Abstract
The fetus requires significant energy for growth and development. Although glucose is a major source of energy for the fetus, other maternal nutrients also appear to promote growth. Thus, the goal of these studies was to determine whether triglyceride-rich remnants are taken up by the placenta and whether maternal dietary lipids, independently of adiposity, can impact fetal growth. To accomplish our first goal, chylomicron particles were duallly labeled with cholesteryl ester and triglycerides. The placenta took up remnant particles/core lipids at rates greater than adipose tissue and skeletal muscle but less than the liver. Although the placenta expresses apoE receptors, uptake of chylomicron remnants and/or core lipids can occur independently of apoE. To determine the impact of dietary lipid on fetal growth, independent of maternal adiposity, females were fed high-fat diets (HFD) for 1 mo; there was no change in adiposity or leptin levels prior to or during pregnancy of dams fed HFD. Fetal masses were greater in dams fed HFD, and mRNA levels of proteins involved in fatty acid oxidation (CPT I, PPARα), but not glucose oxidation (pyruvate kinase) or other regulatory processes (HNF-4α, LXR), were increased with maternal dietary fat. There was also no change in mRNA levels of proteins involved in placental glucose and fatty acid transport, and GLUT1 protein levels in microvillous membranes were similar in placentas of dams fed either diet. Thus, the ability of the placenta to take up chylomicron remnant core lipids likely contributes to accelerated fetal growth in females fed high fat diets.
Keywords: fetus, triglyceride, apolipoprotein E receptors, lymph duct cannulation, Smith-Lemli-Opitz syndrome
the fetus has a significant requirement for energy to support basic metabolism and the extensive cellular proliferation and differentiation that occur throughout gestation. The human fetus increases >500-fold from ≈6 to ≈3,500 g. Until recently, glucose was thought to be the primary source of energy for the fetus. This was based on several lines of evidence (reviewed in Ref. 51). First, trophoblasts express abundant glucose transporters that facilitate glucose transport from the maternal to fetal circulation. Second, high levels of glucose (as could occur with numerous glucose transporters) lead to elevated malonyl-CoA levels, which suppress carnitine palmitoyltransferase I (CPT I), the protein responsible for transporting fatty acids into the mitochondria where they are oxidized. In fact, fetal liver is more sensitive to malonyl-CoA compared with newborn liver. Third, it was thought that fatty acid synthesis did not even occur in the fetus, since mRNA levels of key synthetic proteins were low during gestation and increased postpartum. Finally, fetal growth was known to be driven by maternal glucose levels (53).
In more recent years, it has become apparent that fatty acids are an additional source of energy for the fetus and could play a role in fetal growth. Energy is generated from fatty acids by way of β-oxidation after fatty acyl-CoA units are transported across the mitochondrial membrane via CPT I. In humans, a lack of fetal fatty acid oxidation, as a result of mitrochondrial trifunctional protein (MTP) deficiency or long-chain acyl-CoA dehydrogenase (LCACD) deficiency, can lead to IUGR (intrauterine growth restriction), preterm labor, or intrauterine death (37, 79); MTP is a protein with fatty acid CoA dehydrogenase activity. Likewise, in rodents, either fetuses are smaller or a greater proportion die in utero in MTP knockouts, LCACD knockouts, or LCACD/VLACD double knockouts (15, 31). The role of fatty acids as a source of energy is possibly most apparent in that there is a direct correlation between maternal triglyceride levels and fetal growth in diabetic women with good glycemic control (59). It should be noted that fatty acids also have a structural role and are needed as substrates for membranes in the rapidly growing tissues.
Fetal fatty acids are either taken up from the maternal circulation or synthesized de novo. Energy-ready fatty acids are found in the maternal circulation in two different forms. Fatty acids can be found in their free form, bound to albumin, or as triglycerides. Uptake of free fatty acids by the placenta has been studied more extensively than the uptake of triglycerides, and it is well accepted that free fatty acids are indeed taken up by the placenta either through diffusional mechanisms or by various fatty acid transport proteins expressed by the placenta, including FATP1–4, FATP6, CD36, and FABPpm (reviewed in Refs. 16 and 23). Once taken up, the free fatty acids are either transported transcellulary to the basolateral membrane via an undefined mechanism or utilized by the placenta itself for energy or as membrane substrates. Maternally derived fatty acids are likely secreted into the fetal circulation as fatty acids (14, 27), possibly with the aid of FATPs or CD36 (24). Since the concentration of free fatty acids in the maternal circulation increases during gestation (10, 29), they could have an important role in fetal development.
As with free fatty acids, maternal plasma triglyceride concentrations increase during gestation (21, 46, 73). Triglycerides are carried through the circulation as lipoprotein particles originating in intestine (chylomicrons, CM) and liver (VLDL). Lipoprotein lipase (LPL) and endothelial lipase hydrolyze triglycerides within the lipid-carrying particles to free fatty acids and remnant particles [CM remnants (CMR), intermediate-density lipoproteins (IDL)]. The lipase-generated free fatty acids can be taken up by the placenta as just described for the free fatty acids. The lipase-derived remnant particles (CMR and IDL), as well as VLDL, can be taken up as whole particles via apolipoprotein E (apoE) receptors, since CMR, IDL, and VLDL all contain apoE. The placenta expresses a number of apoE receptors (reviewed in Ref. 76). Homozygous fetuses are viable and fertile when LDLR, VLDLR, and apoER2 are deleted (18, 33, 70), although the relative fetal growth rates are unknown. Fetuses lacking LRP1 or megalin/LRP2 die in utero from the lack of protein expression in the fetus proper (30, 66, 74), although a contributing effect of receptors on nutrient transport and fetal growth has not been ruled out. Remnant particles and/or remnant core lipids that have been shed also can be taken up independently of apoE, as recently demonstrated in the heart (5), possibly by endocytosis/phagocytosis, which is relatively elevated in the placenta (4, 64, 77).
Fatty acids can also be synthesized de novo by the fetus. While oxidation of newly synthesized fatty acids would not generate as much net energy as oxidation of exogenous fatty acids since fatty acid synthesis requires energy (NADPH), the fatty acids synthesized could be used as a source of structural substrates, sparing exogenous fatty acids for oxidation. Fatty acid synthesis also is essential for fetal development and fatty acid synthase (FAS), the multifunctional enzyme that catalyzes the synthesis of saturated fatty acids, is enhanced in highly proliferating tissues (38). The inability to synthesize fatty acids from a lack of FAS or acyl-CoA carboxylase-1 (ACC1) is lethal in the murine model (1, 13).
Understanding what drives growth rates is important for the healthy fetus. Besides the obvious acute importance of fetal growth for fetal well-being, long-term consequences exist, and infants with abnormal fetal growth have an increased risk of developing age-related diseases, including diabetes, atherosclerosis, and obesity (11, 57, 65). With improved understanding of what affects fetal growth, dietary or lifestyle changes can be made by the mother to optimize growth if indicated. Women have been very willing to make necessary changes for the benefit of their babies, as seen by a voluntary decrease in cigarette smoking and drinking during gestation once the adverse effects of these lifestyles to the infant were demonstrated, and by a willingness to modify lifestyle in obese and/or diabetic women (47, 49, 55). Thus, the objective of these studies was to examine whether diet-derived triglycerides (CM) can be taken up by the placenta and whether dietary fat can affect fetal growth. Using dually labeled CM (triglyceride/cholesteryl ester-labeled CM), we found that the lipid core of remnants and/or the remnants themselves are indeed taken up by the placenta at rates greater than those of other peripheral tissues, and more lipid is transported to the fetus when more lipid is given. In addition, fetuses of dams fed a high-fat diet for a short period of time, prior to the occurrence of diet-induced elevated maternal adiposity or leptin levels, had greater masses. Finally, when dams fed a high-fat diet for 3 wk were switched to a low-fat diet at conception, fetuses were smaller. Thus, diet alone can have an impact on fetal growth, likely as a result of the ability of the placenta to take up core lipids of CMR, including triglycerides, and to transport fatty acids from hydrolyzed triglycerides to the fetus.
