Abstract
Recent studies have demonstrated an age-related decline in gonadotropins and a decrease in pituitary responsiveness to GnRH, indicating that aging influences the neuroendocrine components of the female reproductive axis independently of changes in ovarian function. To determine whether aging might also affect the luteinizing hormone (LH) negative and positive feedback responses to gonadal steroids, we administered a controlled, graded sex steroid infusion to 11 younger (45–56 yr) and nine older (70–80 yr) postmenopausal women (PMW) in whom endogenous ovarian steroids and peptides are uniformly low. The doses of estradiol (E2) and progesterone (P) were chosen to mimic levels across the normal follicular phase and have been shown previously to induce negative followed by positive feedback on LH. Similar E2 and P levels were achieved in younger and older PMW (P = 0.4 and 0.3, respectively) and produced a biphasic LH response in all subjects. The early decline in LH to 53% of baseline was not different in older vs. younger PMW. However, the positive feedback effect was attenuated in older compared with younger PMW (peak LH 144.4 ± 19.5 vs. 226.8 ± 22.3 IU/l, respectively, P = 0.01). In conclusion, these studies in PMW demonstrate preservation of short-term steroid negative and positive feedback in response to exogenous E2 and P with aging. Attenuation of positive feedback in older compared with younger PMW is consistent with previous reports of declining GnRH responsiveness with aging.
Keywords: luteinizing hormone, neuroendocrine, postmenopausal women
reproductive senescence in women represents a dynamic phase of complex physiological changes in which irregular follicular development and ovulatory dysfunction eventually give way to the total loss of ovarian function. The depletion of ovarian follicles, accompanied by decreasing levels of inhibin B and anti-mullerian hormone, is the primary mechanism underlying reproductive aging in women (2, 9, 23, 29, 35). After the initial postmenopausal rise in gonadotropins, there is a progressive decline in both luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels in postmenopausal women (PMW) as a function of age that provides evidence for an additional neuroendocrine effect of aging in women that is independent of changes at the ovarian level (12). We have demonstrated comparable sensitivity to the negative feedback effects of chronic low-dose estrogen on LH in younger compared with older PMW (7, 8, 26). However, the question of whether aging attenuates the inhibitory and stimulatory effects of estrogen on LH over a time frame that more closely approximates that of the normal follicular phase has not been investigated.
We have addressed this question in younger and older PMW, in whom endogenous estrogen and inhibin are uniformly low (4), by administering a controlled intravenous steroid infusion that mimics estradiol (E2) and progesterone (P) levels across the follicular phase and results in negative followed by positive feedback in reproductive-aged women (20, 31). This study demonstrates that, whereas short-term estrogen negative and positive feedback are retained after the menopausal transition, estrogen positive feedback is attenuated with aging.
MATERIALS AND METHODS
Subjects.
Young (45–56 yr; n = 11) and old (70–80 yr; n = 9) PMW were studied. All subjects were healthy and had experienced their last menstrual period ≥18 mo previously, and therefore, they were postmenopausal according to the Stages of Reproductive Aging Workshop criteria (28). Subjects were not on any medication known to interact with the neuroendocrine reproductive axis, including over-the-counter medications or herbal remedies. Prolactin, thyroid-stimulating hormone, complete blood count, and renal function tests were normal. Electrocardiograms and mammograms were normal in all subjects, and none had any contraindications to hormone replacement therapy (HRT). Factor V Leiden mutations were absent in all patients. Subjects took 324 mg/day ferrous gluconate beginning 1 mo prior to the inpatient protocol until 1 mo after the study.
The study was approved by the Human Research Committee of the Massachusetts General Hospital, and signed informed consent was obtained from each subject before participation. The study was registered at ClinicalTrials.gov (NCT 00455741).
Experimental protocol.
Subjects were admitted to the Clinical Research Center of the Massachusetts General Hospital for 5 days for a graded intravenous E2 and P infusion protocol that has previously been shown to result in negative followed by positive feedback in normal and PMW (20, 22, 31). A stepwise infusion of E2 was initiated at 1500 on the day of admission and continued for 96 h, simulating the rise in E2 that occurs over the course of the follicular phase (Fig. 1). An infusion of P was started 48 h after the onset of the E2 infusion and continued for 48 h to mimic the low concentrations of P that are present in normal cycles prior to ovulation. Three blood samples were obtained from each subject at 15-min intervals before initiation of the E2 infusion, and samples were then drawn every 4 h throughout the infusions and for 24 h after the infusions were discontinued.
