Abstract
PYY may play a role in modulating satiety and energy expenditure; increasing PYY postprandially has been studied largely in single-meal responses. The diurnal rhythm of PYY and its role in energy balance have not been fully characterized. The purpose of our study was to characterize features of the diurnal rhythm of PYY and determine its role in regulating energy balance. This study was a cross-sectional analysis of 11 subjects in whom 24-h repeated blood sampling was conducted at baseline of a larger prospective study. Breakfast (B), lunch (L), dinner (D), and a snack (S) occurred between 0900 and 1900. Total PYY was assayed every hour from 0800 to 1000, every 20 min from 1000 to 2000, and every hour from 2000 to 0800. PYY variables included total AUC, postprandial peaks, and 24-h mean. Energy balance variables included energy intake, RMR, RQ, and NEAT. PYY postprandial peaks were significantly higher than fasting (P < 0.05). Twenty-four-hour peak PYY occurred after L and was significantly higher than all other peaks (P < 0.05). A cubic curve function accounted for most of the variance in PYY (r2 = 69.9%, P < 0.01). Fasting PYY (0800) correlated with postprandial peaks at B (r = 0.77, P = 0.01), L (r = 0.71, P = 0.01), and D (r = 0.65, P = 0.03). The only significant association between PYY and energy expenditure was that RMR (kcal/24 h) correlated with 24-h mean PYY (r = 0.71, P = 0.013) and total AUC (r = 0.69, P = 0.019). We conclude that PYY displays a meal-driven diurnal rhythm and is correlated to RMR, a major contributor to energy expenditure. Thus, PYY varies in accordance with energy content and RMR, supporting a role for PYY in energy balance modulation.
Keywords: body weight regulation, energy homeostasis
peptide yy (PYY) is secreted from L cells in the gut, where it slows digestion, acting as an “ileal brake” to increase absorption of nutrients (16). PYY1–36, cleaved to PYY3–36 via dipeptidyl peptidase, is secreted in proportion to energy and macronutrient content, where high-fat or high-protein meals stimulate greater PYY secretion than high-carbohydrate meals (1, 5, 15). PYY reaches peak concentrations 1–2 h postprandially and aids in meal cessation (1) and has thus been identified as a satiety hormone.
Fasting as well as postprandial PYY concentrations have been shown to be depressed in obese and elevated in energy-deficient women (3, 13, 20, 31). Findings regarding the effect of weight loss/gain on PYY are conflicting as well as its role in energy balance (10, 14, 19, 24, 29–31). To date, much of the work in humans has been observations of single-meal responses in pathophysiological conditions (3, 13, 15, 19, 20) where normal physiological responses have been perturbed. Thus, studies in individuals without chronic disease may aid in our understanding of the role of PYY in the etiology of disease.
The diurnal rhythms of hormones like ghrelin and leptin have been characterized in terms of responses to meal timing (8, 21, 32), energy content of meals (22), and the impact of energy deficiency (18, 22). The diurnal rhythm of PYY has yet to be fully characterized under normal physiological conditions or in relation to meal timing and meal energy or macronutrient content of meals. Studies of single-meal responses do not address whether PYY responses change across the day due to time of day or as nutrient intake accumulates.
Studies have also suggested a potential role for PYY in the modulation of energy expenditure (EE) (9, 34). In the hypothalamus, PYY binds to inhibitory Y2 receptors (Y2R) in the arcuate nucleus, where neuropeptide Y (NPY) and proopiomelanocortin (POMC) neurons are located. PYY binds to Y2R on NPY neurons, inhibiting orexigenic NPY secretion. This inhibition releases inhibition of POMC neurons, resulting in greater POMC activation and thus secretion of anorexigenic hormones (α-MSH) and increasing EE (26, 27, 36). Guo et al. (14) found a negative correlation between fasting PYY and resting metabolic rate as well as respiratory quotient (RQ) and postprandial peak PYY. Additionally, Sloth and colleagues (33, 34) peripherally infused PYY and showed increases in EE (kJ/day) as well as fat oxidation (lowered RQ) in lean and obese men.
The purpose of this study was to characterize features of the diurnal rhythm of PYY and to explore the role of PYY in energy balance in normal-weight premenopausal women. We hypothesized that meal energy content and timing would be key components in eliciting the 24-h profile of PYY and that PYY would correlate positively to indices of EE.
SUBJECTS AND METHODS
Experimental Design and Subjects
This study was part of a larger prospective study designed to assess the effects of energy restriction on metabolism and reproductive function. We examined the 24-h profile of total PYY and energy balance parameters in subjects during the baseline period. PYY was measured in blood samples from 11 subjects who completed 24-h blood sampling and anthropometric and energy balance measurements on days 2–7 of the follicular phase. Nonsmoking, healthy, nonexercising (<1 h/wk purposeful exercise) women aged 18–30 yr with body weights of 48–73 kg, 15–30% body fat, and BMI between 18 and 25 kg/m2 were included. Exclusion criteria included any evidence of disordered eating or history of an eating disorder, loss/gain of a significant amount of weight (± 2.3 kg) in the past year, or use of hormonal contraceptives or medication that may have altered metabolic hormones. Each subject signed an informed consent form approved by the Biomedical Institutional Review Board of Pennsylvania State University.
