dagenais GR, Moorjani S, Lupien PJ. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med 334: 952–957, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev 19: 608–624, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Magi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JR, Egan JM, Lajunen T, Grarup N, Sparso T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proenca C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O'Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Bottcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Cornelis M, Crawford G, Crisponi L, Day IN, de Geus EJ, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, Hallmans G, Hammond N, Han X, Hartikainen AL, Hassanali N, Hayward C, Heath SC, Hercberg S, Herder C, Hicks AA, Hillman DR, Hingorani AD, Hofman A, Hui J, Hung J, Isomaa B, Johnson PR, Jorgensen T, Jula A, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Lyssenko V, Mahley R, Mangino M, Manning AK, Martinez-Larrad MT, McAteer JB, McCulloch LJ, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Morken MA, Mukherjee S, Naitza S, Narisu N, Neville MJ, Oostra BA, Orru M, Pakyz R, Palmer CN, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Perola M, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Psaty BM, Rathmann W, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Roden M, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Scott LJ, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvanen AC, Tanaka T, Thorand B, Tichet J, Tonjes A, Tuomi T, Uitterlinden AG, van Dijk KW, van Hoek M, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Walters GB, Ward KL, Watkins H, Weedon MN, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zeggini E, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC, Borecki IB, Loos RJ, Meneton P, Magnusson PK, Nathan DM, Williams GH, Hattersley AT, Silander K, Salomaa V, Smith GD, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Dedoussis GV, Serrano-Rios M, Morris AD, Lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WH, Pankow JS, Sampson MJ, Kuusisto J, Laakso M, Hansen T, Pedersen O, Pramstaller PP, Wichmann HE, Illig T, Rudan I, Wright AF, Stumvoll M, Campbell H, Wilson JF, Bergman RN, Buchanan TA, Collins FS, Mohlke KL, Tuomilehto J, Valle TT, Altshuler D, Rotter JI, Siscovick DS, Penninx BW, Boomsma DI, Deloukas P, Spector TD, Frayling TM, Ferrucci L, Kong A, Thorsteinsdottir U, Stefansson K, van Duijn CM, Aulchenko YS, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Waterworth DM, Vollenweider P, Peltonen L, Mooser V, Abecasis GR, Wareham NJ, Sladek R, Froguel P, Watanabe RM, Meigs JB, Groop L, Boehnke M, McCarthy MI, Florez JC, Barroso I. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 42: 105–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Erdmann J, Kallabis B, Oppel U, Sypchenko O, Wagenpfeil S, Schusdziarra V. Development of hyperinsulinemia and insulin resistance during the early stage of weight gain. Am J Physiol Endocrinol Metab 294: E568–E575, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Erdmann J, Mayr M, Oppel U, Sypchenko O, Wagenpfeil S, Schusdziarra V. Weight-dependent differential contribution of insulin secretion and clearance to hyperinsulinemia of obesity. Regul Pept 152: 1–7, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Ferrannini E, Balkau B. Insulin: in search of a syndrome. Diabet Med 19: 724–729, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Feskens EJ, Loeber JG, Kromhout D. Diet and physical activity as determinants of hyperinsulinemia: the Zutphen Elderly Study. Am J Epidemiol 140: 350–360, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Goodarzi MO, Guo X, Taylor KD, Quinones MJ, Saad MF, Yang H, Hsueh WA, Rotter JI. Lipoprotein lipase is a gene for insulin resistance in Mexican-Americans. Diabetes 53: 214–220, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Goodarzi MO, Guo X, Taylor KD, Quiñones MJ, Samayoa C, Yang H, Saad MF, Palotie A, Krauss RM, Hsueh WA, Rotter