Abstract
Frequent episodes of hyperketonemia are associated with a higher incidence of vascular disease. The objective of this study was to examine the hypothesis that hyperketonemia increases monocyte-endothelial cell (EC) adhesion and the development of vascular disease in diabetes. Human U937 and THP-1 monocyte cell lines and human umbilical vein endothelial cells (HUVECs) were cultured with acetoacetate (AA) (0–10 mM) or β-hydroxybutyrate (BHB) (0–10 mM) for 24 h prior to evaluating adhesion and adhesion molecule expression. The results demonstrate a significant (P < 0.01) increase in both U937 and THP-1 adhesion to HUVEC monolayers treated with 4 mM AA compared with control. Equal concentrations of BHB resulted in similar increases in monocyte-EC adhesion. Similarly, treatments of AA or BHB to isolated monocytes from human blood also show increases in adhesion to endothelial cells. intercellular adhesion molecule-1 (ICAM-1) was significantly increased on the surface of HUVECs and an increase in total protein expression with AA treatment compared with control. The expression level of lymphocyte function-associated antigen-1 (LFA-1) was increased in monocytes treated with AA, and LFA-1 affinity was altered from low to high affinity following treatment with both AA and BHB. Monocyte adhesion could be blocked when cells were preincubated with an antibody to ICAM-1 or LFA-1. Results also show a significant increase in IL-8 and MCP-1 secretion in monocytes and HUVECs treated with 0–10 mM AA. These results suggest that hyperketonemia can induce monocyte adhesion to endothelial cells and that it is mediated via increased ICAM-1 expression in endothelial cells and increased expression and affinity of LFA-1 in monocytes.
Keywords: intercellular adhesion molecule-1, lymphocyte function-associated antigen-1, ketosis, type 1 diabetes, inflammation, adhesion molecules
vascular inflammation and cardiovascular disease (CVD) are the leading causes of mortality and morbidity among patients with diabetes. Type 1 diabetes (T1D) is associated with increased vascular complications and is considered a proinflammatory condition (8). Studies in the current literature indicate that T1D is associated with increased oxidative stress and increased levels of various inflammatory biomarkers such as CRP, monocyte activity, sICAM, sE-selectin, sP-selectin, and sCD40L (9, 10, 20). The current literature also contains reports of an increased risk for CVD in T1D (12, 29, 34). In addition to hyperglycemia, T1D patients frequently experience hyperketonemia due to a state of insulin deficiency. T1D is the most common pathological cause of elevated ketone bodies; however, it has also been shown to coexist with hyperglycemia among older T2D patients and in select minority groups with T2D (1, 28, 35, 38). There are three main ketone bodies that are produced when glucose is not readily available: acetoacetate and β-hydroxybutyrate being the most abundant and acetone being the third and least abundant (25). In severe cases, levels of circulating ketone bodies can reach 25 mM compared with normal levels of less than 0.5 mM (21, 25). Although there is a significant amount of literature on the microvascular complications of T1D, little is known about the pathogenesis of CVD in T1D. It is known that diabetic subjects with frequent episodes of hyperketonemia experience increased incidence of vascular disease, morbidity, and mortality (7, 32, 36, 37). The precise biochemical mechanisms by which hyperketonemia increases the development of vascular disease remains unknown.
This study examines the hypothesis that hyperketonemia increases monocyte-endothelial cell (EC) adhesion and the development of vascular disease in diabetes. To examine this hypothesis, we evaluated the adherence between monocytes and endothelial cells treated with acetoacetate or β-hydroxybutyrate. We also measured the levels of proinflammatory chemokine and adhesion molecule expression and activation in both human monocytes and human umbilical vein endothelial cells (HUVEC) exposed to acetoacetate or β-hydroxybutyrate. Our data indicate that physiological concentrations of acetoacetate and β-hydroxybutyrate can contribute to increased monocyte adhesion to endothelial cells by upregulation of intercellular adhesion molecule-1 (ICAM-1) in endothelial cells and lymphocyte function-associated antigen-1 (LFA-1) in monocytes. This provides evidence for a novel mechanism by which hyperketonemia contributes to the excess vascular disease seen in type 1 diabetics.
MATERIALS AND METHODS
Cell culture.
Human monocytic cell lines, human U-937 monocytes and THP-1 monocytes, were purchased from American Type Culture Collection (ATCC, Manassas, VA). These cells were cultured in RPMI 1640 medium supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine. The culture was maintained at 37°C in a humidified atmosphere containing 5% CO2. For treatments, the cells were counted on a hemocytometer using Trypan Blue exclusion and adjusted to approximately 1 × 106 cells/ml in complete medium. In addition, THP-1 monocytes and isolated human monocytes were used in this study to determine whether results were cell type specific.
HUVECs.
HUVECs were purchased from Lonza Walkersville and were cultured to confluence in EGM-2 medium and 5% CO2 in a 37°C humidified atmosphere. The culture was passaged according to standard procedures. For experiments, HUVECs were used within 24 h after reaching confluence, between passages 3 and 10. The vendor certified that the medium had endotoxin concentrations <0.005 EU/ml.
In our experiments, we used concentrations of acetoacetate and β-hydroxybutyrate ranging from 0 to 10 mM. Studies in the literature report blood levels of up to 5 mM acetoacetate and 11 mM β-hydroxybutyrate in diabetic patients (7, 34a). Thus the concentrations used in these experiments are interpretable as physiologically relevant results. The monocyte cell viability was not affected (>90%) at this range of concentrations. In HUVECs, the viability was greater than 90% in cells treated with up to 5 mM acetoacetate but significantly decreased with concentrations higher than 5 mM (16).