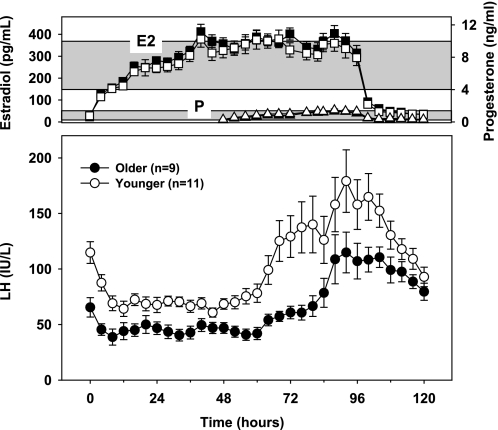
Fig. 1.
Means ± SE for younger (■ and ●) and older (○, □, and ▵) postmenopausal women for estradiol (E2) (□ and ■; top), progesterone (P) [▵ and ▴; top (closed triangles are overlapped by open triangles)], and LH levels (● and ○; bottom) during a graded steroid infusion. Top: the shaded horizontal bars represent means ± SD of the preovulatory E2 (upper) and P (lower) levels in 81 regularly cycling young women over 118 cycles (31), indicating that physiological preovulatory levels of both E2 and P were achieved in response to the infusion. LH is expressed in IU/l as equivalents of the Second International Reference Preparation of human menopausal gonadotropins. See text for conversion of LH to IU/l expressed in relation to the pituitary standard.
Steroid infusions.
The E2 infusion was constituted from a stock of crystalline E2 dissolved in propylene glycol at 17.34 mmol/l, and the P stock solution was similarly prepared with micronized P in propylene glycol at 31.8 mmol/l, as described previously (31). Both stock solutions were passed through a 0.22-μm filter. Five hundred microliters of stock solution was then mixed with 4.5 ml of 25% human serum albumin and added to 500 ml of normal saline in glass bottles. The final concentration of the E2 solution was 0.5 μg/ml; the final concentration of the P solution was 10 ng/ml. The infusions were administered using a Micro 965 Volumetric Infusion Pump (IMED, San Diego, CA) via a closed system and non-PVC fluid-path iv tubing (Accuset; IMED) that reduces steroid binding to its surface. The infusion rates were adjusted to produce E2 and P levels within the normal follicular phase range. For these studies, E2 was infused at a rate of 0.37 nmol·kg−1·h−1 (0.1 μg·kg−1·h−1) for 12 h, 0.5 nmol·kg−1·h−1 (0.135 μg·kg−1·h−1) for 12 h, 0.61 nmol·kg−1·h−1 (0.165 μg·kg−1·h−1) for 12 h, and 0.74 nmol·kg−1·h−1 (0.2 μg·kg−1·h−1) for the remaining 60 h. P was infused at a rate of 4.77 nmol·kg−1·h−1 (1.5 μg·kg−1·h−1) for the first 24 h and 6.36 nmol·kg−1·h−1 (2 μg·kg−1·h−1) for the second 24 h.
Assays.
Serum LH, FSH, and E2 were measured by immunoassay (AxSYM; Abbott Laboratories, Chicago, IL), as described previously (13, 30, 35, 36). P was analyzed using a sequential competitive immunoassay (Immulite 1000; Siemens, Los Angeles, CA). LH and FSH are expressed in international units per liter as equivalents of the Second International Reference Preparation 71/223 of human menopausal gonadotropins (hmg). The following formulas can be used to convert to the Pituitary (pit) Second International Standard 80/552: LH (pit) = 0.41 × (LH hmg) − 0.32 and FSH (pit) = 0.57 × (FSH hmg) − 0.25. The assay sensitivity for both LH and FSH is 1.6 IU/l. The intra-assay coefficients of variation (CVs) for LH and FSH are <7 and <6%, respectively, with interassay CVs for both hormones of <7.4%. The interassay CVs for P are 14.4, 10.6, and 10.8% at concentrations of 1.5, 3.2, and 14.3 ng/ml (4.8, 10.2, and 45.5 nmol/l), respectively.
Statistical analysis.