Screening
Subjects provided information regarding demographics, medical history, menstrual history, and physical activity along with eating attitude questionnaires. A fasting blood sample was obtained between 0600 and 1000 for analysis of a complete blood count and basic chemistry panel and to rule out abnormal pituitary function or metabolic diseases. Psychological stability and the absence/risk of eating disorders were established in an interview under the supervision of a clinical psychologist. Subjects met with a General Clinical Research Center (GCRC) registered dietician to ensure absence of aberrant dietary habits and suitability for a controlled feeding study. Documentation of two to three ovulatory menstrual cycles prior to the study was performed with measurements of midluteal phase serum progesterone and the midcycle urinary LH surge (First Response; Tambrands).
Anthropometrics
Hydrostatic weighing was performed after correcting for residual lung volume to determine body composition. Body density was used to calculate body composition using the Brozek equation (7). Body weight was measured on the same day, with subjects wearing shorts and a tee shirt (without shoes), and recorded to the nearest 0.01 kg.
Energy Balance Parameters
Resting metabolic rate.
Resting metabolic rate (RMR) was measured using a ventilated hood system between 0600 and 1000 following an overnight fast. Subjects lay in the supine position for 20–30 min to acclimate to the room temperature and testing procedures; the hood was placed over each subject's head for 30 min. Expired air was measured every minute for carbon dioxide and oxygen concentration, using a carbon dioxide analyzer (URAS4; Hartmann & Braun, Frankfurt, Germany) and a paramagnetic oxygen analyzer (Magnos 4G, Hartmann & Braun). The values for minutes in which steady state was achieved were averaged to calculate RMR (kcal/day), determined using the Weir equation (40), and RQ.
Physical activity expenditure.
To determine 24-h EE, subjects wore a triaxial activity monitor (AM; RT3 accelerometer; Stayhealthy, Monrovia, CA) 24 h/day for one 7-day period to assess the energy cost of all nonpurposeful exercise EE (kcal). The AM was worn on the left hip for 3 wk of baseline and was not worn during showering/bathing. Subjects recorded weekly AM logs that identified all types of activity and when the monitor was taken off. Because all subjects were sedentary at baseline and thus did not accumulate EE from exercise, RMR (kcal/24 h) and the average daily EE from the AM were added together to determine total 24-h EE.
Free-living dietary intake.
Three-day diet logs were completed by each subject and consisted of records from two weekdays and one weekend day. Subjects recorded any and all energy intake from food and beverage for the entire day. Data were analyzed in Nutritionist Pro (First Data Bank, Indianapolis, IN) by GCRC registered dieticians.
Determination of Baseline Energy Needs
Caloric intake required to maintain weight for each subject (baseline energy needs) was calculated based on the sum of EE from the measurement of 24-h RMR and AM kcals. The prescribed diet was prepared by the GCRC metabolic kitchen and then provided for a 7-day calibration period during baseline where subjects were weighed daily, and ±100-kcal adjustments were made if body weight fluctuated by more than ±1 kg. The 7-day diet was comprised of 55% carbohydrates, 30% fat, and 15% protein and totaled the amount of calories that represented one's individual energy needs.
Twenty-Four-Hour Assessment of PYY Diurnal Rhythm
All subjects were tested in the follicular phase (days 2–7) ≥1 wk after the calibration period. Subjects were instructed to abstain from exercise or caffeine ingestion 24 h prior to the test and to fast as of 2000 the night prior. Subjects arrived at the GCRC at 0730 on the day of testing, where they remained in a supine position with their upper bodies slightly elevated. An intravenous catheter was inserted in a forearm vein whereafter blood samples were obtained every 10 min for 24 h. A total of 488 ml (33 tablespoons) of blood was drawn over the 24-h period. Each sample was allowed to clot at room temperature and subsequently spun in a centrifuge for 15 min at 2,500 rpm. Serum aliquots were transferred to storage tubes and stored in a −80°C freezer until analysis.
All meals for the 24-h sampling protocol were prepared in the GCRC metabolic kitchen. Food items were measured to the nearest gram to achieve the prescribed calorie level. The diet was comprised of 55% carbohydrate, 30% fat, and 15% protein and consisted of three meals and a snack prepared for 0900 (breakfast), 1200 (lunch), 1800 (dinner), and 2100 (snack). Dinner consisted of 503 ± 0.4 kcal, the remainder of which was distributed between breakfast (416 ± 30 kcal), lunch (486 ± 26 kcal), and the snack (66 ± 4 kcal). Subjects knew when meals were to be administered and were required to eat all/only the food provided. The caloric prescription for the 24-h blood sampling period provided subjects with 85% of their calculated baseline energy needs to account for reductions in EE due to inactivity associated with bed rest. Meal composition was as follows: breakfast: 48% carbohydrate, 32% fat, and 20% protein; lunch: 54% carbohydrate, 32% fat, and 14% protein; and dinner: 55% carbohydrate, 30% fat, and 15% protein. A snack comprised of 95% carbohydrate, 2% fat, and 4% protein was provided at 2100. Meals provided were reflective of what subjects typically consumed throughout baseline and consisted of foods like English muffins, orange juice, turkey lunchmeat sandwiches, grapes, and pork stir-fry.