MATERIALS AND METHODS
Animals and diets.
All male and nonpregnant female C57BL/6 mice were purchased from The Jackson Laboratories (Bar Harbor, ME) and fed a pelleted chow (Harlan, Madison, WI). Animals were housed for ≈1 wk in a temperature- and humidity-controlled room and subjected to 12 h of light and 12 h of darkness prior to dietary changes. Females were 10 wk old when they arrived. A majority of the females were fed a breeder chow that contained 9% fat (wt/wt; PMI Nutrition International, Brentwood, MO) for 1–2 wk prior to mating. For breeding, one male was placed in a cage with three females. Presence of a postcopulatory plug was checked when lights came on and denoted 0.5 day postconception (dpc). When a plug was detected, female mice were separated into their own cage. After 5 days of mating, males were separated from remaining nonpregnant females. After 2 days, the process was repeated; females underwent mating until a plug was detected or until three mating cycles were completed.
For a second series of studies, females were fed high-fat or low-fat diets. Initially, females were fed chow (Harlan; 5.8% fat, wt/wt) or a high-fat semipurified diet for 4 wk and then mated; the high-fat diet consisted of 15.6% fat (wt/wt, D12266B; Research Diets, New Brunswick, NJ). In a second set of similar dietary studies, females were fed semipurified diets containing either 4.6% fat (wt/wt, D12489B) or 15.6% fat (wt/wt, D12266B). Food consumption was measured daily for 3 days just prior to mating for each cage of three females. Mating was performed after 3.5 wk on dietary treatment as described in the paragraph above. Animals that had not mated after two cycles were not used for the studies.
In a third series of studies, apoE−/− males and females were mated; mice were a generous gift of Dr. David Hui. Females (10–12 wk old) were fed breeder chow for 3 wk prior to mating. Animals pregnant during the first, and only, week of mating were studied. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati.
CM clearance.
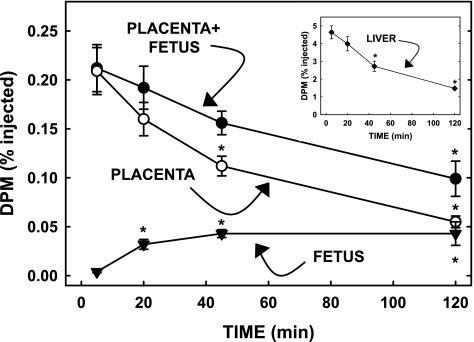
To determine whether diet-derived triglyceride can be taken up by the placenta and ultimately transported to the fetus, CM were dually radiolabeled in vivo with triolein and cholesteryl ester, and CM were collected via a lymph fistula. Since a degradable marker was used (triolein) to radiolabel the core triglyceride, an initial study was performed to determine how quickly exogenously derived triglycerides were oxidized by tissues. Lyposyn II, an intravenous lipid emulsion (Hospira, Lake Forest, IL), was radiolabeled with [3H]triolein (2). Briefly, Lyposyn II was vigorously mixed with [3H]triolein for 3–4 h to obtain a homogenous mixture and used immediately. Pregnant mice (18.5 dpc) were fasted (6 h) and injected intravenously with 5 μCi of 3H. At 5, 20, 45, and 120 min postinjection, animals were euthanized and tissues rapidly collected. The first two fetal units (fetus, placenta, yolk sac) from the right uterine horn and the maternal liver were collected rapidly at different times after injection, separated, washed thoroughly, and saponified. Fatty acids were extracted from tissues, and radiolabel was measured. Results from the fetuses, placentas, and yolk sacs within one dam were averaged, and each tissue was used as an n of 1. Yolk sacs took up negligible radiolabel and were not included in fetal unit results. Results from each of the time points were averaged and plotted for fetuses, placentas, and whole fetal units (fetuses plus placentas). A decrease in dpm in the whole fetal unit would be representative of fatty acid loss due to oxidation (or the unlikely occurrence of secretion back into the maternal circulation). As seen in Fig. 1, fatty acid dpm were greatest in fetal units and maternal liver at 5 min postinjection. There were no significant decreases at 20 min. By 45 min postinjection, dpm were markedly reduced in the fetal unit and the placenta. Dpm decreased faster in the placenta than the in the fetal unit due to transport to the fetus and fatty acid oxidation. Fetal dpm increased most between 5 and 20 min, as lipid taken up by the placenta was transported to the fetus. All remaining studies were performed at 15 min postinjection to allow for significant uptake by the placenta and prior to fatty acid oxidation.
Fig. 1.
Uptake of [3H]triolein by the fetal unit and maternal liver (inset) at different times after injection. Pregnant dams [18.5 days postconception (dpc)] were injected iv with Lyposyn II that had been radiolabeled with [3H]triolein. After 5, 20, 45, and 120 min, fetuses, placentas, and maternal livers were rapidly collected. Tissues were saponified, fatty acids were extracted, and amount of radiolabel was measured. Data are presented as means ± SE (n = 3 litters). Inset: percent radiolabel taken up by maternal liver. *Differences (P < 0.05) from 5-min time point.
CM clearance in placentas and other maternal tissues of mice was studied during early and late postconception. Two days prior to the planned study, donor animals were fitted with a mesenteric lymph duct cannula and a duodenal intubation via the stomach and allowed to recover overnight. In these studies, male Sprague-Dawley rats were used as the donor animals. Donor animals were given a duodenal bolus of [3H]triolein and [14C]cholesterol in Lyposyn II (20% lipid) as done previously (25, 69). Lymph was collected for 6 h on ice and pooled. Lymph was centrifuged at 4°C (25,000 rpm, 60 min, Ti50.3), and the top milky layer containing CM was collected and used within the next 2 days. Pregnant mice at 12.5 and 18.5 dpc were fasted for 6 h and injected intravenously with radiolabeled CM, which were converted to CMR in the circulation, as particles are depleted of lipid via lipase activity. Blood was collected from the tail at 5 min postinjection. At 15 min postinjection, placentas and fetuses and adult liver, skeletal muscle, and inguinal adipose tissue were collected. All placentas and fetuses at 12.5 dpm from each litter were pooled, and fetuses from each litter late in gestation were analyzed separately due to their mass but averaged so that tissues of each litter had an n of 1. The amounts of 3H and 14C in saponified tissues were measured and presented as a percentage of that injected per tissue and per gram of tissue. Uptake of CMR or core lipids was indicated by the presence of 14C. Uptake of fatty acids, as either CMR, core lipids, or lipase-derived fatty acids, was indicated by the presence of 3H.
CM clearance was also determined in mice lacking apoE. Donor radiolabeled CM were collected from male mice lacking apoE as described for the donor rats except that 5 μCi of 3H and 2.5 μCi of 14C were given to donor mice, and CM were collected for 8 h. The CM from donor mice were pooled, isolated by ultracentrifugation, and used the next day. The study was similar to that just described, except that apoE−/− pregnant females were 15.5–18.5 dpc; we did not separate results by gestational age, since results were similar at each age as seen in the previous study.
The uptake and transport of different amounts of exogenous triglyceride were also determined in the placenta and fetus. At 18.5 dpc, female mice were fasted 5–6 h and injected intravenously with Lyposyn II radiolabeled with [3H]triolein as described (2). Animals were given 51.9 ± 6.8 vs. 169.2 ± 13.5 μl of Lyposyn II (11.0 ± 1.5 or 35.6 ± 2.8 μg triglyceride). Fifteen minutes after injection, fetuses and placentas and maternal liver and adipose tissue were collected; the first two fetuses and placentas (fetal units) in the right uterine horn were collected. The amount of radiolabeled 3H in the placentas and fetuses as a percentage of the amount injected was calculated.