Isolation of human monocytes.
Blood was collected in EDTA tubes from fasting healthy adult volunteers, and the peripheral blood mononuclear cells were isolated using Ficoll Hypaque centrifugation. The monocytes were isolated using Monocyte Isolation Kit II (Miltenyi Biotec, Auburn, CA) which uses magnetic separation to deplete non-monocytes (negative selection). The purity of the monocyte population was confirmed, using flow cytometry, by staining an aliquot of cells with anti-CD14-PE (Miltenyi Biotec). This technique yields greater than 86% of cells identified as monocytes. The isolated monocytes were cultured and assayed the same as for monocytic cell lines.
Chemokine assays.
IL-8 and monocyte chemoattractant protein-1 (MCP-1) levels were determined by ELISA methods using commercially available ELISA kits from Pierce-Endogen (Rockford, IL). The intra-assay for each of these assays was <10%. The chemokine concentration was determined by evaluating the supernatant from endothelial cells and monocytes. All appropriate controls and standards specified by the manufacturer's kit were used. The data are expressed as picograms per milliliter of cell supernatant.
ELISA of endothelial cell adhesion molecule expression.
HUVECs were plated on 48-well tissue culture dishes and grown to 80–90% confluence. Primary antibodies or nonbinding IgG diluted to 1:500 in PBS with 2% heat-inactivated FBS were added to each well and incubated for 30 min at 37°C. The cells were washed and then incubated with horseradish peroxide (HRP)-conjugated goat anti-mouse IgG diluted 1:500 in PBS with 2% heat inactivated FBS. The wells were then washed, and the surface ECAM expression was determined by addition of 100 μl of TMB substrate solution. The reaction was stopped with 100 μl of 0.18 M sulfuric acid. The samples were transferred to a 96-well plate and read in a plate reader at OD 450 nm using wells with secondary antibody only as a blank. All samples were done in duplicate.
Western blotting.
The cells were lysed for 1 h with vortexing in RIPA buffer containing protease inhibitors. Lysates were centrifuged for 5 min at 13,000 rpm. Supernatants were collected and the protein levels determined by BCA. Lysates were suspended in SDS sample buffer containing either 4% β-mercaptoethanol or no reducing agent. The contents were mixed and boiled at 100°C for 5 min. The samples were loaded onto an 8% Tris-SDS acrylamide gel and run at 80 V until complete separation. The proteins were transferred to a nitrocellulose membrane (0.2 μM; Bio-Rad Laboratories, Hercules, CA) followed by blocking with 1% BSA prepared in TBS-T (Tris-buffered saline + 0.25% Tween 20) for 1 h. The blot was then incubated with a primary antibody overnight followed by washing and then a 1 h incubation in HRP-conjugated secondary antibody. Protein bands were detected by ECL detection reagents (PerkinElmer, Boston, MA) and exposed on blue X-ray film (Phenix Research Products, Candler, NC).
Flow cytometry.
Surface analysis of cell membrane proteins was done using indirect staining procedures and flow cytometry. Ice-cold reagents/solutions and the presence of sodium azide was used to prevent the modulation and internalization of surface receptors. Incubations were all done at 4°C in the dark to prevent loss of signal. The cells were harvested at the end of the experiment and resuspended to ∼1 × 106 cells/ml in 300 μl of ice-cold PBS, 10% FCS, and 1% sodium azide. An aliquot of 100-μl cell suspension was added to a microtube, followed by addition of primary antibody, and incubated for 30 min. Cells were washed three times by resuspending them in ice-cold PBS. A secondary antibody conjugated to FITC was diluted 1:250 in 3% BSA-PBS, and the cells were resuspended in 100 μl of this solution and incubated 30 min. Cells were washed three times in PBS and resuspended in PBS, 3% BSA, and 1% sodium azide. Cells were directly analyzed using flow cytometry as soon as possible. Cells were analyzed by collecting 10,000 events. Results are expressed as the percentage of positive-stained cells as well as the total mean fluorescence intensity of 10,000 cells. Controls for matched isotype of primary antibodies were analyzed to ensure no nonspecific binding. Unstained cells, both treated and nontreated, were used as a negative control, and cells with secondary antibody only were also analyzed to ensure no nonspecific secondary binding.
Monocyte-EC adhesion.
HUVECs were plated and allowed to grow to confluent monolayers. The ECs were treated with either acetoacetate (0–6 mM) or β-hydroxybutyrate (0–8 mM) for 24 h. Monocytes were loaded with 8 μM CellTracker Green (CMFDA; Invitrogen, Eugene, OR) and then treated with either equal acetoacetate or β-hydroxybutyrate, at concentrations matching those of the ECs. After 24 h, 1 × 106 cells were added to the endothelial monolayers and incubated at 37°C for 30 min. The nonadherent cells were washed away with EC media, and adherent cells were lysed in 0.2% Triton X for quantification. The fluorescent intensity of the monocytes added to the monolayer (input) as well as the nonadherent cells was measured at excitation 485 and emission 528 nm.
Reagents.