ANOVA for repeated measures was used to compare E2 and P levels achieved with infusions in younger and older PMW. The baseline LH was calculated as the arithmetic mean of levels measured at −30, −15, and 0 min prior to the steroid infusion. The negative feedback effect of E2 on LH was assessed by identifying the LH nadir base using a three-point moving average and expressed in absolute terms and in relation to baseline. The LH peak was defined as the highest value. For each individual, the onset of positive feedback was defined as the time when LH first exceeded the mean + 2 SD of the previous three time points and showed a sustained increase. To account for potential changes in the time course of positive feedback and to control for differences in the absolute LH nadir between subjects, the area under the curve (AUC) of the LH response for each subject was calculated as the geometric area above the LH value just prior to the onset of positive feedback until the completion of the study.
Unpaired t-tests were used for comparisons between younger and older PMW. Results are expressed as means ± SE unless otherwise indicated. P < 0.05 was considered significant.
RESULTS
Baseline characteristics.
Older and younger PMW were separated by age but did not differ in body weight or BMI (Table 1). The majority of subjects (n = 17) had undergone natural menopause, whereas one younger and two older subjects had undergone bilateral oophorectomy. Menopausal status was confirmed in all subjects at baseline by low E2 and elevated gonadotropins. Gonadotropins were lower in older compared with younger PMW at baseline, as seen previously (11), whereas no difference in E2 levels was seen between younger and older PMW. Seventy-three percent (8 of 11) of the younger women and 44% (4 of 9) of the older women had never taken HRT; those older women with a history of HRT had been off replacement for a median of 2.0 yr (range 1–10 yr). The younger women were a median of 5.1 yr [interquartile range (IQR) 3.3–9.5] postmenopause and 3.6 yr (IQR 1.6–5.1) postmenopause or HRT, whereas the older women were a median of 22 yr (IQR 19.4–24.1) postmenopause and 11.2 yr (IQR 8.9–13.4) postmenopause or HRT (P < 0.001 for both comparisons).
Table 1.
Characteristics of younger and older postmenopausal subjects
| Younger (n = 11) | Older (n = 9) | P Value | |
|---|---|---|---|
| Age, yr | 49.9 ± 1.1 | 75.2 ± 1.2 | <0.001 |
| Weight, kg | 65.0 ± 3.6 | 67.0 ± 3.2 | 0.7 |
| BMI, kg/m2 | 25.0 ± 1.3 | 26.1 ± 1.0 | 0.5 |
| Baseline | |||
| Estradiol, pg/ml* | 26 ± 2.1 | 27 ± 2.3 | 0.8 |
| LH, IU/l | 121.9 ± 12.5 | 81.5 ± 7.8 | 0.02 |
| FSH, IU/l | 184.0 ± 19.4 | 122.0 ± 13.3 | 0.02 |
Values are expressed as means ± SE.
P values represent younger vs. older comparisons.Gonadotropins are expressed in IU/l as equivalents of the Second International Reference Preparation of human menopausal gonadotropins. Please see text for conversion to IU/l expressed in relation to pituitary standards for LH and FSH.
To convert pg/ml to pmol/l, multiply by 3.67.
Steroid infusion.
E2 levels rose within several hours of the start of the graded E2 infusion (Fig. 1), reaching a mean plateau of 351 ± 7 pg/ml (1,290 ± 25 pmol/l) from 48 to 96 h, which was not statistically different from levels observed in normally cycling women during the late follicular phase (1). There was no difference in mean E2 from 0 to 96 h between younger and older women [285 ± 18 vs. 306 ± 19 pg/ml (1,050 ± 66 vs. 1,120 ± 71 pmol/l), respectively, P = 0.4], nor was there a difference in mean E2 during the 24 h preceding the LH peak [332 ± 36 vs. 309 ± 34 pg/ml (1,220 ± 133 vs. 1,130 ± 123 pmol/l), P = 0.6]. Mean P levels during the infusion were 1.3 ± 0.2 ng/ml (4,130 ± 636 pmol/l), consistent with what is seen prior to ovulation in the late follicular phase of normal cycles (1), and did not differ between younger and older PMW [1.0 ± 0.1 vs. 0.9 ± 0.1 ng/ml (3,180 ± 318 vs. 2,860 ± 318 pmol/l), respectively, P = 0.3].