Real-time PCR.
Fetal livers or placentas were collected rapidly at 13.5 and 18.5 dpc after exsanguination, snap-frozen in liquid nitrogen, and stored at −80°C until use. Tissue RNA was isolated using TRIzol and stored in FORMAzol at −80°C. RNA was treated with RNase-free DNase I and reverse transcribed to cDNA by SuperScript II reverse transcriptase using random hexamers. PCR assays were performed on a Bio-Rad iCycler iQ Real-Time PCR Detection System using SYBR Green as our fluorophore; primers will be given upon request. A serial dilution of a randomly picked sample was used to generate a standard curve for each gene examined. This standard curve was used to calculate the relative levels of mRNA for the gene of interest and the reference/housekeeping gene (cyclophilin).
Microvillous membrane preparation and western blotting.
Microvillous membranes (MVM) were isolated from placentas of dams at 18.5 dpc fed high-fat and low-fat diets as described for mice using MgCl2 precipitation (34), a modification of human MVM preparations (20, 32). The proteins of the membranes were then separated by gel electrophoresis using a prepoured gradient gel, and the relative amount of GLUT1 (Millipore, Billerica, MA) in microvillous membranes was determined by Western blotting using ECL Plus as described (81); similar gel loading was verfied after stripping membranes and reprobing with an antibody to β-actin (34).
Maternal leptin levels and adiposity.
Mice fed diets with different levels of triglyceride for 3.5 to 4 wk were weighed, and body fat mass was measured by MRI (Echo MRI; Echo Medical Systems, Houston, TX). A sample of fasted blood (6-h fast) was collected as well. Mice were mated, and blood and tissues collected at 18.5 dpc in the fed state; retroperitoneal and inguinal fat pads were weighed. Leptin levels in maternal blood collected pre-pregnancy and during gestation were measured by LINCOplex by the Mouse Metabolic Phenotype Center of the University of Cincinnati.
Fetal plasma metabolites and percent fetal fat.
Fetuses (18.5 dpc) from dams fed high-fat corn oil (HFCO) diets or chow were weighed and decapitated. Blood was collected from all fetuses and pooled and plasma was collected. One body from each litter was weighed and cut into ≈5-mm pieces. Fetal fat was extracted in Folch, with moderate heat and extract poured into preweighed glass tubes. Percent fat was determined gravimetrically. Insulin levels were measured by RIA and glucose levels enzymatically by the Mouse Metabolic Phenotype Center of the University of Cincinnati.
De novo fatty acid synthesis.
Pregnant females (18.5 dpc) were injected intraperitoneally with 25 mCi of [3H]H2O under light anesthesia (62). After 1 h, animals were anesthetized and exsanguinated. Fetal livers were removed from the bodies. Two fetal livers were saponified in alcoholic KOH. Fatty acids were extracted, and extracts were assayed for 3H content. The rates of synthesis are presented as nanomoles of [3H]H2O incorporated into fatty acids per hour per gram of tissue.
Statistics.
Data are presented as means ± SE. Differences between two groups [early vs. late gestation and chow/low-fat corn oil (LFCO) vs. HFCO] were determined by two-tailed Student's t-tests (P < 0.05). To determine differences in uptake between tissues of pregnant dams, values for each tissue at both gestational ages were averaged (no difference between 12.5 and 18.5 dpc existed), and differences between peripheral tissues were determined by one-way ANOVA (P < 0.05). If a treatment effect occurred, differences between groups were evaluated by the Student-Newman-Keuls method (P < 0.05). One-way ANOVAs were also used to determine differences in uptake at different times postinjection of radiolabeled [3H]triolein.
RESULTS
It is well accepted that the placenta will take up fatty acids from the maternal circulation. However, the ability to take up triglyceride-containing particles, such as VLDL, IDL, or CMR is not defined. Thus, CMs were radiolabeled in vivo with core lipids that could be hydrolyzed by lipases to fatty acids (triglyceride) and/or retained within the CM core (cholesteryl ester and triglyceride); the radiolabels were esterified to triglyceride and cholesteryl ester in enterocytes (84). Since triglycerides and not the nondegradable fatty acid analog BMIPP were incorporated into the CM core in vivo, a preliminary time course was needed to establish the best time postinjection to perform the studies prior to oxidation. As shown in Fig. 1 and described in materials and methods, samples needed to be collected by 20 min postinjection to measure triglyceride uptake, and so uptake was measured at 15 min in remaining studies. In the inset of Fig. 1, we found that the decrease in dpm in the fetal unit paralleled that in the liver, another tissue with high fatty acid oxidation rates.
Although the placenta is known to take up lipase-derived fatty acids, it is unknown whether the resultant remnant particle also was taken up. To study uptake of the remnant particle, we dually labeled the CM with [14C]cholesteryl ester as well as [3H]triglyceride. The presence of 14C would indicate uptake of the CMR-cholesteryl ester plus shed core lipids, whereas the presence of 3H would be indicative of uptake of CMR-triglyceride plus lipase-derived fatty acids plus shed core lipids. As in other studies, clearance of 3H was greater than that of 14C: there was 27.9 ± 3.4 and 51.8 ± 3.8% dpm in plasma of 3H and 14C at 5 min, respectively, and 22.1 ± 3.1 and 33.9 ± 3.0% dpm at 15 min.
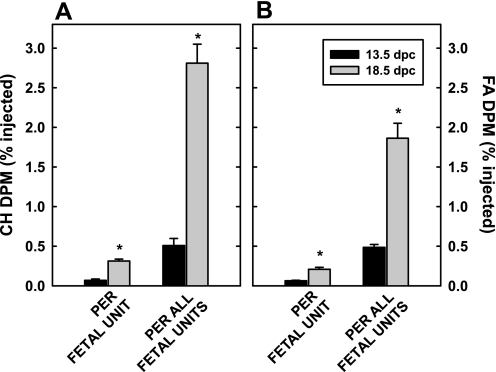
When uptake was presented per fetal unit (fetus + placenta), more [14C]cholesteryl ester (Fig. 2A) as well as [3H]fatty acid and [3H]triglyceride (Fig. 2B) were taken up later in gestation (P < 0.001). It should be noted that there was no detectable [14C]cholesterol in the fetus during this short study, as expected from previous studies from this laboratory (9, 75), whereas there was 3H as expected from Fig. 1. The differences in uptake were maintained when uptake was determined for all fetal units (Fig. 2, A and B; P < 0.001). Interestingly, lipid uptake late in gestation comprised a significant portion of uptake by the whole body. To compare uptake with other tissues, data were converted to uptake per gram of placenta, since the placenta was responsible for the uptake of lipids. There was no statistical change in percent dose of cholesterol taken up per gram of tissue by the placenta between early and late gestation; 1.47 ± 0.26 vs. 1.92 ± 0.28% injected cholesterol was taken up per gram of placenta at 12.5 and 18.5 dpc, respectively. There was little effect of gestational age in the maternal tissues as well, and results from each gestational age were averaged. As expected, each gram of liver took up much more CMR than other tissues (Fig. 3A). Compared with other peripheral tissues, the placenta took up more [14C]cholesteryl ester per gram of tissue than skeletal muscle (P < 0.001) and inguinal adipose tissue (P = 0.003). As seen in Fig. 3B, the liver also took up more [3H]fatty acids plus [3H]triglyceride than other tissues, although the amount was less than that taken up as [14C]cholesteryl ester. As with CMR, the placenta took up more [3H]fatty acids plus [3H]triglyceride compared with other peripheral tissues (P < 0.005). Ratios of uptake of 14C:3H were 2.80 ± 0.27, 0.53 ± 0.06, 1.05 ± 0.10, and 1.25 ± 0.12 for the liver, muscle, adipose, and placenta, respectively.