All chemicals were obtained from Sigma Chemical (St. Louis, MO) unless stated otherwise. EC growth medium was purchased from Clonetics (San Diego, CA). The following primary antibodies used in this study were: rabbit polyclonal anti-CD11a, for detection of LFA-1, and mouse monoclonal anti-β-actin (Abcam, Cambridge, MA); mouse monoclonal anti-E-selectin, -ICAM-1, and -vascular cell adhesion molecule-1 (VCAM-1) (Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal anti-ICAM-1 for blocking (US Biological, Swampscott, MA); mouse monoclonal anti-CD11a for blocking (Abcam), mouse monoclonal anti-CD11+CD18 (mAb24) for activation epitope, and mouse monoclonal IgG1 (Abcam). Secondary antibodies used were goat anti-rabbit conjugated to FITC (Abcam), goat anti-mouse conjugated to HRP, Bio-Rad Laboratories (Hercules, CA), goat anti-rabbit conjugated to HRP (Millipore Temecula, CA).
Statistical analysis.
Results are expressed as means ± SE. Student's t-test was used to compare the differences between treatments using Sigma plot statistical software (SPSS, Chicago, IL). A P value of <0.05 was considered significant.
RESULTS
Hyperketonemia increases monocyte cell adhesion to ECs.
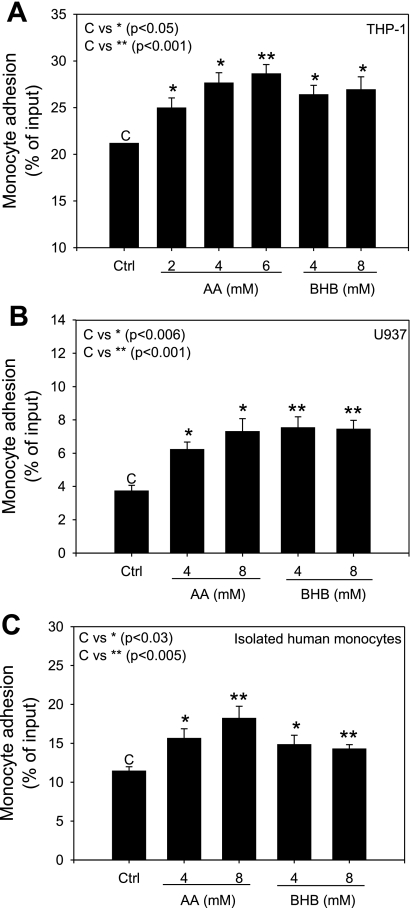
To determine whether hyperketonemic conditions would affect the adhesion of monocytes to EC layers, adhesion assays were performed between monocytes and ECs treated with acetoacetate or β-hydroxybutyrate for 24 h. We observed a dose-dependent increase in monocyte adherence to ECs when they were treated with acetoacetate using both U937 and THP-1 monocytes. There was also an increase in adherence in cells treated with β-hydroxybutyrate, but the increase was uniform and not affected by the β-hydroxybutyrate concentration (Fig. 1, A and B). Additionally, we confirmed the increase in monocyte adhesion by using isolated human monocytes under identical assay conditions (Fig. 1C).
Fig. 1.
Monocyte-endothelial cell (EC) adherence. Human umbilical vein ECs (HUVECs) were seeded and allowed to grow to confluent monolayers. ECs and monocytes were treated with acetoacetate (AA; 0–8 mM) or β-hydroxybutyrate (BHB; 0–8 mM) for 24 h. Adhesion assays were carried out as described in materials and methods. Values are expressed as %input fluorescence. A: THP-1 monocytes. B: U937 monocytes. C: isolated human monocytes. Values are means ± SE; n = 3.
Hyperketonemia induces ICAM-1 expression in ECs.
ICAM-1 is the predominant EC ligand for binding of LFA-1. As mentioned earlier, T1D patients have increased blood levels of biomarkers of inflammation, including s-ICAM (9). To determine whether hyperketonemia may influence ICAM-1 expression levels, HUVEC monolayers were treated with increasing concentrations of acetoacetate or β-hydroxybutyrate for 24 h. Surface expression of ICAM-1, VCAM-1, and E-selectin were all determined by ELISA. In addition, total protein expression of ICAM-1 was determined using Western blot. The results indicate that, of the three ECAMs evaluated, ICAM-1 surface expression levels increased by almost 30% compared with those of untreated cells when treated with acetoacetate (6 mM; Fig. 2B, left). Consistent with the increase in surface expression of ICAM-1, Western blotting also indicated that there was a significant increase in total protein when HUVECs were treated with acetoacetate (8 mM) compared with the control (Fig. 2A). Neither E-selectin nor VCAM-1 showed any significant change in surface expression when treated with acetoacetate or β-hydroxybutyrate (Fig. 2B). There was no significant change in E-selectin or VCAM-1 compared with a nonbinding control. TNF-α was used as a positive control and resulted in a significant increase in surface expression for ICAM-1 (Control 0.191 vs. TNF-α 1.114) and VCAM-1 (Control 0.124 vs. TNF-α 0.498); however, no significant increase was detected for E-selectin (control 0.131 vs. TNF-α 0.173). These data suggest that physiological concentrations of acetoacetate can induce ICAM-1 expression in ECs.
Fig. 2.