PYY Radioimmunoassay Analysis
Serum samples were assayed for total circulating PYY hourly from 0800 to 1000, every 20 min from 1000 to 2000, and hourly from 2000 to 0800. PYY was assayed using a radioimmunoassay (Millipore, Billerica, MA). The sensitivity of the assay was 10 pg/ml, and the intra- and interassay coefficients of variation were 2.9 and 7.1%, respectively.
Data Analysis
Fasting PYY was designated as 0800. Postprandial peak PYY was defined as the highest PYY concentration (pg/ml) obtained after meal administration and prior to subsequent meal administration (1–2 h postprandially). PYY nadirs were determined to be the lowest PYY preprandial concentration before each meal. Twenty-four-hour mean PYY was the average of all PYY concentrations (pg/ml) observed in the 24-h analysis. Total area under the curve (AUC) was calculated by the trapezoidal rule. Four-hour-blocked AUC was defined as meal response AUC for the time between 0800 and 1200, 1200 and 1600, 1600 and 2000, and 2000 and 0000 to capture the breakfast, lunch, dinner, and snack responses, respectively, and 0000 and 0400 and 0400 and 0800 to capture the nocturnal AUCs. Meal rises were defined as the change in PYY that occurred from meal administration to the peak postprandial PYY concentration (pg/ml). Last, 24-h peak PYY was considered the highest concentration of PYY (pg/ml) obtained in the entire 24-h sampling period.
Statistical Analyses
To determine the presence of a diurnal rhythm, 24-h PYY profiles were analyzed using ANOVA with repeated measurements to determine differences in meal-related postprandial peaks across the day. When main effects were detected, post hoc analyses were performed by means of paired sample t-tests employing the Bonferroni correction factor. Regression curve-fit analysis was also employed to determine what, if any, curvilinear function best fit the 24-h profile of PYY. To determine the relationship between fasting PYY and meal-related PYY features, Pearson correlations were performed to assess the relationship between fasting PYY (pg/ml) and 24-h mean PYY, AUC variables, and meal peaks/nadirs. Pearson correlations were calculated to determine the association between PYY and energy balance parameters such as body composition variables, 24-h sampling meal energy and macronutrient content, 3-day dietary intake and macronutrient composition, RMR, and RQ. A P value of <0.05 was considered statistically significant. Data are reported as means ± SE, and all analyses were performed using SPSS software (version 18.0; SPSS, Chicago, IL).
RESULTS
Subjects
Subject demographics are shown in Table 1. Subjects were healthy, normal-weight, premenopausal women. Subjects were sedentary at baseline (<1 h purposeful exercise/wk) and had remained weight stable (no significant weight loss/gain ± 2.3 kg) for ≥1 year prior to the study.
Table 1.
Subject characteristics
| Variable | Means ± SE | Range |
|---|---|---|
| Age, yr | 20 ± 0.6 | 18–24 |
| Height, cm | 166.7 ± 1.4 | 159.4–175.3 |
| Body weight, kg | 58.5 ± 1.4 | 51.1–66.5 |
| BMI, kg/m2 | 21.1 ± 0.6 | 18.4–23.9 |
| %Body fat | 27.0 ± 1.6 | 19.7–36.8 |
| Fat mass, kg | 15.9 ± 1.2 | 10.7–22.2 |
| FFM, kg | 42.6 ± 1.2 | 37.4–49.6 |
BMI, body mass index; FFM, fat-free mass.
PYY Diurnal Rhythm
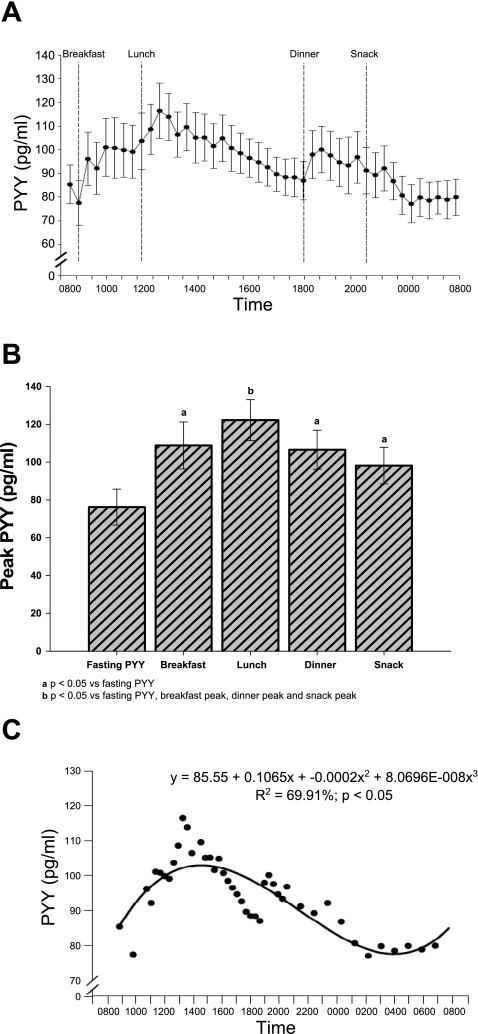
Figure 1 illustrates the 24-h profile of total circulating PYY. PYY exhibits a meal-driven diurnal rhythm characterized by elevated postprandial concentrations after every meal that were significantly higher than fasting PYY. The 24-h peak PYY concentration, observed after lunch, was significantly higher than fasting PYY and all other postprandial peaks across the day (Fig. 1B). This was likely due to an additive effect of breakfast and lunch calories combined, which was correlated to the lunch postprandial peak PYY concentration (r = 0.64, P = 0.04). Presumably, the between-meal duration of 3 h between breakfast and lunch allowed for a greater lunch postprandial PYY peak than the 6 h between lunch and dinner, although the energy content of lunch and dinner was not significantly different (P = 0.52). Additionally, despite the difference in absolute PYY concentrations between lunch and dinner, the meal rises at lunch and dinner were not significantly different (P = 0.90).