Fig. 2.
Uptake of chylomicron (CM)-cholesteryl ester and -fatty acid (FA) plus triglyceride by fetal units (placenta + fetus). CM labeled with [14C]cholesteryl ester (A) and [3H]triolein (B) were injected iv into pregnant dams (12.5 and 18.5 dpc). Twenty minutes after injection, tissues were collected and saponified, lipids were extracted, and amount of radiolabel was measured. Data are presented as means ± SE (n = 4 litters). *Differences (P < 0.05) between amount of 14C or 3H in fetal units at different gestational ages.
Fig. 3.
Uptake of CM-cholesteryl ester and -FA plus triglyceride per gram of fetal unit (PLA) and maternal liver, skeletal muscle (SM), and inguinal adipose tissue (AT). Pregnant dams (12.5 and 18.5 dpc) were injected with CM dually labeled with [14C]cholesteryl ester (A) and [3H]triolein (B); pregnant dams are the same as those described in Fig. 2. Data are presented as means ± SE (n = 4 litters). Data from each tissue in early and late gestation were averaged, as values were similar at both gestational ages (see text). Different letters depict differences (P < 0.05) in uptake from the fetal unit in peripheral tissues.
To begin to define the mechanism responsible for uptake of core lipids of CMR, we 1) determined whether apoE receptors were expressed in the placenta and 2) determined whether core lipids could be taken up independently of apoE. LPL and all the apoE receptors, including the LDLR, LRP, apoER2, VLDLR, LR11, and LRP2, were expressed by the placenta (Fig. 4). There was a marked increase in LRP, apoER2, and VLDLR mRNA levels with gestational age. We then studied the uptake of particles when apoE was absent from lipoproteins and animals. Particles were cleared more slowly in apoE−/− mice (50.3 ± 4.9 and 58.0 ± 4.5% dpm in plasma of 3H and 14C at 5 min, respectively, and 35.8 ± 3.1 and 52.3 ± 2.4% dpm at 15 min) compared with apoE+/+ mice. Uptake was 2.51 ± 0.18% of injected 14C dpm per gram in the placenta of apoE−/− females (n = 5) compared with 1.69 ± 0.19% in the placenta of apoE+/+ females (Fig. 3). This was in contrast to the liver, which took up only 5.11 ± 0.36% injected 14C dpm per gram of tissue when apoE was absent from particles compared with 19.6 ± 32.10% in the liver of control mice; ratios of 14C:3H were 0.88 ± 0.06 and 1.20 ± 0.05 for the liver and placenta, respectively.
Fig. 4.
Relative mRNA levels of proteins associated with lipid uptake in placentas of dams at 13.5 and 18.5 dpc. mRNA levels of placental genes of interest compared with cyclophilin are presented. Data are presented as means ± SE (n = 3–5 litters). *Significant differences (P < 0.05) between mRNA levels at different stages of gestation.
Since previous studies have shown that dietary fat can lead to an increase in fetal growth and/or that fetal growth is correlated to plasma lipid levels (34, 36, 59), we next determined whether the placenta would transport more exogenous lipid to the fetus if there was acutely more lipid in the maternal circulation. Animals were injected with ≈11 or ≈36 μg of triglyceride (20% of Lyposyn II) radiolabeled with [3H]triolein. As seen in Fig. 5A, the fetal unit took up a similar percentage of dpm regardless of the dose given. Although the percentage of dose transported to the fetus was less when more lipid was given, there was still more lipid presented to the fetus of dams given threefold more lipid. It is unlikely that the effect was due solely to changes in fatty acid oxidation since the livers, a tissue with high fatty acid oxidation rates, of dams given threefold more lipid also contained threefold more lipid after 15 min (183.4 ± 30.8 vs. 439.8 ± 101.8 μl lipid).
Fig. 5.
Uptake of different amounts of triglyceride by the placenta and transport to the fetus. Lyposyn II was radiolabeled with [3H]triolein and injected iv into pregnant dams (18.5 dpc). Triglyceride mass injected was ≈10 or ≈30 μg. Twenty minutes postinjection, fetuses and placentas were collected rapidly and saponified, lipids were extracted, and amount of radiolabel was measured. Data are presented as means ± SE (n = 5 litters) of percent injected (A) and μg lipid taken up (B). *Differences (P < 0.05) between tissues of dams injected with different triglyceride mass.
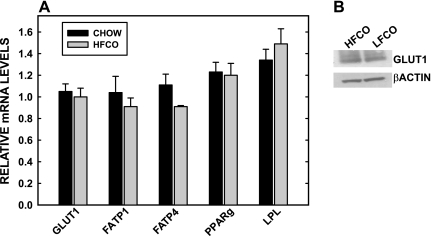
We then determined the effect of chronic hyperlipidemia on fetal metabolism and growth. Initially, basic rodent chow with 5% fat and a semipurified diet with 15% fat were fed to mice for 4 wk. With this short time period of feeding, there was no change in maternal adiposity or leptin levels at the time of mating (Table 1). At 18.5 dpc, there was an ≈20% increase (P = 0.02) in fetal masses in dams fed the HFCO diet vs. chow. The increase was not due to an increase in fetal fat, in that fat mass was the same in fetuses of dams fed either diet (3.14 ± 0.17 vs. 2.97 ± 0.29%). There was a marked change in fat metabolism in the fetus (Fig. 6), however, as seen by a 50% increase in CPT I mRNA levels, the rate-limiting step for fatty acid oxidation, but no change in pyruvate kinase, an enzyme within glycolysis that is regulated by glucose. PPARα mRNA levels were also increased (28%) in fetuses of dams fed the high-fat diet. Other regulatory genes were not affected, however, including hepatocyte nuclear factor (HNF)-4α and ABCG1 [ABCG1 is a downstream target of activated liver X receptor (LXR)]. TNF-1α, an inflammatory marker, was not affected by dietary lipid in the fetal liver either. mRNA levels of genes involved in glucose and fatty acid uptake by the placenta were measured, and there was no change in placental mRNA levels of GLUT1, GLUT4, FATP1 and -4, LRP, PPARγ, and LPL (Fig. 7A). Since mRNA and total protein levels of GLUT1 may not change but the amount of GLUT1 on the MVM could be different in animals fed the high-fat diet (34), we also measured the amount of GLUT1 protein on MVM of dams fed HFCO or LFCO diets. GLUT1 protein expression in the MVM were similar when high-fat diets were fed compared with low-fat diets prior to any changes in adiposity (Fig. 7B).
Table 1.
Adiposity of female mice fed chow or HFCO diet for 4 wk
| DIET |
||
|---|---|---|
| Parameters | Chow | HFCO |
| Weight, g | 19.6 ± 0.5 | 20.6 ± 0.6 |
| Fat mass, g | 7.1 ± 1.8 | 9.1 ± 1.8 |
| Leptin, ng/ml | 0.106 ± 0.031 | 0.094 ± 0.022 |
Values are means ± SE; n = 5–6 litters. Weight, fat mass (measured by MRI), and leptin levels (measured by LINCOplex) in female mice fed chow or high-fat corn oil (HFCO) diet for 4 wk.
Fig. 6.
Fetal masses (A) and relative mRNA levels of proteins associated with lipid metabolism in fetal livers (B) of dams fed chow or a high-fat corn oil (HFCO) diet. Dams were fed diets for 4 wk, mated, and studied at 18.5 dpc. Relative amounts of fetal liver mRNA of genes involved in lipid oxidation and regulation compared with cyclophilin are presented. Data are presented as means ± SE (n = 3–5 litters). *Significant differences (P < 0.05) between fetal livers of dams fed different amounts of dietary fat.
Fig. 7.