ECAM protein expression in HUVECs. A: total protein expression of intercellular adhesion molecule-1 (ICAM-1). HUVECs were plated in 100-mm dishes and treated with AA or BHB (0–8 mM). ICAM-1 was detected via Western blotting as described in materials and methods. An 8% gel was loaded with 20 μg of protein extract. Arbitrary units were determined using image J software and expressed per tubulin. Representative blot of ≥3 different experiments. B: surface expression of E-selectin, vascular cell adhesion molecule-1 (VCAM-1), and ICAM-1, and nonbinding IgG control was determined by ELISA as described in materials and methods. Values are means ± SE; n = 3.
Hyperketonemia increases expression and activation of LFA-1 in monocytes.
LFA-1 is a member of the integrin family and is composed of a common β-subunit (CD18) and a distinct α-subunit (CD11a). LFA-1 functions to mediate leukocyte adhesion to its primary endothelial adhesion ligand ICAM-1 (33). To determine whether hyperketonemia alters the expression level of LFA-1 in human monocytes in response to hyperketonemic conditions, we treated monocytes with increasing concentrations of acetoacetate or β-hydroxybutyrate for 24 h. After 24 h, the protein expression level of LFA-1 in U937 and THP-1 monocytes was determined by Western blot (Fig. 3A). An antibody for the α-subunit CD11a was used to distinguish LFA-1 from other similar integrin family members. These results indicated that total protein expression levels of LFA-1 adhesion molecules were increased in response to both acetoacetate and β-hydroxybutyrate. Increased expression of LFA-1 in monocytes suggests that it plays a role in the increased adhesive property of rolling monocytes in the blood stream.
Fig. 3.
Lymphocyte function-associated antigen-1 (LFA-1) expression in monocytes. A: total protein expression. Human U937 monocytes were plated at 1 × 106 cells/ml and treated with AA (0–10 mM) or BHB (0–12 mM) for 24 h. Protein expression of CD11a and β-actin was determined by Western blotting by loading 10 μg of protein extract into an 8% gel. Bands were quantified using image J software and expressed per actin. Left: U937 monocytes. Right: THP-1 monocytes. B: surface expression of LFA-1. Following treatments, surface expression was determined by flow cytometry as described in Flow cytometry. Data are expressed as %CD11a + cells from the parent/gated population. C: mean fluorescence intensity (MFI) of 10,000 events. Left: U937 monocytes. Right: THP-1 monocytes. Values are means ± SE; n = 3.
Since activation of leukocytes has also been known to regulate the rapid mobilization of β2-integrins from intracellular peroxidase-negative granules (5, 26), and total protein expression levels increase following treatment with acetoacetate and β-hydroxybutyrate, surface expression of LFA-1 was analyzed. The results indicated that U937 cells expressed LFA-1 at much lower levels than did THP-1 cells, 20% vs. 96% (Fig. 3B). However, when treated with acetoacetate or β-hydroxybutyrate, surface expression of LFA-1 increased significantly with acetoacetate (>2 mM) and β-hydroxybutyrate (>8 mM) in U937 monocytes (Fig. 3B, left). THP-1 monocytes did not show any change in surface expression with acetoacetate or β-hydroxybutyrate treatment (Fig. 3B, right). These data suggest that physiological concentrations of both acetoacetate and β-hydroxybutyrate result in upregulation of integrin expression, which may contribute to the recruitment and adhesion of an increased number of monocytes.
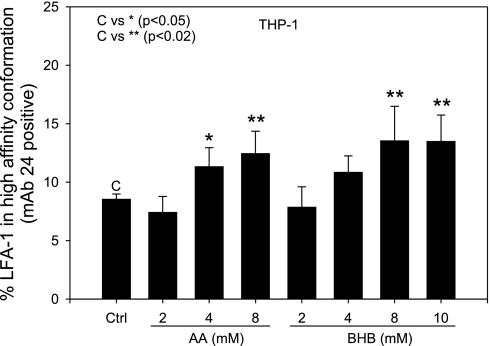
Integrins exist in a resting or inactive state on mononuclear cells; upon chemotactic stimulation or other means of cell activation, the receptors redistribute to high-density clusters and into a high-affinity state. To address the affinity of the CD11/CD18 molecule, we used a specific antibody that recognizes the activation epitope of β2-integrin in a high-affinity conformation (mAb24, Abcam). Figure 4 shows that physiological concentrations of both acetoacetate and β-hydroxybutyrate induced the high-affinity conformation of CD11/CD18. This provides evidence for a role for CD11/CD18 in the mechanism by which hyperketonemia activates monocytes for cell adhesion.
Fig. 4.
LFA-1 affinity. Human THP-1 monocytes were plated at 1 × 106 cells/ml and treated with AA (0–8 mM) or BHB (0–10 mM) for 24 h. mAb24 was added directly to the sample and incubated for 1 h prior to the end of the experiment to minimize loss of binding due to possible conformation changes by mechanical and physical stress during sample preparation. Following this, samples were processed as described in Flow cytometry. Values are means ± SE; n = 3.
ICAM-1 and LFA-1 play an important role in mediating monocyte-EC adhesion in acetoacetate-induced adhesion. Since ICAM-1 was upregulated on the surface of ECs exposed to acetoacetate and adherence of monocytes to the EC monolayer was increased in hyperketonemic conditions, we investigated the role of ICAM-1 in mediating monocyte-EC adhesion. To determine this, we used a monoclonal antibody that specifically blocks ICAM-1 binding and a different antibody specific for blocking CD11a. Mouse IgG was used as a control. We show that by blocking either ICAM-1 on ECs or CD11a in THP-1 monocytes, we were able to reduce the monocyte adherence to near control levels (Fig. 5). Because LFA-1 was also activated in THP-1 monocytes treated with BHB, we show that, by inhibiting CD11a in BHB treated cells, adherence was reduced below that observed in the control (Fig. 5). These data provide evidence for a novel mechanism mediating adherence between monocytes and ECs during hyperketonemic conditions.