Fig. 1.
A: composite 24-h profile of total peptide YY (PYY; pg/ml), illustrating meal administration time points. B: fasting and postprandial meal peaks of total PYY (pg/ml). C: regression curve-fit analysis of 24-h total PYY (pg/ml), illustrating a cubic curve function (n = 11). Data are expressed as means ± SE; P < 0.05.
To further characterize the diurnal rhythm of PYY, total AUC was partitioned into 4-h blocks of time to illustrate meal-related AUC. The lunch response AUC from 1200 to 1600 was significantly higher than the breakfast response AUC from 0800 to 1200 (P = 0.001). Also, the nocturnal AUC blocks (0000–0400, P = 0.003; and 0400–0800, P = 0.006) were significantly lower than the 0800–1200 AUC block. No significant difference was found when the dinner response AUC (1600–2000, P = 0.54) was compared with the breakfast response. Figure 1C displays the result of a regression curve-fit analysis, where a cubic curvilinear function accounted for a significant amount of the variance in 24-h PYY (r2 = 69.9%, P < 0.01).
Fasting PYY and 24-h Diurnal Rhythm
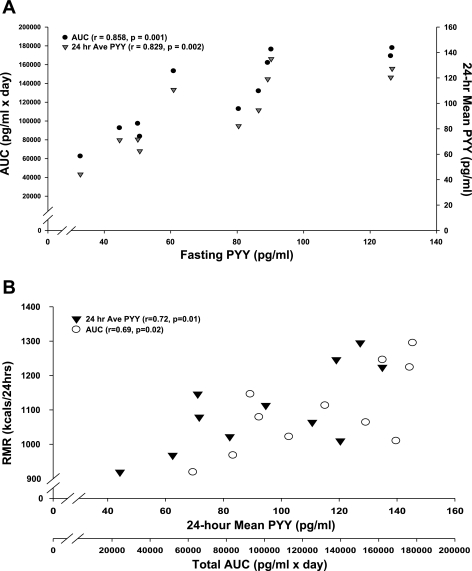
Fasting PYY (pg/ml; 0800) correlated with postprandial meal peaks at breakfast (r = 0.77, P = 0.01), lunch (r = 0.71, P = 0.01), and dinner (r = 0.65, P = 0.03) as well as total AUC (r = 0.86, P = 0.001) and 24-h mean PYY (r = 0.83, P = 0.002). Figure 2A depicts the relationship between fasting PYY and total AUC and the 24-h mean PYY. Additionally, fasting PYY correlated significantly with total energy intake (kcal/day; r = 0.63, P = 0.04) provided on the day of 24-h blood sampling as well as combined energy intake from breakfast and lunch (kcal; r = 0.64, P = 0.03) and total energy intake from carbohydrate (g; r = 0.63, P = 0.04), fat (g; r = 0.64, P = 0.03), and protein (g; r = 0.60, P = 0.05). These same meal energy content variables also showed significant positive correlation with total AUC and 24-h mean PYY (Table 2).
Fig. 2.
A: scatter plot illustrating Pearson correlations between fasting PYY (pg/ml) and area under the curve (AUC; pg/ml × day) and 24-h mean PYY (pg/ml). B: scatter plot illustrating Pearson correlations between resting metabolic rate (RMR; kcal/24 h) and 24-h mean PYY (pg/ml) and AUC (pg·ml−1·day). P < 0.05.
Table 2.