Relative mRNA levels of proteins associated with nutrient uptake in placentas of dams fed high- or low-fat diets. Dams are the same as those described in Fig. 6. A: relative amounts of mRNA of genes of interest compared with cyclophilin are presented. Data are presented as means ± SE (n = 3–5 litters). *Significant differences (P < 0.05) between placentas of dams fed different amounts of dietary fat. B: relative amounts of GLUT1 in microvillous membranes of placentas of dams fed high- or low-fat diets was measured by Western blotting, using β-actin as a marker for loading.
Since these diets consisted of a different base diet (chow vs semipurified), dams were fed semipurified diets with either lower (5%) or higher (15%) levels of fat for ≈4 wk. Dams consumed similar amounts of food (8.3 ± 0.5 vs. 7.7 ± 0.4 g per cage of three dams fed HFCO or LFCO diets, respectively), such that those fed the high-fat diet were indeed consuming more fat. As with the chow and HFCO diets, fetal masses were greater in dams fed HFCO vs. LFCO diets (Table 2). Plasma leptin levels collected at the time of the study were similar in dams fed either diet (3.49 ± 0.50 vs. 3.58 ± 0.46 ng/ml for dams fed HFCO vs. LFCO diets, respectively). Maternal fat pad masses were also similar at the time of the study regardless of diet (retroperitoneal: 0.115 ± 0.020 vs. 0.105 ± 0.013 g for HFCO vs. LFCO, respectively; inguinal: 0.417 ± 0.038 vs. 0.376 ± 0.020 g for HFCO vs. LFCO, respectively). Finally, to determine whether the composition of the diet fed during gestation made a difference in fetal masses, dams were fed the HFCO diet for 1 mo and then were either switched to the LFCO diet at 0.5 dpc or were retained on the HFCO diet. Fetuses of dams fed the LFCO diet during gestation were smaller than those fed the HFCO diet before and during gestation (Table 2). Even though fetal growth rates varied, there was no difference in fetal plasma insulin or glucose concentrations; fetal plasma glucose concentrations were 35.7 ± 4.7 vs. 41.2 ± 5.3 mg/dl and fetal insulin concentrations were 0.87 ± 0.15 vs. 0.99 ± 0.29 ng/ml for fetuses of dams fed low-fat vs. high-fat diets, respectively.
Table 2.
Masses of fetuses in dams fed HFCO diets before gestation and HFCO or LFCO diets during gestation
| DIET |
||
|---|---|---|
| Dietary regimen | LFCO | HFCO |
| Dams fed LFCO or HFCO before and during gestation | 0.740 ± 0.045 | 0.827 ± 0.010* |
| Dams fed HFCO before and LFCO or HFCO during gestation | 0.774 ± 0.024 | 0.893 ± 0.061* |
Values are means ± SE; n = 5–6 litters.
P < 0.05 from fetuses in dams fed low-fat corn oil (LFCO) diets.
Finally, we measured the relative levels of mRNA for proteins involved in the endogenous source of fatty acids, i.e., those synthesized de novo. In the placenta (Fig. 8A), there was an increase in FAS and SREBP-1a between early and late gestation but not in ACC or SREBP-1c. In the fetal liver (Fig. 8B), FAS increased with gestation, whereas ACC remained relatively constant between early and late gestation, similar to that which occurs in the placenta. In contrast to the placenta, SREBP-1a was similar with gestational age, whereas SREBP-1c increased in fetal livers as gestation progressed.
Fig. 8.
Relative mRNA levels of proteins associated with fatty acid synthesis in placentas (A) and fetal livers (B) of dams at 13.5 and 18.5 dpc. Relative amounts of mRNA of genes of interest compared with cyclophilin are presented. Data are presented as means ± SE (n = 3–5 litters). *Significant differences (P < 0.05) between placentas at different stages of gestation.
DISCUSSION
Since Pedersen first documented the increased rate of fetal growth in fetuses of diabetic women (53), the ability of maternal glucose to affect fetal growth has been well accepted. Glucose has two major routes by which it can manipulate fetal growth. First, glucose is transported readily across the placenta and is a primary source of energy for the fetus. Fetal glucose is also a stimulus for insulin secretion by the fetal pancreas, and insulin is known to be trophic. One might hypothesize that if maternal glucose can be maintained in diabetic women, then fetal growth rates would be stabilized and constant. Interestingly, when maternal glucose levels are controlled in diabetic women, fetal growth rates are not constant but are directly related to maternal lipid concentrations (59). Thus, maternal lipids likely play an important role in fetal growth.
Free fatty acids or lipase-generated fatty acids can be taken up by the placenta and trophoblasts and transported to the fetus (reviewed in Ref. 22). However, no in vivo studies have examined clearance of the partially hydrolyzed remnant lipoprotein particle. The ability to take up lipoprotein particles is important, since most dietary lipids enter the body by way of CM. In addition, there is relatively more triglyceride in the circulation than fatty acids late in gestation (350 vs. 10 mg/dl) (29). Although the lipid core of the CM is hydrolyzed prior to uptake of the particle, a significant amount of triglyceride remains associated with the newly formed remnant particle after lipase activity (52). In addition to the dietary sources of lipid, the liver synthesizes triglyceride-rich VLDL, and the levels increase as gestation progresses as an additional source of lipid for the fetus (26). Thus, we set out to determine 1) if maternal dietary lipids, specifically in the form of CM, could be taken up as remnant particles or as core lipids as well as lipase-derived free fatty acids and 2) if fetuses of dams with similar adiposity and fed a high-fat diet for a short period of time were larger than fetuses of dams fed low-fat or chow diets.
Importantly, the core lipids of CMR were taken up by the placenta, as evidenced by the presence of 14C (primarily as cholesteryl ester). Fatty acids were also taken up. If free fatty acids were taken up independently of the remnant particle, then there would be more radiolabeled fatty acids in the placenta vs. cholesterol, similar to that measured in the skeletal muscle (ratio CH:FA of 0.53). If the whole particle were taken up without uptake of free fatty acids, then the ratio CH:FA would be similar to that in the liver (ratio of 2.80). Since the ratio CH:FA in the placenta was close to 1 (1.25), similar amounts of cholesteryl ester and fatty acids/triglycerides were taken up. Thus, there was likely hydrolysis of lipids by LPL generating the remnant. The newly generated fatty acids as well as the remnant particle or core lipids were taken up in similar amounts. Compared with uptake by other extrahepatic tissues, the placenta took up more triglyceride plus fatty acids and more cholesterol per gram of tissue than skeletal muscle or adipose tissue.
It has been shown that the uptake of remnant particles or their lipid core is mediated by LPL (5), as is the uptake of fatty acids (2). LPL may play a critical role in fetal metabolism, since LPL mRNA levels decrease in peripheral tissues (44) and stay equal or increase per gram of placenta (28, 42), possibly to direct triglyceride-containing particles to the fetal unit. Surprisingly, fetal growth rates in women lacking LPL with reduced placental LPL activity are normal (43). However, endothelial lipase, a second placental triglyceride hydrolase (8, 40), might be elevated when LPL activity is reduced as occurs in the LPL knockout mouse (40, 43). The newly generated remnants, regardless of which lipase is active, can now be taken up by trophoblasts. We initially thought that particles were taken up exclusively via apoE receptors, since all the receptors are present in the placenta. However, we also found that either the remnants or the core lipids can be taken up independently of apoE; core lipids are likely shed from particles during LPL hydrolysis (5). Our data are consistent with previously reported results from our laboratory, in that there is a large receptor-independent component of lipoprotein uptake by the placenta, larger than the liver (77), that could be the result of the increased endocytosis and phagocytosis that occur in the placenta (4, 64). Not all uptake is receptor independent, as various receptors and transport proteins mediate uptake of LPL-derived fatty acids by the trophoblasts (16, 23). Although we know that CMR or core lipids can be taken up independently of apoE, likely at a substantial rate, we cannot determine what proportion of lipid was taken up via apoE receptors vs. that taken up independently of apoE, since the particles lacking apoE were cleared more slowly, thereby making a greater proportion available for uptake by the placenta. Regardless, the main point is that diet-derived lipids, including triglyceride, can be taken up by the placenta as either whole particles or as core lipids.