Fig. 5.
Blocking ICAM-1 and CD11a inhibits monocyte-EC adhesion. HUVECs and monocytes were treated with 4 mM AA or 8 mM BHB for 24 h. Following treatment, HUVEC monolayers were treated with 5 mg/ml mouse IgG antibody or 5 mg/ml ICAM-1 blocking antibody for 30 min at 37°C. Then THP-1 monocytes (1 × 106 cells) were added to the endothelial monolayer and incubated for 30 min at 37°C. When CD11a was being blocked, monocytes were treated with blocking antibody (diluted 1:50) 30 min prior to adding them to the treated endothelial layer. Adhesion assays were carried out as described in materials and methods. Data are expressed as %input fluorescence of 3 different experiments. Values are means ± SE; n = 3.
Hyperketonemia increases proinflammatory chemokine secretion in ECs and monocytes.
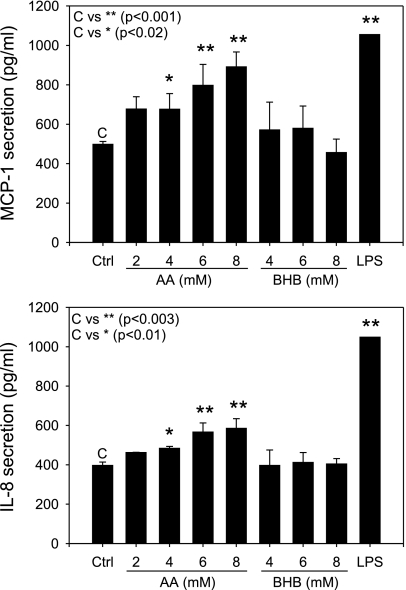
The chemokines IL-8 and MCP-1 are highly inducible chemoattractant proteins and modulate monocyte recruitment via chemotaxis and adhesion by altering adhesion molecule affinity. Figure 6 shows the effect of ketone bodies acetoacetate and β-hydroxybutyrate on IL-8 and MCP-1 secretion from ECs. The results indicate that there was a dose-dependent increase in levels of both chemokines IL-8 and MCP-1 when the cells were treated with acetoacetate but not with β-hydroxybutyrate (Fig. 6). LPS was used as a positive control to show that the cells secreted the chemokines under activated conditions. These results indicate that, at physiological concentrations of acetoacetate, there is a low level of inflammation in ECs that stimulates chemokine production.
Fig. 6.
Chemokine secretion in ECs. Confluent monolayers of HUVECs were treated with AA (0–8 mM) or BHB (0–8 mM) for 24 h. Monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8) levels were determined by assaying the supernatant using an ELISA kit. LPS (4 μg/ml) was used as a positive control. Data are representative of 4 different experiments. Values are means ± SE; n = 4.
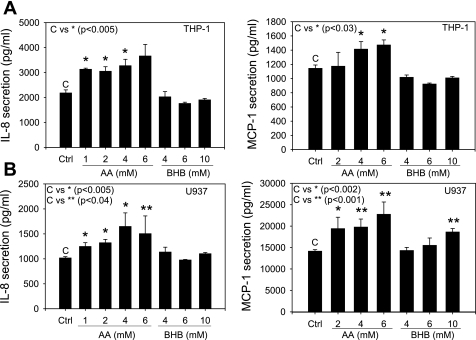
Monocytes also secrete chemokines in response to various stimuli, which contributes to the overall increased level of chemokines in the serum of diabetic patients. We evaluated chemokine production in monocytes in response to hyperketonemic conditions. The results indicate that both activated THP-1 and U937 monocytes secrete significantly higher levels of MCP-1 and IL-8 when treated with physiological concentrations of acetoacetate (Fig. 7). β-Hydroxybutyrate had little or no effect on IL-8 secretion in THP-1 or U937 monocytes but created a small increase in MCP-1 secretion in U937 monocytes but not THP-1 (Fig. 7).
Fig. 7.
Chemokine secretion in monocytes. Activated monocytes were treated with AA (0–6 mM) or BHB (0–10 mM) for 24 h. ELISA was completed on cell supernatant for IL-8 and MCP-1. A: THP-1 monocytes. B: U937 monocytes. Values are means ± SE; n = 4.
DISCUSSION
Several markers of vascular inflammation have been shown to be influenced by the presence of hyperketonemia (15–20). It has been reported that acetoacetate, but not β-hydroxybutyrate, increases lipid peroxidation and growth inhibition in cultured human ECs (16) as well as lowering glutathione levels in human erythrocytes (19). It has also been reported that acetoacetate increases TNF-α and IL-6 secretion in cultured monocytes and in hyperketonemic diabetic patients (17, 18). There is no previous study that has examined the role of hyperketonemia in the adhesivity of monocytes to the endothelium. Overall, there is very little evidence concerning the role hyperketonemia may play in vascular inflammation and its contribution to the risk of developing CVD in diabetes. In this study, we make a novel contribution to the literature that indicates that hyperketonemia may influence the progression of CVD in diabetes. The present study demonstrates that hyperketonemia increases adhesion of monocytes to ECs. Our results provide evidence for the role of LFA-1 expression in monocytes and ICAM-1 expression in ECs in the increased monocyte adhesion to ECs.
