“Stress is life and life is stress.” The words of Hans Selye from the 1940s still ring true today. Selye recognized that physical, emotional, and environmental challenges (stressors) elicit a variety of physiological responses and that our ability to respond and adapt to these stressors is critical to our survival. In the 50 years since these initial observations, we have learned a great deal about the complex system that allows us to maintain homeostasis in the resting state and to respond appropriately to stressors, yet much remains to be learned about the regulation of this system.
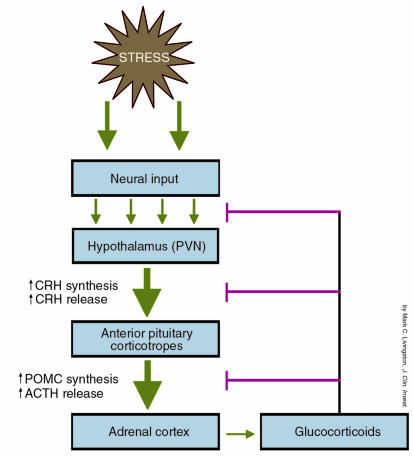
A key component of this stress system is the hypothalamic-pituitary-adrenal (HPA) axis (Figure 1). In response to a stressful stimulus, neural inputs from the central and peripheral nervous system converge on a small nucleus in the hypothalamus (the paraventricular nucleus, or PVN) and signal for increased synthesis and release of corticotropin-releasing hormone (CRH, also known as CRF). This 41–amino acid peptide is released into the hypophyseal portal blood and carried to the anterior pituitary, where it binds to CRH receptors on the corticotropes. As with other Gs protein–coupled membrane receptors, CRH receptors stimulate production of the intracellular second messenger cAMP. Activation of this pathway results in increased production of proopiomelanocortin (POMC) and increased release of adrenocorticotropic hormone (ACTH) and β-endorphin, bioactive proteolytic products of POMC. While CRH is widely regarded as the major hypothalamic releasing factor for ACTH, other hypothalamic compounds such as vasopressin, oxytocin, and norepinephrine can also stimulate ACTH release (at much lower potencies) or potentiate CRH-induced ACTH secretion (reviewed in ref. 1). ACTH is carried via the blood to its target organ, the adrenal cortex, where it stimulates secretion of glucocorticoids (corticosterone in rodents or cortisol in humans). Glucocorticoids then mediate a variety of metabolic effects that help the body to respond to the stressor; for example, they stimulate protein catabolism and gluconeogenesis while inhibiting peripheral glucose uptake.
Figure 1.
Schematic representation of the hypothalamic-pituitary-adrenal (HPA) axis. The initial changes in the axis in response to a stressful stimulus are shown in red. Stress increases CRH synthesis and release from neurons in the paraventricular nucleus (PVN) of the hypothalamus. CRH is carried to the anterior pituitary, where it binds to CRH receptors on corticotropes, resulting in increased synthesis of POMC and release of ACTH. ACTH, in turn, stimulates production of glucocorticoids from the adrenal cortex. Glucocorticoids signal for a variety of metabolic changes that allow the body to respond to the stressor. They also provide negative feedback (shown in red) at numerous levels in the HPA axis to terminate the stress response and maintain homeostasis.
Glucocorticoids also play a critical role in turning off the stress signal to maintain homeostasis in the HPA axis. Elevated glucocorticoid levels reduce the synthesis and release of CRH from the hypothalamus and suppress POMC production and ACTH release in the anterior pituitary. Glucocorticoids also feed back at higher brain centers to modulate the neural inputs to the hypothalamus. Thus, this finely tuned stress system allows us to respond quickly to stressors by increasing CRH, ACTH, and glucocorticoid release but then to return the system to its basal unstimulated state to maintain homeostasis. Altered HPA activation or impaired negative feedback regulation by glucocorticoids is associated with a variety of clinical endocrine and psychiatric disorders, including Cushing’s disease, depression, anxiety disorders, and anorexia. A clear understanding of the regulatory mechanisms in this axis is critical to our ability to develop new treatments for these disorders.
It is not surprising, then, that changes in the glucocorticoid status of an animal result in altered POMC synthesis and ACTH release. Yet the individual contributions of hypothalamic CRH or peripheral glucocorticoids (at various concentrations) to these pituitary corticotrope changes remain controversial. Many studies have examined this question using physiological alterations such as adrenalectomy in the presence or absence of corticosterone replacement, pharmacological treatments, or neuroanatomical lesions (reviewed in ref. 2). An alternative approach, adopted by Muglia et al. in this issue of the JCI (3), involves the use of genetically altered mice — in this case, a CRH-deficient mouse.
The CRH-deficient mouse was created in 1995 by Muglia and colleagues using homologous recombination (4). These mice are completely CRH-deficient and exhibit chronic glucocorticoid insufficiency. Not surprisingly, they also have a dramatically impaired stress response. Interestingly, however, plasma ACTH levels in these mice appear to be normal despite the absence of CRH and altered glucocorticoid levels. Now, Muglia and colleagues have used this interesting mouse model to address the individual roles of glucocorticoids and/or CRH in POMC synthesis and ACTH release (3). Their results suggest that in CRH-deficient mice, alterations in peripheral glucocorticoid levels can regulate POMC synthesis and ACTH production, but that changes in ACTH release in response to altered glucocorticoid levels require the presence of CRH.
While we often associate increased synthesis with increased secretion, these events are clearly distinct. Numerous studies in anterior pituitary cultures have demonstrated decreased POMC transcription by glucocorticoids and increased POMC transcription by CRH (5). The identification and characterization of the genomic sequences and transcription factors mediating these changes in POMC transcription remain areas of intense study. Anterior pituitary cell cultures also show calcium-dependent increases in ACTH secretion with CRH treatment or increased levels of intracellular cAMP. In contrast, glucocorticoids alone have little or no effect on basal ACTH secretion but decrease CRH- or cAMP-stimulated ACTH secretion (5, 6). These in vitro results are consistent with the in vivo data presented from the CRH-deficient mice. Additional studies on the regulation of ACTH secretion may elucidate the key roles of CRH and elevated cAMP levels in calcium-mediated ACTH release and identify the molecular mechanisms by which glucocorticoids inhibit ACTH secretion. These results may have important implications in the treatment of patients following chronic glucocorticoid administration or deficiency.
References
- 1.Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43:425–473. [PubMed] [Google Scholar]
- 2.Dallman MF, et al. Regulation of ACTH secretion: variations on a theme of B. Recent Prog Horm Res. 1987;43:113–173. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- 3.Muglia LJ, et al. Corticotropin-releasing hormone links pituitary adrenocorticotropin gene expression and release during adrenal insufficiency. J Clin Invest. 2000;105:1269–1277. doi: 10.1172/JCI5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muglia LJ, Jacobsen L, Dikkes P, Majzoub J. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373:427–432. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- 5.Gagner JP, Drouin J. Tissue-specific regulation of pituitary proopiomelanocortin gene transcription by CRH, cAMP, and glucocorticoids. Mol Endocrinol. 1987;1:677–682. doi: 10.1210/mend-1-10-677. [DOI] [PubMed] [Google Scholar]
- 6.Shipston MJ. Mechanism(s) of early glucocorticoid inhibition of ACTH secretion from anterior pituitary corticotropes. Trends Endocrinol Metab. 1995;6:261–266. doi: 10.1016/1043-2760(95)00149-2. [DOI] [PubMed] [Google Scholar]