Knowing that maternally derived CMR/core lipids as well as fatty acids are taken up by the placenta, we next set out to determine whether more dietary fat would be transported to the fetus or retained in the placenta when pregnant females consumed a high-fat diet. Animals were given an intravenous bolus of ≈10 or ≈30 μg of lipid to parallel our diets of 5 vs. 15% fat. Not surprisingly, the same relative percentage of dosed lipid was taken up by the placenta for the higher as well as for the lower dose. However, the relative percentage of dosed lipid transported to the fetus was less in the dams given more lipid. Even though proportionately less lipid was transported to the fetus when more lipid was given, the absolute amount transported was still greater in the dams given threefold more lipid. These data suggest that the placenta will mediate the amount of lipid to be transported to the fetus. There are several different possibilities to account for the difference in fatty acids taken up vs. that transported. First, the transport or export process across and out of trophoblasts could be saturated, since the process is likely protein mediated. This would seem disadvantageous to the placenta, since lipotoxicity can occur in tissues with elevated lipid uptake (60). Second, the placenta could retain any lipid it needs based on its requirements, i.e., for energy or membrane formation. Third, the placenta could transport lipid based on the needs of the fetus. Fourth, the placenta could secrete excess lipid into the maternal circulation, since the placenta can indeed synthesize new lipoproteins, although the newly synthesized lipoproteins could be secreted into the fetal circulation (41).
In parallel with these acute hyperlipidemic studies, dams were fed 5 or 15% fat for a long enough time frame to enter a new steady state of lipid metabolism but a short enough time frame that an increase in maternal adiposity did not occur. Expressions of fetal proteins that are regulated by fatty acids were measured as an indication of excess lipids being transported to the fetus. There was an increase in PPARα mRNA levels, an indication of activation of this transcription factor by dietary fatty acids (17). Importantly, the downstream target of PPARα, CPT I, was also increased. An increase in CPT I does indicate an increase in fatty acid oxidation and thereby energy production. The activation appeared to be specific for PPARα in that ABCG1, a downstream target of LXR, was not affected by high-fat diets. In adults, LXR is affected by unsaturated fatty acids (17). It is not unusual for the fetus to respond differently to exogenous factors compared with adults, however, since fetal sterol synthesis does not change in response to exogenous cholesterol and polyunsaturated fatty acids as do adults (61, 81). Likewise, the lack of effect on fetal HNF-4α might not be unexpected, since the previously reported increase in this signaling factor occurred in fetal livers of monkeys fed diets with higher levels of saturated fat and cholesterol compared with our mice, which were fed more unsaturated fat and no cholesterol (45). A major similarity of the monkey and mouse studies, however, is that maternal fat does indeed affect fetal metabolism. TNF-1α mRNA was also not affected, which suggests no fetal inflammation even though dams were fed n-6, a proinflammatory fat.
We also measured mRNA levels of proteins involved with fatty acid synthesis since an increase in fatty acids from the endogenous source could spare the exogenous source for various processes, such as membrane formation. FAS mRNA levels increased with gestational age in both the placenta and liver. SREBP-1 mRNA levels were also increased, though 1c was increased in the fetal liver with gestation whereas 1a was increased in the placenta. Alhough 1a is usually low and unregulated in adults, it is not surprising that it is regulated in fetal tissues since it is found in rapidly growing cultured cells (63). Despite the fact that SREBP-1 is not processed in the neonate (7), it does appear to be processed to the mature, active form late in gestation (6). Fatty acid synthesis did indeed occur in the fetal liver at 18.5 dpc (13,507 ± 1,505 nmol 3H2O converted to fatty acids/g liver), and was greater than that in the neonate (80).
The mechanism for how maternal fat affects fetal growth is currently unknown. It could be simply that the oxidation of fat in the fetus spares glucose from oxidation, leading to fetal hyperglycemia as occurs during diabetes, hyperinsulinemia, and fetal overgrowth. Alternatively, more glucose could be transported as occurs when high-fat diets lead to increased adiposity and adipokine levels (34, 35). It seems unlikely that hyperglycemia was a contributing factor in the current study, since there was no difference in insulin and glucose concentrations in the fetal circulation and no increase in GLUT1 levels in MVM, and pyruvate kinase mRNA levels were not affected and glucose will enhance hepatic pyruvate kinase levels (78). An alternative is that fatty acids and/or fatty acid oxidation specifically can affect growth. Others have shown that fatty acids can lead to an increase in mTOR either directly (19, 71) or via a change in AMPK activity resulting from β-oxidation (48). An increase in mTOR in combination with elevated SREBP-1c promotes cellular and organ growth in Drosophila (54). The question remains whether an increase in an exogenous source of fatty acids plus mTOR could affect growth in murine fetuses. A change in mTOR in the placenta also could affect fetal growth via upregulation of amino acid transport (56). More studies are required to delineate the dietary fat-dependent, maternal adiposity-independent effect on fetal growth.
In addition to the fat-induced effects, the ability of the placenta to take up dietary cholesterol in the form of remnants could be important for fetuses with the Smith-Lemli-Opitz syndrome (SLOS). SLOS fetuses have reduced rates of cholesterol biosynthesis that are associated with a myriad of adverse phenotypical changes (reviewed in Ref. 3). Since recent studies have shown the ability of maternal cholesterol to be transported to the fetus (reviewed in Ref. 76), the ability to take up diet-derived cholesterol could be an important route to enhance sterol balance in utero in SLOS fetuses and thereby possibly improve pregnancy outcome.
These studies also have major implications for the general population. Large human newborns and rodents exposed to increased nutrients have an increased risk of obesity and/or diabetes later in life (11, 12, 39, 50, 57, 58, 65, 67, 68). It is not known whether the same effect is mediated when fetal growth is altered via different pathways, i.e., increased placental transport of fat, glucose, and/or amino acid. However, offspring of animals that were pair fed a high-fat diet and did not become obese did not develop altered metabolism (72). Since fetal sizes were not measured in these previous studies, it is unclear how these data relate to large offspring of dams that are not obese. In the current study, we showed that diet alone can affect fetal growth, even when the high-fat diet is switched at conception. This is not surprising, since we also showed that whole triglyceride-containing remnant particles can be taken up by the placenta. These studies could lead the way to new methods used to dissect out the impact of fetal growth, independent of glucose, on developmental programming and long term health.
GRANTS
These studies were supported by Grants HD-34089 and DK-059630 from the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Thomas Jones for excellent technical assistance. We would also like to thank Drs. Heubi, Davidson, and Jandacek for their insightful comments on the manuscript.