Estrogen negative feedback.
In response to the E2 infusion, LH initially decreased to a lower absolute nadir of 36.3 ± 4.3 IU/l in older compared with 58.6 ± 4.5 IU/l in younger PMW (P = 0.002; Fig. 1). However, as in previous studies, baseline LH levels were lower in older than in younger PMW, and the decrease in LH was not different when expressed as a percent decrease from baseline LH (53.2 ± 2.3 and 52.8 ± 3.0% for older and younger PMW, respectively, P = 0.9). These data suggest that aging affects baseline LH without altering sensitivity to short-term E2 negative feedback in women.
Estrogen positive feedback.
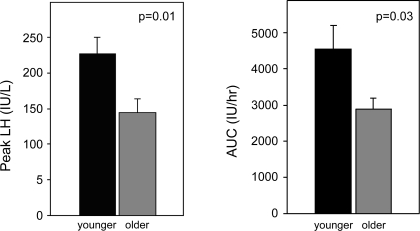
With continued steroid infusion, LH levels increased above baseline in all subjects to levels consistent with those observed in normal women at the time of the endogenous LH surge (1). Although the time of onset of the surge was not influenced by aging (58.7 ± 6.1 vs. 64.4 ± 4.8 h in older and younger PMW, respectively, P = 0.5), peak LH was 36% lower in older compared with younger PMW (144.4 ± 19.5 vs. 226.8 ± 22.3 IU/l, respectively, P = 0.01; Figs. 1 and 2). Peak LH was not significantly different between older and younger PMW when adjusted for baseline LH (121.7 ± 30.7% increase in older vs. 114.8 ± 15.9% in younger, P = 0.8). However, the AUC for LH, which takes into account both the pattern and the peak of the LH increase and controls for differences in the absolute nadir between older and younger PMW, was reduced in older compared with younger PMW (2,885.5 ± 316.7 IU·l−1·h vs. 4,544.6 ± 636.3 IU·l−1·h, respectively; P = 0.03) (Fig. 2). There was no difference in the peak LH response in women with a history of HRT use compared with those without hormone exposure (P = 0.3). Taken together, these results suggest that although steroid positive feedback is retained after menopause, it is attenuated with aging.
Fig. 2.
Peak LH (see Fig. 1 and text for discussion of reference standards used) and LH area under the curve (AUC) are attenuated in older compared with younger postmenopausal women in response to a controlled steroid infusion. AUC was calculated to control for differences in the absolute LH nadir between subjects and accounts for any differences in the time course of positive feedback between the 2 groups.
DISCUSSION
There is now ample evidence that aging of the neuroendocrine components of the reproductive axis occurs in women (12), with a decline in gonadotropin-releasing hormone (GnRH) pulse frequency (13) and pituitary responsiveness (27) but a concomitant increase in overall GnRH quantity (8). The current studies add estrogen positive feedback to this list of age-related changes. In studies in which E2 and P were carefully controlled and mimicked values achieved during normal menstrual cycles, we have now demonstrated a differential effect of aging on steroid negative and positive feedback such that the magnitude of the positive feedback LH response to ovarian steroids is attenuated with aging in women, whereas the magnitude of estrogen negative feedback is unaffected.
These studies used a validated protocol that is based on the known importance of both dose and duration of estrogen exposure for generation of positive feedback on LH (16, 40). In this protocol, recreation of physiological follicular phase E2 and P results in negative followed by positive feedback on LH in reproductive-aged women (20, 31), with peak LH levels mimicking those at the time of the endogenous LH surge in normally cycling women (1). By manipulating the time at which the estrogen infusion is stopped, previous studies have shown that the increase in LH using this infusion paradigm reflects positive feedback rather than merely a release from negative feedback (31). The biphasic LH response to this steroid infusion protocol in PMW is similar to that in reproductive-aged women, suggesting that it is a valid model with which to examine the effects of aging on estrogen negative and positive feedback.
The subjects in this study were separated by both age and time from estrogen exposure, and thus it is possible that the effect of aging may be mediated by time from estrogen exposure rather than chronological age itself. However, in a previous study demonstrating decreased pituitary responsiveness to GnRH with aging (27), we found no direct relationship between years from estrogen exposure (i.e., menopause or HRT) and pituitary responsiveness.