The monocyte/macrophage is a pivotal cell in atherogenesis and inflammation. The adhesion of monocytes to the endothelium followed by transmigration into the subendothelial layer is a key event in the early pathogenesis of atherosclerosis (23). This is a tightly regulated process in which adhesion molecules and chemokines play crucial roles (13). Although it is a normal process, observation of many inflammatory disease conditions indicates that increased monocyte adhesion results in an overinfiltration of monocytes into the arterial intima. CVD consists of a number of different diseases that involve the heart and blood vessels, most of which have similar causes, mechanisms, and treatments. Atherogenesis is a common denominator among most vascular diseases, and it is well accepted that inflammation drives this process.
The adhesion between monocytes and ECs was increased in response to both acetoacetate and β-hydroxybutyrate. Adhesion, which was assessed by the increase in mean fluorescence, was significantly increased in both monocyte cell lines and isolated human monocytes (Fig. 1). The increase was dose responsive when cells were treated with acetoacetate but not β-hydroxybutyrate. β-Hydroxybutyrate treatment resulted in a persistent increase that was not dose responsive. These observations suggest that β-hydroxybutyrate and acetoacetate may be signaling via different mechanisms to enhance adhesion.
Adhesion molecules such as sICAM-1 or LFA-1 have been found to be elevated in T1D patients (11, 27). Our results indicate that both ICAM-1 in ECs and LFA-1 in monocytes was upregulated in hyperketonemic conditions. Total protein expression of LFA-1 was upregulated in U937 and THP-1 monocytes treated with acetoacetate and β-hydroxybutyrate (Fig. 3A). Surface analysis gave similar results in U937 monocytes treated with acetoacetate but not β-hydroxybutyrate (Fig. 3B). In U937 monocytes, β-hydroxybutyrate treatment caused a slight increase in surface LFA-1 but not to the same degree, as indicated by Western blotting for total protein. The surface expression of LFA-1 in THP-1 monocytes remains consistent with ketone body treatment, which differs from observations seen in total protein analysis. It has never been indicated that LFA-1 contains storage granules; however, this could be a reason for the increase in total protein and not the surface expression.
Acetoacetate and β-hydroxybutyrate have been shown to increase oxidative stress via ROS production (3, 16, 31) and decreased intracellular GSH in red blood cells (19). Acetoacetate has also been shown to increase monocyte secretion of TNF-α, which could be inhibited by specific inhibitors of protein kinase A (H-89), p38-mitogen-activated protein kinase (MAPK; SB-203580), and nuclear transcription factor (NF-κB-SN50) (18). Studies by Abdelmegeed et al. have shown that acetoacetate can activate extracellular signal-regulated kinase (ERK1/2) and p38 MAPK (3), as well as inducing CYP2E1 protein expression in primary cultured rat hepatocytes (2). Overall, this suggests a role for oxidative stress as well as NF-κB and MAPK signaling as potential mechanisms of hyperketonemia-induced chemokine secretion and adhesion molecule regulation in monocytes and ECs.
An increase in integrin surface expression does not necessarily indicate an increase in adhesive properties of the monocytes, since integrins are also regulated by conformational changes. Integrins switch from an inactive conformation to an active conformation upon activation by various stimuli, mostly chemokines or adhesion molecules. We show that both acetoacetate and β-hydroxybutyrate induce LFA-1 in THP-1 monocytes into the high-affinity conformation (Fig. 4). Although the surface expression remained consistent in THP-1 monocytes, the conformation changed to the high-affinity conformation, which may explain the increase in adhesion of β-hydroxybutyrate-treated cells, since neither LFA-1 nor ICAM-1 surface expression was increased with β-hydroxybutyrate treatment. Previous studies in the literature have also reported that, when treated with phosphatidylcholine hydroperoxide, the expression of LFA-1 does not increase in THP-1 monocytes, but the activation state changes, resulting in increased adhesion to ICAM-1 (4). These data suggest that physiological concentrations of both acetoacetate and β-hydroxybutyrate may result in upregulation and activation of adhesion molecules as a way to recruit an increased number of monocytes to the vascular endothelium.
We evaluated the role of ICAM-1 and LFA-1 in mediating acetoacetate- or β-hydroxybutyrate-induced adhesion (Fig. 5). Since ICAM-1 was not increased in ECs treated with β-hydroxybutyrate, we did not do these experiments in β-hydroxybutyrate-treated cells. However, we did block β-hydroxybutyrate-induced adhesion by using an LFA-1 antibody. By blocking ICAM-1 with an antibody specific to the LFA-1 binding site, acetoacetate-induced adhesion was almost completely attenuated. Blocking with LFA-1 antibody also attenuated both acetoacetate- and β-hydroxybutyrate-induced monocyte-EC adhesion. LFA-1 blocked adhesion slightly more than that caused by ICAM-1 alone, suggesting that other ICAM-1 ligands such as MAC-1 may also be increased and may be responsible for a small amount of adhesion. Since both acetoacetate and β-hydroxybutyrate increased monocyte adhesion to ECs and the adhesion can be almost completely blocked by either an ICAM-1 antibody or a LFA-1 antibody, it seems plausible to say that hyperketonemia mediates monocyte-EC cell adhesion primarily via the ICAM-LFA-1 interaction.