Pearson correlation coefficients between PYY and meal-related parameters
| Peak |
Nadir |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Fasting | AUC | 24-h Mean | B | L | D | B | L | D | N |
| Total 24-h, kcal | 0.63* | 0.70* | 0.71* | 0.68* | 0.63* | 0.67* | 0.69* | 0.72* | 0.62* | 0.67* |
| Total fat, g | 0.64* | 0.70* | 0.72* | 0.69* | 0.64* | 0.68* | 0.70* | 0.72* | 0.62* | 0.68* |
| Total CHO, g | 0.63* | 0.73* | 0.74* | 0.72* | 0.69* | 0.64* | 0.73* | 0.78* | 0.65* | 0.71* |
| Total protein, g | 0.60* | 0.67* | 0.69* | 0.68* | 0.61* | 0.69* | 0.68* | 0.69* | 0.61* | 0.64* |
| Breakfast, kcal | 0.37 | 0.49 | 0.52 | 0.54 | 0.43 | 0.65* | 0.49 | 0.48 | 0.49 | 0.42 |
| Lunch, kcal | 0.72* | 0.70* | 0.69* | 0.62* | 0.64* | 0.48 | 0.69* | 0.74* | 0.55 | 0.72* |
| B + L, kcal | 0.64* | 0.70* | 0.72* | 0.70* | 0.64* | 0.69* | 0.71* | 0.72* | 0.63* | 0.68* |
| D, kcal | −0.25 | −0.26 | −0.25 | −0.17 | −0.29 | −0.15 | −0.20 | −0.33 | −0.18 | −0.13 |
| Snack, kcal | 0.34 | 0.44 | 0.46 | 0.40 | 0.43 | 0.37 | 0.42 | 0.58 | 0.35 | 0.34 |
PYY, peptide YY; AUC, area under the curve; B, breakfast; L, lunch; D, dinner; N, nocturnal; CHO carbohydrate.
P < 0.05.
Day of 24-h Sampling
PYY concentrations expressed in a variety of ways were significantly correlated with the energy content from all meals across the day. Table 2 illustrates correlations between total kilocalories, macronutrient and meal energy content, and PYY peaks and nadirs, fasting PYY, AUC, and 24-h mean concentrations across the day.
PYY and Energy Balance
Table 3 depicts energy balance parameters for both energy intake and expenditure. Energy intake is reported from 3-day diet logs in terms of average total daily intake (kcal) as well as macronutrient content (g). EE variables include total daily caloric expenditure (kcal/24 h), RMR (kcal/24 h and kcal·kg fat-free mass−1·24 h−1), RQ, and EE from AM (kcal/24h).
Table 3.
Energy balance parameters
| Means ± SE | Range | |
|---|---|---|
| Dietary intake (3-day diet logs) | ||
| Total daily intake, kcal | 1,806 ± 124 | 1,315–2459 |
| CHO, g | 240.8 ± 15.7 | 193.5–324.3 |
| Fat, g | 61.7 ± 6.0 | 28.1–89.0 |
| Protein, g | 69.2 ± 5.1 | 42.5–92.5 |
| Alcohol, g | 4.1 ± 2.0 | 0.02–18.6 |
| %CHO | 51.9 ± 1.6 | 41.3–58.0 |
| %Fat | 33.5 ± 1.7 | 27.1–37.8 |
| %Protein | 13.0 ± 0.7 | 8.0–16.0 |
| %Alcohol | 1.5 ± 0.8 | 0.01–7.5 |
| Energy expenditure | ||
| Total 24-h, kcal | 1,740 ± 71 | 1,396–2,088 |
| RMR, kcal/24 h | 1,099 ± 36 | 919–1,295 |
| RMR, kcal·kg FFM−1·24 h−1 | 26.0 ± 1.2 | 21.7–33.3 |
| RQ | 0.88 ± 0.01 | 0.83–0.95 |
| Activity monitor, kcal/24 h | 641 ± 53 | 477–975 |
RMR, resting metabolic rate; RQ, respiratory quotient.
Body Weight and Body Composition
Table 4 illustrates Pearson correlations between PYY, body composition, and energy balance parameters. No significant correlation was found between fasting PYY, 24-h mean PYY, or AUC and any measurement of body composition.
Table 4.
Pearson correlation coefficients between PYY and energy balance parameters
| Fasting PYY, pg/ml | Total AUC, pg·ml−1· day | 24-h Mean PYY, pg/ml | |
|---|---|---|---|
| Body weight, kg | 0.19 | 0.08 | 0.07 |
| BMI, kg/m2 | −0.04 | −0.10 | −0.07 |
| %Body fat | 0.03 | −0.10 | −0.10 |
| Fat mass, kg | 0.11 | −0.04 | −0.05 |
| FFM, kg | 0.13 | 0.14 | 0.13 |
| Dietary intake (3-day diet logs) | |||
| Daily intake, kcal | 0.21 | 0.30 | 0.27 |
| CHO, g | 0.28 | 0.24 | 0.19 |
| Fat, g | 0.13 | 0.28 | 0.26 |
| Protein, g | 0.10 | 0.19 | 0.21 |
| Alcohol, g | 0.04 | 0.24 | 0.22 |
| Energy expenditure | |||
| Total 24-h, kcal | 0.35 | 0.45 | 0.48 |
| RMR, kcal/24 h | 0.52 | 0.69* | 0.72* |
| RMR, kcal·kg FFM−1·24 h−1 | 0.30 | 0.42 | 0.45 |
| RQ | 0.28 | 0.31 | 0.33 |
| Activity monitor, kcal | 0.11 | 0.14 | 0.15 |
P < 0.05.