We have reported previously that 1 mo of estrogen administration produces decreases in mean LH (7), hypothalamic GnRH secretion (8), and direct inhibition of LH at the pituitary (26) that do not differ between younger and older PMW. Similarly, in the current study, the negative feedback response to short-term E2 administration was not influenced by aging. Interestingly, the E2 levels achieved and the percent suppression of LH were greater than in our longer-term studies in a similar subject population, suggesting that the effect of aging on estrogen negative feedback on LH is true across a range of low doses of E2. Studies of short-term estrogen negative feedback by other groups have been less consistent, demonstrating either enhanced or attenuated negative feedback with aging. However, these studies have had methodological limitations such as the inclusion of perimenopausal women in whom endogenous estrogen exposure may influence the results (33), the use of different estrogen doses in the two groups being compared (32), or the use of clomiphene citrate as an estrogen agonist (24) since clomiphene citrate may have both agonist and antagonist properties, depending on the endogenous estrogen milieu.
It is intriguing that the neurophysiological pathways responsible for generation of the LH surge in response to exogenous but physiological steroid administration appear to be intact in women into the eighth decade of life despite years without endogenous ovarian steroids. The absolute LH levels achieved in PMW were comparable with those in young reproductive-aged women during both endogenous (1) and induced (31) surges, although the percent increase relative to baseline LH levels was less due to the long-term lack of steroid negative feedback on baseline LH levels in PMW. In an observational study of regularly cycling women in their 30s to 50s, Lee at al. (19) found no change in midcycle LH profiles with aging, and perimenopausal women have ovulatory cycles for several years after initially entering into the menopause transition (5), consistent with the preservation of positive feedback. These findings are also consistent with the results of a large observational study in perimenopausal women in which fewer than 4% of women failed to ovulate in the face of periovulatory estrogen levels (34). Taken together, these data suggest that the decreased LH positive feedback response demonstrated in the current studies is a relatively late event in reproductive aging.
These findings in women contrast with those in other animal species, in which diminished estrogen positive feedback is a relatively early event in the process of reproductive aging. In intact middle-aged rats, the proestrous LH surge is blunted by ∼40% compared with young rats prior to any overt changes in estrous cyclicity (37). The observation that these findings were replicated in ovariectomized rats treated with E2 (25, 38) suggests that this effect is independent of other changes in ovarian secretion that accompany aging in rodents analogous to the current study.
Attenuation of estrogen positive feedback in rodents is attributed to the effects of aging at the hypothalamus secondary to an imbalance between stimulatory (glutamate, norepinephrine) and inhibitory (GABA, opioids) inputs (reviewed in Refs. 3, 6, and 39), a diminished diurnal rhythm of suprachiasmatic nucleus input (17), and reduced responsiveness to kisspeptin (18). However, there is considerable evidence that estrogen positive feedback in women is mediated primarily at the pituitary, with hypothalamic GnRH secretion playing only a permissive role (14, 15, 21). Neuroanatomic studies in PMW undergoing a graded steroid infusion similar to that used in the current studies indicate that negative feedback is associated with a decrease in metabolic activity in the medial basal hypothalamus followed by increased pituitary, but not hypothalamic, activity associated with positive feedback (22), suggesting that in PMW, as in reproductive-aged women, the positive feedback effect resides primarily at the pituitary.
We have shown previously that pituitary responsiveness to GnRH is decreased with aging (27) and hypothesize that attenuation of the LH surge with aging in response to a controlled steroid infusion may be an additional manifestation of pituitary aging. The mechanisms through which aging may alter pituitary responsiveness to GnRH and estrogen positive feedback are unknown. Recent studies demonstrate a decrease in pituitary volume with aging in women (10), but it is unclear whether this translates into changes in gonadotrope number, morphometry, or function. Additional possibilities include decreased expression of estrogen receptors or changes in intracellular signaling as a function of aging.
In summary, the current studies in PMW, in which the rising physiological steroid levels of the normal follicular phase have been reproduced, now demonstrate that the neuroendocrine mechanisms responsible for estrogen feedback remain intact well after the menopause. Whereas estrogen negative feedback remains robust with aging, estrogen positive feedback is attenuated. These studies add to an increasing body of work that demonstrates that aging impacts the central components of the reproductive axis in women and provide support for differential effects of aging on the hypothalamus and pituitary.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-AG-13241 and M01-RR-01066. N. D. Shaw received fellowship support from the NIH (5T32-HD-007396) and from the Scholars in Clinical Science Program of Harvard Catalyst [the Harvard Clinical and Translational Science Center (Award no. UL1 RR 025758) and financial contributions from Harvard University and its affiliated academic health care centers]. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, or its affiliated academic health care centers, the National Center for Research Resources, or the NIH.