REFERENCES
- 1. Abu-Elheiga L, Matzuk MM, Kordari P, Oh W, Shaikenov T, Gu Z, Wakil SJ. Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically lethal. Proc Natl Acad Sci USA 102: 12011–12016, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Augustus AS, Kako Y, Yagyu H, Goldberg IJ. Routes of FA delivery to cardiac muscle: modulation of lipoprotein lipolysis alters uptake of TG-derived FA. Am J Physiol Endocrinol Metab 284: E331–E339, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Battaile KP, Steiner RD. Smith-Lemli-Opitz syndrome: the first malformation syndrome associated with defective cholesterol synthesis. Mol Genet Metab 71: 154–162, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Bevilacqua E, Hoshida MS, Amarante-Paffaro A, Albieri-Borges A, Zago Gomes S. Trophoblast phagocytic program: roles in different placental systems. Int J Dev Biol 54: 495–505, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Bharadwaj KG, Hiyama Y, Hu Y, Huggins LA, Ramakrishnan R, Abumrad NA, Shulman GI, Blaner WS, Goldberg IJ. Chylomicron- and VLDL-derived lipids enter the heart through different pathways: in vivo evidence for receptor and non-receptor mediated fatty acid uptake. J Biol Chem 285: 37976–37986, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bobard A, Hainault I, Ferré P, Foufelle F, Bossard P. Differential regulation of sterol regulatory element-binding protein 1c transcriptional activity by insulin and liver X receptor during liver development. J Biol Chem 280: 199–206, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Botolin D, Jump DB. Selective proteolytic processing of rat hepatic sterol regulatory element binding protein-1 (SREBP-1) and SREBP-2 during postnatal development. J Biol Chem 278: 6959–6962, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Broedl UC, Maugeais C, Millar JS, Jin W, Moore RE, Fuki IV, Marchadier D, Glick JM, Rader DJ. Endothelial lipase promotes the catabolism of apoB-containing lipoproteins. Circ Res 94: 1554–1561, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Burke KT, Colvin PL, Myatt L, Graf GA, Schroeder F, Woollett LA. Transport of maternal cholesterol to the fetus is affected by maternal plasma cholesterol concentrations in the Golden Syrian hamster. J Lipid Res 50: 1146–1155, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burt RL, Leake NH, Pulliam RP. Regulation of plasma NEFA in pregnancy and the puerperium. Preliminary observations. Obstet Gynecol 17: 215–221, 1961 [PubMed] [Google Scholar]
- 11. Catalano PM. Obesity and pregnancy—the propagation of a viscous cycle? J Clin Endocrinol Metab 88: 3505–3506, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG 113: 1126–1133, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Chirala SS, Chang H, Matzuk M, Abu-Elheiga L, Mao J, Mahon K, Finegold M, Wakil SJ. Fatty acid synthesis is essential in embryonic development: fatty acid synthase null mutants and most of the heterozygotes die in utero. Proc Natl Acad Sci USA 100: 6358–6363, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coleman RA, Haynes EB. Synthesis and release of fatty acids by human trophoblast cells in culture. J Lipid Res 28: 1335–1341, 1987 [PubMed] [Google Scholar]
- 15. Cox KB, Hamm DA, Millington DS, Matern D, Vockley J, Rinaldo P, Pinkert CA, Rhead WJ, Lindsey JR, Wood PA. Gestational, pathologic and biochemical differences between very long-chain acyl-CoA dehydrogenase deficiency and long-chain acyl-CoA dehydrogenase deficiency in the mouse. Hum Mol Genet 10: 2069–2077, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Duttaroy AK. Transport of fatty acids across the human placenta: a review. Prog Lipid Res 48: 52–61, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Forman BM, Chen J, Evans RM. The peroxisome proliferator-activated receptors: ligands and activators. Ann NY Acad Sci 804: 266–275, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Frykman PK, Brown MS, Yamamoto T, Goldstein JL, Herz J. Normal plasma lipoproteins and fertility in gene-targeted mice homozygous for a disruption in the gene encoding very low density lipoprotein receptor. Proc Natl Acad Sci USA 92: 8453–8457, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ginsgras AA, White PJ, Chouinard PY, Julien P, Davis TA, Dombrowski L, Couture Y, Dubreuil P, Myre A, Bergeron K, Marette A, Thivierge MC. Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt-mTOR-S6K1 pathway and insulin sensitivity. J Physiol 579: 269–284, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, Marconi AM, Pardi G, Sibley CP. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res 42: 514–519, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Hadden DR, McLaughlin C. Normal and abnormal maternal metabolism during pregnancy. Sem Fetal Neon Med 14: 66–71, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Haggarty P. Fatty acid supply to the human fetus. Ann Rev Nutr 30: 237–255, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Haggarty P. Placental regulation of fatty acid delivery and its effect on fetal growth—a review. Placenta 23: S28–S38, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Hanebutt FL, Demmeimair H, Schiessl B, Larque E, Koletzko B. Long-chain polyunsaturated fatty acid (LC-PUFA) transfer across the placenta. Clin Nutr 27: 685–693, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Hayashi H, Nutting DF, Fujimoto K, Cardelli JA, Black D, Tso P. Transport of lipid and apolipoproteins A-I and A-IV in intestinal lymph of the rat. J Lipid Res 31: 1613–1625, 1990 [PubMed] [Google Scholar]
- 26. Herrera E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eu J Clin Nutr 54 Suppl: S47–S51, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Herrera E, Amusquivar E, Lopez-Soldedo I, Ortega H. Maternal lipid metabolism and placental lipid transfer. Horm Res 65: 59–64, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Herrera E, Lasunción MA, Gomez-Coronado D, Aranda P, López-Luna P, Maier I. Role of lipoprotein lipase activity on lipoprotein metabolism and the fate of circulating triglycerides in pregnancy. Am J Obstet Gynecol 158: 1575–1583, 1988 [DOI] [PubMed] [Google Scholar]
- 29. Herrera E, Ortega H, Alvino G, Giovannini N, Amusquivar E, Cetin I. Relationship between plasma fatty acid profile and antioxidant vitamins during normal pregnancy. Eur J Clin Nutr 58: 1231–1238, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Herz J, Clouthier DE, Hammer RE. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell 71: 411–421, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Ibdah JA, Paul H, Zhao Y, Binford S, Salleng K, Cline M, Matern D, Bennett MJ, Rinaldo P, Strauss AW. Lack of mitochondrial trifunctional protein in mice causes neonatal hypoglycemia and sudden death. J Clin Invest 107: 1403–1409, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Illsley NP, Wang ZQ, Gray A, Sellers MC, Jacobs MM. Simultaneous preparation of paired, syncytial, microvillous and basal membranes from human placenta. Biochem Biophys Acta 1029: 218–226, 1990 [DOI] [PubMed] [Google Scholar]
- 33. Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest 92: 883–893, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J 23: 271–278, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kamohara L, Burcelin R, Malaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature 25: 374–376, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Knopp RH, Magee ML, Walden CE, Bonet B, Benedetti TJ. Prediction of infant birth weight by GDM screening tests: Importance of plasma triglycerides. Diabetes Care 15: 1605–1613, 1992 [DOI] [PubMed] [Google Scholar]