Dietary Intake from 3-Day Diet Logs
Average daily energy intake obtained from 3-day diet logs is shown as total energy intake (1,806 ± 124 kcal/day) and divided into macronutrient content per gram and percentage of carbohydrate, fat, protein, and alcohol (Table 3). To determine the role of PYY in chronic energy intake, PYY variables were correlated to variables obtained from the 3-day diet logs; however, no significant correlations were found (Table 4).
EE
Table 4 also exemplifies that significant correlations were found between RMR (kcal/24 h) and 24-h mean PYY (r = 0.71, P = 0.013) as well as total AUC (r = 0.69, P = 0.019). Figure 2B depicts the relationship between RMR and PYY 24-h mean and total AUC. Breakfast (r = 0.65, P = 0.03), lunch (r = 0.69, P = 0.02), and dinner (r = 0.66, P = 0.03) postprandial peaks were also positively correlated with RMR (kcal/24 h). No other significant correlations were found among any characteristics of PYY and body composition, chronic energy intake, or EE.
DISCUSSION
Diurnal Rhythm of PYY
To our knowledge, we are the first to report that PYY displays features of an energy-driven diurnal rhythm entrained by meal timing in healthy premenopausal women. Several groups have reported the 24-h profile of PYY; however, these studies were in reference to pathophysiological conditions and obtained samples at time intervals where capturing meal responses may be compromised (12, 13, 28, 37). Total energy and macronutrient content of the 24-h sampling period were positively correlated to fasting PYY and all meal-related PYY parameters, suggesting a meal-driven PYY response. In contrast to our data, Guo et al. (14) found no correlation between fasting PYY and 24-h intake in obese and lean individuals. Additionally, postprandial peaks are all significantly elevated above fasting, which demonstrates that the diurnal rhythm of PYY is driven by meal timing as well.
Fasting PYY Correlates
Positive correlations between fasting PYY and postprandial peaks as well as total AUC and 24-h mean PYY provide evidence that higher fasting PYY concentrations are associated with a greater PYY response over the entire 24-h period. This suggests that higher fasting PYY may be associated with enhanced satiety and subsequent weight regulation and thus serves as a proxy indicator of PYY across the day. In support of our findings, a study by le Roux et al. (20) suggests that declines in fasting and postprandial PYY concentrations were associated with decreased satiety in obese compared with lean subjects, whereas Batterham et al. (4) has shown that infusions of PYY lead to enhanced satiety in humans.
PYY and Energy Intake
Our data demonstrate that there was one PYY peak that is significantly higher than all other postprandial peaks even when there was no significant difference in meal energy content between lunch and dinner. Our finding that the meal rise is not significantly different between lunch and dinner thus indicates that the meal-induced PYY rise is dependent on the caloric content of the meal. Presumably, PYY was highest after lunch because the time between breakfast and lunch was relatively short (3 h), and thus PYY concentrations remained elevated from the breakfast meal. This may suggest that allowing more time between meals permits more time for digestion/absorption and diminished PYY secretion. Additionally, our data best fit (highest r2 and lowest P values) a cubic curvilinear function illustrating that PYY response is significantly altered across the day, highest at lunch and lowest after an overnight fast.
Several studies suggest that more frequent meals across the day may allow for beneficial metabolic effects as well as greater 24-h satiety (6, 11, 35). Because the PYY response is meal driven, more frequent meals across the day may allow for the maintenance of higher circulating PYY and perhaps greater 24-h satiation. Whether changing meal timing would affect peak PYY concentrations is unknown; however, we speculate that this would modify the 24-h pattern we observed. Little is known about the diurnal rhythm of PYY; therefore, it may be advantageous to explore other modulators of PYY beyond what we have investigated.
PYY is considered a satiety hormone among others that displays similar meal response patterns. Glucagon-like peptide-1 (GLP-1) and cholecystokinin (CCK) also act in concert in response to nutrient ingestion to comprise the satiety response. GLP-1 is cosecreted with PYY from L cells in the gut, and thus it is possible that these two hormones share a similar diurnal rhythm. It has been shown that both PYY and GLP-1 concentrations rise 5–30 min postprandially, which is likely due to vagal stimulation before nutrients reach the gut. Additionally, the effects of these peptides act as an “ileal brake” (17). GLP-1, like PYY, has been shown to be reduced in obesity (39). However, GLP-1 has been shown to be reduced, whereas PYY has been shown to be elevated, in young anorexic females (38).
CCK is secreted from I cells and acts similarly to PYY and GLP-1. It rises 10–30 min postprandially and, like PYY, responds more robustly to fat and protein ingestion more so than carbohydrate (23). Additionally, the release of PYY has been demonstrated in dogs to be dependent upon CCK (25). Last, unlike PYY, fasting CCK has been shown to be elevated in obese women and decreased in anorexia nervosa compared with lean control subjects (2).