DISCLOSURES
The authors have nothing to declare.
REFERENCES
- 1.Adams JM, Taylor AE, Schoenfeld DA, Crowley WF, Jr, Hall JE. The midcycle gonadotropin surge in normal women occurs in the face of an unchanging gonadotropin-releasing hormone pulse frequency. J Clin Endocrinol Metab 79: 858–864, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Block E. Quantitative morphological investigations of the follicular system in women; variations at different ages. Acta Anat (Basel) 14: 108–123, 1952 [DOI] [PubMed] [Google Scholar]
- 3.Brann DW, Mahesh VB. The aging reproductive neuroendocrine axis. Steroids 70: 273–283, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, Dennerstein L. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab 84: 4025–4030, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Burger HG, Robertson DM, Baksheev L, Collins A, Csemiczky G, Landgren BM. The relationship between the endocrine characteristics and the regularity of menstrual cycles in the approach to menopause. Menopause 12: 267–274, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Downs JL, Wise PM. The role of the brain in female reproductive aging. Mol Cell Endocrinol 299: 32–38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill S, Lavoie HB, Bo-Abbas Y, Hall JE. Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J Clin Endocrinol Metab 87: 2297–2302, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Gill S, Sharpless JL, Rado K, Hall JE. Evidence that GnRH decreases with gonadal steroid feedback but increases with age in postmenopausal women. J Clin Endocrinol Metab 87: 2290–2296, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Gougeon A, Ecochard R, Thalabard JC. Age-related changes of the population of human ovarian follicles: increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biol Reprod 50: 653–663, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Grams AE, Gempt J, Stahl A, Forschler A. Female pituitary size in relation to age and hormonal factors. Neuroendocrinology 92: 128–132, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Hall JE. Neuroendocrine physiology of the early and late menopause. Endocrinol Metab Clin North Am 33: 637–659, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Hall JE. Neuroendocrine changes with reproductive aging in women. Semin Reprod Med 25: 344–351, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hall JE, Lavoie HB, Marsh EE, Martin KA. Decrease in gonadotropin-releasing hormone (GnRH) pulse frequency with aging in postmenopausal women. J Clin Endocrinol Metab 85: 1794–1800, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Hall JE, Taylor AE, Martin KA, Rivier J, Schoenfeld DA, Crowley WF., Jr Decreased release of gonadotropin-releasing hormone during the preovulatory midcycle luteinizing hormone surge in normal women. Proc Natl Acad Sci USA 91: 6894–6898, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karsch FJ, Bowen JM, Caraty A, Evans NP, Moenter SM. Gonadotropin-releasing hormone requirements for ovulation. Biol Reprod 56: 303–309, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Keye WR, Jr, Jaffe RB. Strength-duration characteristics of estrogen effects on gonadotropin response to gonadotropin-releasing hormone in women. I. Effects of varying duration of estradiol administration. J Clin Endocrinol Metab 41: 1003–1008, 1975 [DOI] [PubMed] [Google Scholar]
- 17.Krajnak K, Kashon ML, Rosewell KL, Wise PM. Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA but not arginine vasopressin mRNA in the suprachiasmatic nuclei of female rats. J Neurosci 18: 4767–4774, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lederman MA, Lebesgue D, Gonzalez VV, Shu J, Merhi ZO, Etgen AM, Neal-Perry G. Age-related LH surge dysfunction correlates with reduced responsiveness of hypothalamic anteroventral periventricular nucleus kisspeptin neurons to estradiol positive feedback in middle-aged rats. Neuropharmacology 58: 314–320, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SJ, Lenton EA, Sexton L, Cooke ID. The effect of age on the cyclical patterns of plasma LH, FSH, oestradiol and progesterone in women with regular menstrual cycles. Hum Reprod 3: 851–855, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Liu JH, Yen SS. Induction of midcycle gonadotropin surge by ovarian steroids in women: a critical evaluation. J Clin Endocrinol Metab 57: 797–802, 1983 [DOI] [PubMed] [Google Scholar]