Chemokines are known to play a crucial role in directing movement of mononuclear cells from the circulation into inflamed tissue and serving as potent agonists for activation of monocytes and other cells of the immune network (24). They are among the most important proinflammatory intercellular mediators of leukocyte trafficking. The chemokines MCP-1 and IL-8 have been well studied, as has their influence on atherosclerotic lesion formation. Both MCP-1−/− and CCR2−/− mice show reduction of lesion size in the apoE−/− mouse atherosclerosis model (6, 14). Papadopoulou et al. (30) showed that inhibition of MCP-1, IL-8, or GROα using blocking antibodies reduced monocyte adhesion to human atherosclerotic plaques. Therefore, MCP-1 and IL-8 serve as important and sensitive inflammatory biomarkers of inflammation known to directly influence monocyte infiltration and adhesion. Our results show that acetoacetate increases chemoattractant protein secretion in both monocytes and ECs (Figs. 6 and 7). The increase in MCP-1 and IL-8 in HUVECs was dose dependent when the cells were treated with acetoacetate and was significant at concentrations of 4 mM (Fig. 6). This suggests that a greater chemokine gradient along the EC surface creates an environment more liable to cause passing leukocytes to undergo chemotaxis toward the inflamed EC layer. Ultimately, this would lead to a higher level of monocyte recruitment and adhesion. Monocytes were also shown to contribute to the overall increase in chemokine secretion from both U937 and THP-1 cell lines. Treatment with acetoacetate resulted in a near dose-responsive increase in chemokine secretion (Fig. 7). One result that may be cell type specific is the dose-response increase seen in MCP-1 secretion from activated U937 monocytes. There was a dose-response increase in MCP-1 secretion when U937 monocytes were treated with 0–10 mM β-hydroxybutyrate, with significant results seen at 10 mM; however, no increase in IL-8 secretion was observed. These results suggest that hyperketonemia may serve as a stimulus for monocyte activation potentiating the chemokine signaling and overall increase in serum concentrations of chemokines in diabetics.
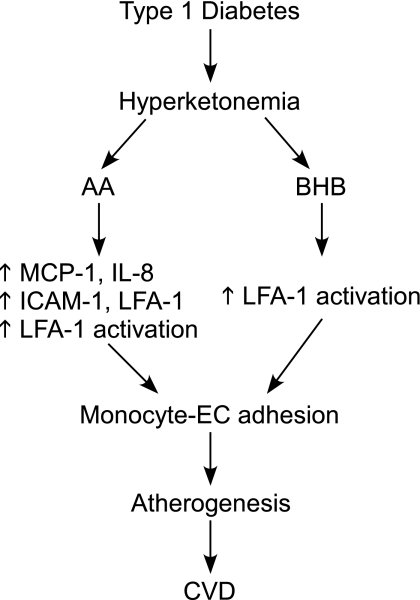
Inflammation is now established as a driving force for the development of atherosclerosis- and diabetes-associated complications. This study for the first time demonstrates the role and the underlying mechanisms by which hyperketonemia increases monocyte adhesion to the vascular endothelium. Figure 8 provides a working model for the mechanisms by which hyperketonemia may contribute to increased CVD in T1D. Overall, these results suggest that hyperketonemia can create a low level of inflammation in the EC layer and circulating monocytes. This condition provides not only a chemokine gradient amenable to monocyte recruitment but also EC and monocyte surfaces primed for cell adhesion. Thus, hyperketonemia contributes to the inflammatory conditions that potentiate the adhesion of monocytes to ECs and the risk for CVD in T1D.
Fig. 8.
Proposed schema of hyperketonemia leading to accelerated cardiovascular disease (CVD) in type 1 diabetes.
GRANTS
This work was supported by grants from National Institute of Diabetes and Digestive and Kidney Diseases and the Office of Dietary Supplements of the National Institutes of Health (RO1 DK-072433) and the Malcolm Feist Endowed Chair in Diabetes.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Georgia Morgan for excellent editing of the manuscript.