PYY and Energy Balance
The role of PYY in energy balance is unclear, as evidenced by several disparities in the current literature that may be the result of varying populations being studied (14, 19, 20, 31). Contrary to our finding, one study showed a negative correlation between fasting PYY and indices of EE like RMR (kcal/24 h) and RQ in obese individuals in response to a single meal (14). However, our subjects were normal-weight sedentary women, and our results reflect the complete 24-h profile of PYY in response to three meals. This discrepancy may suggest a mechanistic disparity in pathophysiological conditions where the positive correlation seen in our study is lost in obesity, where blunted fasting and circulating PYY occur (20), or in energy deficiency or gastric bypass patients, where PYY is elevated (19, 31). This correlation may exemplify a role for PYY in EE under normal physiological conditions, where higher circulating PYY may be driving metabolism throughout the entire 24-h period and/or vice versa. It is possible that greater circulating concentrations of PYY may be acting due to activation of hypothalamic receptors where PYY binds to the Y2R and inhibits NPY secretion and its orexigenic effects. Y2R binding also activates POMC neurons, enhancing anorexigenic effects and increasing EE (26, 27). To the contrary, higher resting metabolisms may be driving a greater secretion of PYY; however, a mechanism by which metabolism may stimulate PYY secretion has yet to be elucidated.
Alhough PYY correlated with dietary intake from the day of the 24-h blood sampling, no correlations were found in relation to 3-day diet logs, suggesting that PYY plays a role in short-term satiety and meal cessation as opposed to chronic energy intake. Additionally, there were no correlations found between any PYY parameter and any measurement of body composition, and thus PYY may not play a role in long-term energy storage.
Limitations
This study was originally designed to measure physiological responses to meals occurring at known times of the day. Measurements of satiety like visual analog scales were not utilized, and thus this study can only speculate as to the effect of greater responses in PYY on satiety. Additionally, total PYY was assayed, which includes both PYY1–36 and PYY3–36; however, when measuring 24-h PYY it may be beneficial to capture both forms of PYY since both forms may be physiologically relevant (34).
Conclusions
PYY can be characterized as having a meal-driven diurnal rhythm, as illustrated by significant correlations between PYY and numerous meal-related parameters exemplifying that meal timing as well as caloric load of a meal elicit postprandial responses, contributing to the 24-h profile. PYY's role in EE is correlated to absolute resting metabolism; however, a defining role in this area needs further attention. Our data further support the hypothesis that PYY plays a significant role in energy balance as a satiety hormone and correlate of EE.
GRANTS
This work was supported by National Institutes of Health Grants 1-R01-HD-39245-01A1 (N. I. Williams) and M01-RR-10732. B. R. Hill is supported by the Intercollegiate Graduate Degree Program in Physiology at Pennsylvania State University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Heather Leidy and the staff that spent numerous hours on the original study for their contributions. We also appreciate the cooperation of the study volunteers.
REFERENCES
- 1. Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 89: 1070–1077, 1985 [DOI] [PubMed] [Google Scholar]
- 2. Baranowska B, Radzikowska M, Wasilewska-Dziubinska E, Roguski K, Borowiec M. Disturbed release of gastrointestinal peptides in anorexia nervosa and in obesity. Diabetes Obes Metab 2: 99–103, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med 349: 941–948, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418: 650–654, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab 4: 223–233, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Bellisle F, McDevitt R, Prentice AM. Meal frequency and energy balance. Br J Nutr 77, Suppl 1: S57–S70, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Brozek J, Grande F, Anderson JT, Keys A. Densitometric analysis of body composition: revision of some quantitative assumptions. Ann NY Acad Sci 110: 113–140, 1963 [DOI] [PubMed] [Google Scholar]
- 8. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50: 1714–1719, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Edelsbrunner ME, Herzog H, Holzer P. Evidence from knockout mice that peptide YY and neuropeptide Y enforce murine locomotion, exploration and ingestive behaviour in a circadian cycle- and gender-dependent manner. Behav Brain Res 203: 97–107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Essah PA, Levy JR, Sistrun SN, Kelly SM, Nestler JE. Effect of weight loss by a low-fat diet and a low-carbohydrate diet on peptide YY levels. Int J Obes (Lond) 34: 1239–1242, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farshchi HR, Taylor MA, Macdonald IA. Beneficial metabolic effects of regular meal frequency on dietary thermogenesis, insulin sensitivity, and fasting lipid profiles in healthy obese women. Am J Clin Nutr 81: 16–24, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Germain N, Galusca B, Grouselle D, Frere D, Billard S, Epelbaum J, Estour B. Ghrelin and obestatin circadian levels differentiate bingeing-purging from restrictive anorexia nervosa. J Clin Endocrinol Metab 95: 3057–3062, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Germain N, Galusca B, Le Roux CW, Bossu C, Ghatei MA, Lang F, Bloom SR, Estour B. Constitutional thinness and lean anorexia nervosa display opposite concentrations of peptide YY, glucagon-like peptide 1, ghrelin, and leptin. Am J Clin Nutr 85: 