- 21.Martin KA, Welt CK, Taylor AE, Smith JA, Crowley WF, Jr, Hall JE. Is GnRH reduced at the midcycle surge in the human? Evidence from a GnRH-deficient model. Neuroendocrinology 67: 363–369, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Ottowitz WE, Dougherty DD, Fischman AJ, Hall JE. [18F]2-fluoro-2-deoxy-d-glucose positron emission tomography demonstration of estrogen negative and positive feedback on luteinizing hormone secretion in women. J Clin Endocrinol Metab 93: 3208–3214, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab 65: 1231–1237, 1987 [DOI] [PubMed] [Google Scholar]
- 24.Rossmanith WG, Reichelt C, Scherbaum WA. Neuroendocrinology of aging in humans: attenuated sensitivity to sex steroid feedback in elderly postmenopausal women. Neuroendocrinology 59: 355–362, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Rubin BS, Lee CE, King JC. A reduced proportion of luteinizing hormone (LH)-releasing hormone neurons express Fos protein during the preovulatory or steroid-induced LH surge in middle-aged rats. Biol Reprod 51: 1264–1272, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Shaw ND, Histed SN, Srouji SS, Yang J, Lee H, Hall JE. Estrogen negative feedback on gonadotropin secretion: evidence for a direct pituitary effect in women. J Clin Endocrinol Metab 95: 1955–1961, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw ND, Srouji SS, Histed SN, McCurnin KE, Hall JE. Aging attenuates the pituitary response to gonadotropin-releasing hormone. J Clin Endocrinol Metab 94: 3259–3264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Climacteric 4: 267–272, 2001 [PubMed] [Google Scholar]
- 29.Sowers MR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, Zhang D, Harlow S, Randolph JF., Jr Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab 93: 3478–3483, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor AE, Khoury RH, Crowley WF., Jr A comparison of 13 different immunometric assay kits for gonadotropins: implications for clinical investigation. J Clin Endocrinol Metab 79: 240–247, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Taylor AE, Whitney H, Hall JE, Martin K, Crowley WF., Jr Midcycle levels of sex steroids are sufficient to recreate the follicle-stimulating hormone but not the luteinizing hormone midcycle surge: evidence for the contribution of other ovarian factors to the surge in normal women. J Clin Endocrinol Metab 80: 1541–1547, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Tsai CC, Yen SS. Acute effects of intravenous infusion of 17-beta-estradiol on gonadotropin release in pre- and post-menopausal women. J Clin Endocrinol Metab 32: 766–771, 1971 [DOI] [PubMed] [Google Scholar]
- 33.Van Look PF, Lothian H, Hunter WM, Michie EA, Baird DT. Hypothalamic-pituitary-ovarian function in perimenopausal women. Clin Endocrinol (Oxf) 7: 13–31, 1977 [DOI] [PubMed] [Google Scholar]
- 34.Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA 292: 2991–2996, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Welt CK, McNicholl DJ, Taylor AE, Hall JE. Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab 84: 105–111, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Welt CK, Pagan YL, Smith PC, Rado KB, Hall JE. Control of follicle-stimulating hormone by estradiol and the inhibins: critical role of estradiol at the hypothalamus during the luteal-follicular transition. J Clin Endocrinol Metab 88: 1766–1771, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Wise PM. Alterations in proestrous LH, FSH, and prolactin surges in middle-aged rats. Proc Soc Exp Biol Med 169: 348–354, 1982 [DOI] [PubMed] [Google Scholar]
- 38.Wise PM. Estradiol-induced daily luteinizing hormone and prolactin surges in young and middle-aged rats: correlations with age-related changes in pituitary responsiveness and catecholamine turnover rates in microdissected brain areas. Endocrinology 115: 801–809, 1984 [DOI] [PubMed] [Google Scholar]
- 39.Yin W, Gore AC. Neuroendocrine control of reproductive aging: roles of GnRH neurons. Reproduction 131: 403–414, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Young JR, Jaffe RB. Strength-duration characteristics of estrogen effects on gonadotropin response to gonadotropin-releasing hormone in women. II. Effects of varying concentrations of estradiol. J Clin Endocrinol Metab 42: 432–442, 1976 [DOI] [PubMed] [Google Scholar]