JI. Determination and use of haplotypes: ethnic comparison and association of the lipoprotein lipase gene and coronary artery disease in Mexican-Americans. Genet Med 5: 322–327, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Goodarzi MO, Taylor KD, Guo X, Quinones MJ, Cui J, Li X, Hang T, Yang H, Holmes E, Hsueh WA, Olefsky J, Rotter JI. Variation in the gene for muscle-specific AMP deaminase is associated with insulin clearance, a highly heritable trait. Diabetes 54: 1222–1227, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Goran MI, Bergman RN, Cruz ML, Watanabe R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care 25: 2184–2190, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Haffner SM, Stern MP, Hazuda HP, Pugh J, Patterson JK, Malina R. Upper body and centralized adiposity in Mexican Americans and non-Hispanic whites: relationship to body mass index and other behavioral and demographic variables. Int J Obes 10: 493–502, 1986 [PubMed] [Google Scholar]
- 16. Haffner SM, Stern MP, Watanabe RM, Bergman RN. Relationship of insulin clearance and secretion to insulin sensitivity in non-diabetic Mexican Americans. Eur J Clin Invest 22: 147–153, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care 25: 1177–1184, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Hanson RL, Pratley RE, Bogardus C, Narayan KM, Roumain JM, Imperatore G, Fagot-Campagna A, Pettitt DJ, Bennett PH, Knowler WC. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol 151: 190–198, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Harris MI, Cowie CC, Gu K, Francis ME, Flegal K, Eberhardt MS. Higher fasting insulin but lower fasting C-peptide levels in African Americans in the US population. Diabetes Metab Res Rev 18: 149–155, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Heid IM, Jackson AU, Randall JC, Winkler TW, Qi L, Steinthorsdottir V, Thorleifsson G, Zillikens MC, Speliotes EK, Magi R, Workalemahu T, White CC, Bouatia-Naji N, Harris TB, Berndt SI, Ingelsson E, Willer CJ, Weedon MN, Luan J, Vedantam S, Esko T, Kilpelainen TO, Kutalik Z, Li S, Monda KL, Dixon AL, Holmes CC, Kaplan LM, Liang L, Min JL, Moffatt MF, Molony C, Nicholson G, Schadt EE, Zondervan KT, Feitosa MF, Ferreira T, Allen HL, Weyant RJ, Wheeler E, Wood AR, Estrada K, Goddard ME, Lettre G, Mangino M, Nyholt DR, Purcell S, Smith AV, Visscher PM, Yang J, McCarroll SA, Nemesh J, Voight BF, Absher D, Amin N, Aspelund T, Coin L, Glazer NL, Hayward C, Heard-Costa NL, Hottenga JJ, Johansson A, Johnson T, Kaakinen M, Kapur K, Ketkar S, Knowles JW, Kraft P, Kraja AT, Lamina C, Leitzmann MF, McKnight B, Morris AP, Ong KK, Perry JR, Peters MJ, Polasek O, Prokopenko I, Rayner NW, Ripatti S, Rivadeneira F, Robertson NR, Sanna S, Sovio U, Surakka I, Teumer A, van Wingerden S, Vitart V, Zhao JH, Cavalcanti-Proenca C, Chines PS, Fisher E, Kulzer JR, Lecoeur C, Narisu N, Sandholt C, Scott LJ, Silander K, Stark K, Tammesoo ML, Teslovich TM, Timpson NJ, Watanabe RM, Welch R, Chasman DI, Cooper MN, Jansson JO, Kettunen J, Lawrence RW, Pellikka N, Perola M, Vandenput L, Alavere H, Almgren P, Atwood LD, Bennett AJ, Biffar R, Bonnycastle LL, Bornstein SR, Buchanan TA, Campbell H, Day IN, Dei M, Dorr M, Elliott P, Erdos MR, Eriksson JG, Freimer NB, Fu M, Gaget S, Geus EJ, Gjesing AP, Grallert H, Grassler J, Groves CJ, Guiducci C, Hartikainen AL, Hassanali N, Havulinna AS, Herzig KH, Hicks AA, Hui J, Igl W, Jousilahti P, Jula A, Kajantie E, Kinnunen L, Kolcic I, Koskinen S, Kovacs P, Kroemer HK, Krzelj V, Kuusisto J, Kvaloy K, Laitinen J, Lantieri O, Lathrop GM, Lokki ML, Luben RN, Ludwig B, McArdle WL, McCarthy A, Morken MA, Nelis M, Neville MJ, Pare G, Parker AN, Peden JF, Pichler I, Pietilainen KH, Platou CG, Pouta A, Ridderstrale M, Samani NJ, Saramies J, Sinisalo J, Smit JH, Strawbridge RJ, Stringham HM, Swift AJ, Teder-Laving M, Thomson B, Usala G, van Meurs JB, van Ommen GJ, Vatin V, Volpato CB, Wallaschofski H, Walters GB, Widen E, Wild SH, Willemsen G, Witte DR, Zgaga