967–971, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Guo Y, Ma L, Enriori PJ, Koska J, Franks PW, Brookshire T, Cowley MA, Salbe AD, Delparigi A, Tataranni PA. Physiological evidence for the involvement of peptide YY in the regulation of energy homeostasis in humans. Obesity (Silver Spring) 14: 1562–1570, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Helou N, Obeid O, Azar ST, Hwalla N. Variation of postprandial PYY 3–36 response following ingestion of differing macronutrient meals in obese females. Ann Nutr Metab 52: 188–195, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Huda MS, Wilding JP, Pinkney JH. Gut peptides and the regulation of appetite. Obes Rev 7: 163–182, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Kim BJ, Carlson OD, Jang HJ, Elahi D, Berry C, Egan JM. Peptide YY is secreted after oral glucose administration in a gender-specific manner. J Clin Endocrinol Metab 90: 6665–6671, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Laughlin GA, Yen SS. Hypoleptinemia in women athletes: absence of a diurnal rhythm with amenorrhea. J Clin Endocrinol Metab 82: 318–321, 1997 [DOI] [PubMed] [Google Scholar]
- 19. le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 243: 108–114, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 147: 3–8, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Leidy HJ, Dougherty KA, Frye BR, Duke KM, Williams NI. Twenty-four-hour ghrelin is elevated after calorie restriction and exercise training in non-obese women. Obesity (Silver Spring) 15: 446–455, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Leidy HJ, Williams NI. Meal energy content is related to features of meal-related ghrelin profiles across a typical day of eating in non-obese premenopausal women. Horm Metab Res 38: 317–322, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest 75: 1144–1152, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lien LF, Haqq AM, Arlotto M, Slentz CA, Muehlbauer MJ, McMahon RL, Rochon J, Gallup D, Bain JR, Ilkayeva O, Wenner BR, Stevens RD, Millington DS, Muoio DM, Butler MD, Newgard CB, Svetkey LP. The STEDMAN project: biophysical, biochemical and metabolic effects of a behavioral weight loss intervention during weight loss, maintenance, and regain. OMICS 13: 21–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin HC, Chey WY, Zhao X. Release of distal gut peptide YY (PYY) by fat in proximal gut depends on CCK. Peptides 21: 1561–1563, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Murphy KG, Bloom SR. Gut hormones in the control of appetite. Exp Physiol 89: 507–516, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Murphy KG, Dhillo WS, Bloom SR. Gut peptides in the regulation of food intake and energy homeostasis. Endocr Rev 27: 719–727, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Paik KH, Jin DK, Lee KH, Armstrong L, Lee JE, Oh YJ, Kim S, Kwon EK, Choe YH. Peptide YY, cholecystokinin, insulin and ghrelin response to meal did not change, but mean serum levels of insulin is reduced in children with Prader-Willi syndrome. J Korean Med Sci 22: 436–441, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pfluger PT, Kampe J, Castaneda TR, Vahl T, D'Alessio DA, Kruthaupt T, Benoit SC, Cuntz U, Rochlitz HJ, Moehlig M, Pfeiffer AF, Koebnick C, Weickert MO, Otto B, Spranger J, Tschöp MH. Effect of human body weight changes on circulating levels of peptide YY and peptide YY3–36. J Clin Endocrinol Metab 92: 583–588, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Roth CL, Enriori PJ, Harz K, Woelfle J, Cowley MA, Reinehr T. Peptide YY is a regulator of energy homeostasis in obese children before and after weight loss. J Clin Endocrinol Metab 90: 6386–6391, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Scheid JL, Williams NI, West SL, VanHeest JL, De Souza MJ. Elevated PYY is associated with energy deficiency and indices of subclinical disordered eating in exercising women with hypothalamic amenorrhea. Appetite 52: 184–192, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Schoeller DA, Cella LK, Sinha MK, Caro JF. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest 100: 1882–1887, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sloth B, Davidsen L, Holst JJ, Flint A, Astrup A. Effect of subcutaneous injections of PYY1–36 and PYY3–36 on appetite, ad libitum energy intake, and plasma free fatty acid concentration in obese males. Am J Physiol Endocrinol Metab 293: E604–E609, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A. Effects of PYY1–36 and PYY3–36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab 292: E1062–E1068, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Smeets AJ, Westerterp-Plantenga MS. Acute effects on metabolism and appetite profile of one meal difference in the lower range of meal frequency. Br J Nutr 99: 1316–1321, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Stanley S, Wynne K, Bloom S. Gastrointestinal Satiety Signals III. Glucagon-like peptide 1, oxyntomodulin, peptide YY, and pancreatic polypeptide. Am J Physiol Gastrointest Liver Physiol 286: G693–G697, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Suneja M, Murry DJ, Stokes JB, Lim VS. Hormonal regulation of energy-protein homeostasis in hemodialysis patients: an anorexigenic profile that may predispose to adverse cardiovascular outcomes. Am J Physiol Endocrinol Metab 300: E55–E64, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tomasik PJ, Sztefko K, Malek A. GLP-1 as a satiety factor in children with eating disorders. Horm Metab Res 34: 77–80, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Verdich C, Toubro S, Buemann B, Lysgård Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int J Obes Relat Metab Disord 25: 1206–1214, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949 [DOI] [PMC free article] [PubMed] [Google Scholar]