- 37. Kurtz DM, Rinaldo P, Rhead WJ, Tian L, Millington DS, Vockley J, Hamm DA, Brix AE, Lindsey JR, Pinkert CA, O'Brien WE, Wood PA. Targeted disruption of mouse long-chain acyl-CoA dehydrogenase gene reveals crucial roles for fatty acid oxidation. Proc Natl Acad Sci USA 95: 15592–15597, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kusakabe T, Maeda M, Hoshi N, Sugino T, Watanabe K, Fukuda T, Suzuki T. Fatty acid synthase is expressed mainly in adult hormone-sensitive cells or cells with high lipid metabolism and in proliferating fetal cells. J Histochem Cytochem 48: 613–622, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Levin BE, Dunn-Meynell AA. Maternal obesity alters adiposity and monoamine function in genetically predisposed offspring. Am J Physiol Regul Integr Comp Physiol 283: R1087–R1093, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Lindegard MLS, Olivecrona G, Christoffersen C, Krasky D, Hannibal J, Petersen BL, Zechner R, Damm P, Nielsen LB. Endothelial and lipoprotein lipases in human and mouse placenta. J Lipid Res 46: 2399–2346, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Madsen EM, Lindegaard MLS, Andersen CB, Damm Pl Nielsen LB. Human placenta secretes apolipoprotein B-100-containing lipoproteins. J Biol Chem 279: 55271–55276, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Magnusson-Olsson AL, Hamark B, Ericsson A, Wennergren M, Jansson T, Powell TL. Gestational and hormonal regulation of human placental lipoprotein lipase. J Lipid Res 47: 2551–2561, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Magnusson-Olsson AL, Lager S, Jacobsson B, Jansson T, Powell TL. Effect of maternal triglycerides and free fatty acids on placental LPL in cultured primary trophoblast cells and in a case of maternal LPL deficiency. Am J Physiol Endocrinol Metab 293: E24–E30, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Martin-Hidalgo A, Holm C, Belfrage P, Schotz MC, Herrera E. Lipoprotein lipase and hormone-sensitive lipase activity and mRNA in rat adipose tissue during pregnancy. Am J Physiol Endocrinol Metab 266: E930–E935, 1994 [DOI] [PubMed] [Google Scholar]
- 45. McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 119: 323–335, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Montes A, Walden CE, Knopp RH, Cheung M, Chapman MB, Albers JJ. Physiologic and supraphysiologic increases in lipoprotein lipids and apoproteins in late pregnancy and postpartum. Possible markers for the diagnosis of “prelipemia”. Arterosclerosis 4: 407–417, 1984 [DOI] [PubMed] [Google Scholar]
- 47. Morisset AS, St. Yves A, Veillette J, Weisnagel SJ, Tchernof A, Robitaille J. Prevention of gestational diabetes mellitus: a review of studies on weight management. Diabetes Metab Res Rev 26: 17–25, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation—AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol 574: 63–71, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nelson SM, Mathews P, Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Human Reprod Update 16: 255–275, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nivoit P, Morens C, Van Assche FA, Jansen EHJM, Poston L, Remache C, Reusens B. Established diet-induced obesity in female rats leads to offspring hyperphagia, adiposity and insulin resistance. Diabetologia 52: 1133–1142, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Oey NA, Den Boer MEJ, Wuburg FA, Vekemans JA, Steiner C, Wanders RJA, Waterham HR, Ruiter JPN, Attie-Bitach T. Long-chain fatty acid oxidation during early human development. Pediatr Res 57: 755–759, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Pattnaik NM, Zilversmit DB. Effect of size and competition by lipoproteins and apolipoproteins on the uptake of chylomicrons and chylomicron remnants by hepatoma cells in culture. Biochim Biophys Acta 617: 335–346, 1980 [DOI] [PubMed] [Google Scholar]
- 53. Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol 16: 330–342, 1954 [DOI] [PubMed] [Google Scholar]
- 54. Portsmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab 8: 224–236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol 21: 521–526, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roos S, Powell TL, Jansson T. Placental mTOR links maternal nutrient availability to fetal growth. Biochem Soc Trans 37: 295–298, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Samaras TT, Elrick H, Storms LH. Birthweight, rapid growth, cancer, longevity: a review. J Natl Med Assoc 95: 1170–1183, 2003 [PMC free article] [PubMed] [Google Scholar]
- 58. Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EHJM, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance. A novel murine model of developmental programming. Hypertension 51: 383–392, 2008 [DOI] [PubMed] [Google Scholar]
- 59. Schaefer-Graf UM, Graf K, Kulbacka I, Kjos SL, Dudenhausen J, Vetter K, Herrera E. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 31: 1858–1863, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol 14: 281–287, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Schmid KE, Woollett LA. Differential effects of polyunsaturated fatty acids on sterol synthesis rates in adult and fetal tissues of the hamster: consequence of altered sterol balance. Am J Physiol Gastrointest Liver Physiol 285: G796–G803, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Shimano H, Horton JD, Hammer RE, Shimomura I, Brown MS, Goldstein JL. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J Clin Invest 98: 1575–1584, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest 99: 846–854, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sibley C, Glazier J, D'Souza S. Placental transporter activity and expression in relation to fetal growth. Exp Physiol 82: 389–402, 1997 [DOI] [PubMed] [Google Scholar]
- 65. Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care 21: B142––B149., 1998 [PubMed] [Google Scholar]
- 66. Spoelgen R, Hammes A, Anzenberger U, Zechner D, Andersen OM, Jerchow B, Willnow TE. LRP2/megalin is required for patterning of the ventral telecephalon. Development 132: 405–414, 2005 [DOI] [PubMed] [Google Scholar]
- 67. Srinivasan M, Katewa SD, Palaniyappan A, Pandya JD, Patel MS. Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. Am J Physiol Endocrinol Metab 291: E792–E799, 2006 [DOI] [PubMed] [Google Scholar]
- 68. Taylor PD, McConnell JM, Khan IY, Holemans K, Lawrence KM, Asare-Anane H, Persaud SJ, Jones PM, Petrie L, Hanson MA, Poston L. Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. Am J Physiol Regul Integr Comp Physiol 288: R234–R239, 2005 [DOI] [PubMed] [Google Scholar]
- 69. Trevaskis NL, Tso P, Rider T, Charman WN, Porter CJH, Jandacek R. Tissue uptake of DDT is independent of chylomicron metabolism. Arch Toxicol 80: 196–200, 2006 [DOI] [PubMed] [Google Scholar]
- 70. Trommsdorff M, Gothardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and apoE receptor 2. Cell 97: 689–701, 1999 [DOI] [PubMed] [Google Scholar]
- 71. Vinciguerra M, Veyrat-Durebex C, Moukl MA, Rubbia-Brandt L, Rohner-Jeanrenaud F, Foti M. PTEN down-regulation by unsaturated fatty acids triggers hepatic steatosis via an NF-kBp65/mTOR-dependent mechanism. Gastroenterology 134: 268–280, 2008 [DOI] [PubMed] [Google Scholar]
- 72. White CL, Purpera MN, Morrison CD. Maternal obesity is necessary for programming effect of high-fat diet on offspring. Am J Physiol Regul Integr Comp Physiol 296: R1464–R1472, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Williams PF, Simons LA, Turle JR. Plasma lipoproteins in pregnancy. Hormone Res 7: 83–90, 1976 [DOI] [PubMed] [Google Scholar]
- 74. Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, Burns DK, Herz J. Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci USA 93: 8460–8464, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Woollett LA. Origin of cholesterol in the fetal Golden Syrian hamster: contribution of de novo sterol synthesis and maternal-derived lipoprotein cholesterol. J Lipid Res 37: 1246–1257, 1996 [PubMed] [Google Scholar]
- 76. Woollett LA. Where does fetal and embryonic cholesterol originate and what does it do? Ann Rev Nutr 28: 97–114, 2008 [DOI] [PubMed] [Google Scholar]
- 77. Wyne KL, Woollett LA. Transport of maternal LDL and HDL to the fetal membranes and placenta of the Golden Syrian hamster is mediated by receptor-dependent and receptor-independent processes. J Lipid Res 39: 518–530, 1998 [PubMed] [Google Scholar]
- 78. Yamada K, Noguchi T. Nutrient and hormonal regulation of pyruvate kinase gene expression. Biochem J 337: 1–11, 1999 [PMC free article] [PubMed] [Google Scholar]
- 79. Yang Z, Zhao Y, Bennett MJ, Strauss AW, Ibdah JA. Fetal genotypes and pregnancy outcomes in 35 families with mitochondrial trifunctional protein mutations. Am J Obstet Gynecol 187: 715–720, 2002 [DOI] [PubMed] [Google Scholar]
- 80. Yao L, Horn PS, Heubi JE, Woollett LA. The liver plays a key role in whole body sterol accretion of the neonatal Golden Syrian hamster. Biochim Biophys Acta 1771: 550–557, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yao L, Jenkins K, Horn PS, Lichtenberg MH, Woollett LA. Inability to fully suppress sterol synthesis rates with exogenous sterol in embryonic and extraembyronic fetal tissues. Biochim Biophys Acta 1171: 1372–1379, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]