L, Zitting P, Beilby JP, James AL, Kahonen M, Lehtimaki T, Nieminen MS, Ohlsson C, Palmer LJ, Raitakari O, Ridker PM, Stumvoll M, Tonjes A, Viikari J, Balkau B, Ben-Shlomo Y, Bergman RN, Boeing H, Smith GD, Ebrahim S, Froguel P, Hansen T, Hengstenberg C, Hveem K, Isomaa B, Jorgensen T, Karpe F, Khaw KT, Laakso M, Lawlor DA, Marre M, Meitinger T, Metspalu A, Midthjell K, Pedersen O, Salomaa V, Schwarz PE, Tuomi T, Tuomilehto J, Valle TT, Wareham NJ, Arnold AM, Beckmann JS, Bergmann S, Boerwinkle E, Boomsma DI, Caulfield MJ, Collins FS, Eiriksdottir G, Gudnason V, Gyllensten U, Hamsten A, Hattersley AT, Hofman A, Hu FB, Illig T, Iribarren C, Jarvelin MR, Kao WH, Kaprio J, Launer LJ, Munroe PB, Oostra B, Penninx BW, Pramstaller PP, Psaty BM, Quertermous T, Rissanen A, Rudan I, Shuldiner AR, Soranzo N, Spector TD, Syvanen AC, Uda M, Uitterlinden A, Volzke H, Vollenweider P, Wilson JF, Witteman JC, Wright AF, Abecasis GR, Boehnke M, Borecki IB, Deloukas P, Frayling TM, Groop LC, Haritunians T, Hunter DJ, Kaplan RC, North KE, O'Connell JR, Peltonen L, Schlessinger D, Strachan DP, Hirschhorn JN, Assimes TL, Wichmann HE, Thorsteinsdottir U, van Duijn CM, Stefansson K, Cupples LA, Loos RJ, Barroso I, McCarthy MI, Fox CS, Mohlke KL, Lindgren CM. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet 42: 949–960, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki-Jarvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology 135: 122–130, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Lau C, Faerch K, Glumer C, Tetens I, Pedersen O, Carstensen B, Jorgensen T, Borch-Johnsen K. Dietary glycemic index, glycemic load, fiber, simple sugars, and insulin resistance: the Inter99 study. Diabetes Care 28: 1397–1403, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Lorenzo C, Haffner SM, Stancakova A, Laakso M. Relation of direct and surrogate measures of insulin resistance to cardiovascular risk factors in nondiabetic finnish offspring of type 2 diabetic individuals. J Clin Endocrinol Metab 95: 5082–5090, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Mayer EJ, Newman B, Austin MA, Zhang D, Quesenberry CP, Jr, Edwards K, Selby JV. Genetic and environmental influences on insulin levels and the insulin resistance syndrome: an analysis of women twins. Am J Epidemiol 143: 323–332, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Mooy JM, Grootenhuis PA, de Vries H, Bouter LM, Kostense PJ, Heine RJ. Determinants of specific serum insulin concentrations in a general Caucasian population aged 50 to 74 years (the Hoorn Study). Diabet Med 15: 45–52, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied Linear Statistical Models. Chicago: McGraw Hill/Irwin, 1996 [Google Scholar]
- 27. Okosun IS, Liao Y, Rotimi CN, Prewitt TE, Cooper RS. Abdominal adiposity and clustering of multiple metabolic syndrome in White, Black and Hispanic Americans. Ann Epidemiol 10: 263–270, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 163: 427–436, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Plaisance EP, Grandjean PW, Judd RL, Jones KW, Taylor JK. The influence of sex, body composition, and nonesterified fatty acids on serum adipokine concentrations. Metabolism 58: 1557–1563, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pradhan AD, Manson JE, Hendrix SL, Johnson KC, Wagenknecht LE, Haan MN, Weidner G, Lacroix AZ, Cook NR. Cross-sectional correlates of fasting hyperinsulinaemia in post-menopausal women of different ethnic origin. Diabet Med 23: 77–85, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 19: 708–723, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med 19: 527–534, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Weiss R, Dziura JD, Burgert TS, Taksali SE, Tamborlane WV, Caprio S. Ethnic differences in beta cell adaptation to insulin resistance in obese children and adolescents. Diabetologia 49: 571–579, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42: 121–130, 1986 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.