REFERENCES
- 1. American Diabetes Association Type 2 diabetes in children and adolescents. Diabetes Care 23: 381–389, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Abdelmegeed MA, Carruthers NJ, Woodcroft KJ, Kim SK, Novak RF. Acetoacetate induces CYP2E1 protein and suppresses CYP2E1 mRNA in primary cultured rat hepatocytes. J Pharmacol Exp Ther 315: 203–213, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Abdelmegeed MA, Kim SK, Woodcroft KJ, Novak RF. Acetoacetate activation of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase in primary cultured rat hepatocytes: role of oxidative stress. J Pharmacol Exp Ther 310: 728–736, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Asai A, Okajima F, Nakagawa K, Ibusuki D, Tanimura K, Nakajima Y, Nagao M, Sudo M, Harada T, Miyazawa T, Oikawa S. Phosphatidylcholine hydroperoxide-induced THP-1 cell adhesion to intracellular adhesion molecule-1. J Lipid Res 50: 957–965, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bainton DFML, Kishimoto TK, Springer TA. Leukocyte adhesion receptors are stored in peroxidase-negative granules of human neutrophils. J Exp Med 166: 1641–1653, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394: 894–897, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Candiloros HMS, Zeghari N, Donner M, Drouin P, Ziegler O. Decreased erythrocyte membrane fluidity in poorly controlled IDDM. Influence of ketone bodies. Diabetes Care 18: 549–551, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Devaraj S, Cheung AT, Jialal I, Griffen SC, Nguyen D, Glaser N, Aoki T. Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes 56: 2790–2796, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes 55: 774–779, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Fasching P, Veitl M, Rohac M, Streli C, Schneider B, Waldhausl W, Wagner OF. Elevated concentrations of circulating adhesion molecules and their association with microvascular complications in insulin-dependent diabetes mellitus. J Clin Endocrinol Metab 81: 4313–4317, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Fogelstrand L, Hulthe J, Hultén LM, Wiklund O, Fagerberg B. Monocytic expression of CD14 and CD18, circulating adhesion molecules and inflammatory markers in women with diabetes mellitus and impaired glucose tolerance. Diabetologia 47: 1948–1952, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Fuller JH, Stevens LK, Wang SL. Risk factors for cardiovascular mortality and morbidity: the WHO multinational study of vascular disease in diabetes. Diabetologia 44: S54–S64, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol 27: 2292–2301, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell 2: 275–281, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Hoffman WH, Cheng C, Passmore GG, Carroll JE, Hess D. Acetoacetate increases expression of intercellular adhesion molecule-1 (ICAM-1) in human brain microvascular endothelial cells. Neurosci Lett 334: 71–74, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Jain SK, Kannan K, Lim G. Ketosis (acetoacetate) can generate oxygen radicals and cause increased lipid peroxidation and growth inhibition in human endothelial cells. Free Radic Biol Med 25: 1083–1088, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Jain SK, Kannan K, Lim G, Matthews-Greer J, McVie R, Bocchini JA., Jr Elevated blood interleukin-6 levels in hyperketonemic type 1 diabetic patients and secretion by acetoacetate-treated cultured U937 monocytes. Diabetes Care 26: 2139–2143, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Jain SK, Kannan K, Lim G, McVie R, Bocchini JA., Jr Hyperketonemia increases tumor necrosis factor-(alpha) secretion in cultured U937 monocytes and type 1 diabetic patients and is apparently mediated by oxidative stress and cAMP deficiency. Diabetes 51: 2287–2293, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Jain SK, McVie R. Hyperketonemia can increase lipid peroxidation and lower glutathione levels in human erythrocytes in vitro and in type 1 diabetic patients. Diabetes 48: 1850–1855, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Jain SK, McVie R, Bocchini JA., Jr Hyperketonemia (ketosis), oxidative stress and type 1 diabetes. Pathophysiology 13: 163–170, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Jain SK, Rains JL, Croad JL. High glucose and ketosis (acetoacetate) increases, and chromium niacinate decreases, IL-6, IL-8, and MCP-1 secretion and oxidative stress in U937 monocytes. Antioxidants Redox Signaling 9: 1581–1590, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Libby P. Inflammation in atherosclerosis. Nature 420: 868–874, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Liehn EA, Zernecke A, Postea O, Weber C. Chemokines: inflammatory mediators of atherosclerosis. Arch Physiol Biochem 112: 229–238, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Lori L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev 15: 412–426, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Miller LJ, Bainton DF, Borregaard N, Springer TA. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest 80: 535–544, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mysliwiec J, Kretowski A, Kinalski M, Kinalska I. CD11a expression and soluble ICAM-1 levels in peripheral blood in high-risk and overt type 1 diabetes subjects. Immunol Lett 70: 69–72, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Newton CA, Raskin P. Diabetic ketoacidosis in type 1 and type 2 diabetes mellitus: clinical and biochemical differences. Arch Intern Med 164: 1925–1931, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications. Diabetes 55: 1463–1469, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Papadopoulou C, Corrigall V, Taylor PR, Poston RN. The role of the chemokines MCP-1, GRO-[alpha], IL-8 and their receptors in the adhesion of monocytic cells to human atherosclerotic plaques. Cytokine 43: 181–186, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelletier A, Coderre L. Ketone bodies alter dinitrophenol-induced glucose uptake through AMPK inhibition and oxidative stress generation in adult cardiomyocytes. Am J Physiol Endocrinol Metab 292: E1325–E1332, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Roe TF, Crawford TO, Huff KR, Costin G, Kaufman FR, Nelson JMD. Brain infarction in children with diabetic ketoacidosis. J Diabet Complic 10: 100–108, [DOI] [PubMed] [Google Scholar]
- 33.Simon SI, Green CE. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng 7: 151–152, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. High risk of cardiovascular disease in patients with type 1 diabetes in the U.K. Diabetes Care 29: 798–804, 2006 [DOI] [PubMed] [Google Scholar]
- 34a.Stephens JM, Sulway MJ, Watkins PJ. Relationship of blood acetoacetate and 3-hydroxybutyrate in diabetes. Diabetes 20: 485–489, 1971 [DOI] [PubMed] [Google Scholar]
- 35.Umpierrez G, Kitabchi A. Diabetic ketoacidosis: risk factors and management strategies. Treatments Endocrinol 2: 95–108, 2003 [DOI] [PubMed] [Google Scholar]
- 36.White NH. Diabetic ketoacidosis in children. Endocrinol Metab Clin North Am 29: 657–682, 2000 [DOI] [PubMed] [Google Scholar]
- 37.White NH. Management of diabetic ketoacidosis. Rev Endocr Metab Dis 4: 343–353, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Yared Z, Chiasson J. Ketoacidosis and the hyperosmolar hyperglycemic state in adult diabetic patients. Diagnosis and treatment. Minervia Medica 94: 409–418, 2003 [PubMed] [Google Scholar]