Abstract
Several receptors linked to the adenylyl cyclase signaling pathway stimulate electrical activity and calcium influx in endocrine pituitary cells, and a role for an unidentified sodium-conducting channel in this process has been proposed. Here we show that forskolin dose-dependently increases cAMP production and facilitates calcium influx in about 30% of rat and mouse pituitary cells at its maximal concentration. The stimulatory effect of forskolin on calcium influx was lost in cells with inhibited PKA (cAMP-dependent protein kinase) and in cells that were haploinsufficient for the main PKA regulatory subunit but was preserved in cells that were also haploinsufficient for the main PKA catalytic subunit. Spontaneous and forskolin-stimulated calcium influx was present in cells with inhibited voltage-gated sodium and hyperpolarization-activated cation channels but not in cells bathed in medium, in which sodium was replaced with organic cations. Consistent with the role of sodium-conducting nonselective cation channels in PKA-stimulated Ca2+ influx, cAMP induced a slowly developing current with a reversal potential of about 0 mV. Two TRP (transient receptor potential) channel blockers, SKF96365 and 2-APB, as well as flufenamic acid, an inhibitor of nonselective cation channels, also inhibited spontaneous and forskolin-stimulated electrical activity and calcium influx. Quantitative RT-PCR analysis indicated the expression of mRNA transcripts for TRPC1 >> TRPC6 > TRPC4 > TRPC5 > TRPC3 in rat pituitary cells. These experiments suggest that in pituitary cells constitutively active cation channels are stimulated further by PKA and contribute to calcium signaling indirectly by controlling the pacemaking depolarization in a sodium-dependent manner and directly by conducting calcium.
Keywords: adenylyl cyclase; adenosine 3′,5′-cyclic monophosphate; calcium transients; transient receptor potential channels; gonadotrophs; lactotrophs; somatotrophs
the membrane potential of isolated pituitary cells in vitro is not stable but oscillates from resting potentials of −65 to −50 mV, reflecting the balance between the activity of depolarizing and hyperpolarizing channels. When membrane potential oscillations reach the threshold level, cells generate action potentials (APs); in vitro firing of APs and calcium transients has been observed in frog, mouse, porcine, ovine, and bovine endocrine pituitary cells. Spontaneous firing of APs has also been observed in situ in rat pituitary slices (32). Pituitary cells express at least two subtypes of voltage-gated calcium channels (Cav; T- and L-type), which contribute to the firing of APs and the accompanied changes in intracellular calcium concentrations ([Ca2+]i). These cells also express high and low tetrodotoxin (TTX)-sensitive voltage-gated Na+ (Nav) channels (38, 39, 42), but the resting membrane potential and firing of APs are not affected by a blockage of these channels (32). However, when extracellular Na+ is substituted with large organic cations, the resting membrane potential rapidly reaches about −85 mV, a value close to the equilibrium potential of K+, suggesting the constitutive activity of a Na+-conducting channel (28, 29). Such prominent hyperpolarization of the plasma membrane in the absence of bath Na+ causes abolition of the spontaneous firing of APs in gonadotrophs, lactotrophs, somatotrophs, and immortalized pituitary cells (18). Several pathways could account for such background Na+ conductance, including Na+-coupled transporters, Nav via the window conductance, hyperpolarization-activated/cyclic nucleotide-modulated (HCN) channels, the recently characterized Na+-leak channel NALCN, and transient receptor potential (TRP) channels.
Several investigations have suggested that the Na+-conducting channels are also responsible for the facilitation of electrical activity and Ca2+ influx in the pituitary somatotrophs by Gs-coupled growth hormone-releasing hormone (GHRH) receptors. Kato et al. (12) showed that GHRH-stimulated hormone secretion by somatotrophs was suppressed in cells bathed in Na+-deficient medium. The same group also showed that GHRH depolarizes somatotrophs, which was greatly suppressed by removal of bath Na+ (13). Others also showed the dependence of GHRH-induced Ca2+ influx on depolarizing Na+ conductance (21, 22). It has been reported that GHRH increases the total Nav current in somatotrophs, which was blocked by low levels of TTX (11), and that GHRH facilitates a TTX-insensitive Nav current in cultured somatotrophs from growth hormone-green fluorescent protein transgenic mice, but in a cAMP-independent and protein kinase C-dependent manner (42). In addition to GHRH receptors, pituitary cells express several other subtypes of Gs-coupled receptors, in which activation causes stimulation of the adenylyl cyclase (AC) signaling pathway, including corticotropin-releasing hormone and vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptors (32). The relevance of Na+ conductance in the action of these receptors has not been studied.
Here, we used rat and mouse anterior pituitary cells, as well as GH3 immortalized pituitary cells, to examine the role of Na+ in spontaneous and cAMP-stimulated calcium [Ca2+]i transients. Because there is no common Gs-coupled receptor among these cells, we used forskolin, an activator of AC. Single cell recordings were used for measurements of electrical activity, membrane currents and [Ca2+]i, and cyclic nucleotide intracellular content and release were measured in mixed pituitary cell populations. To dissociate between direct effect of cAMP on HCN channels and its indirect effect, through a cAMP-dependent kinase (PKA), we used two inhibitors of HCN channels, ZD7288 and Cs+, and two inhibitors of PKA, H89 and RP-cAMPs, on forskolin-stimulated electrical activity and calcium signaling. To investigate directly the role of PKA in the regulation of these channels, we also used two mouse models with altered PKA signaling: Prkar1a+/− and Prkar1a+/−Prkaca+/− (15, 37). We expected that Prkar1a haploinsufficiency will be accompanied by elevated PKA activity in pituitary cells. This in turn should cause the loss of stimulatory action of the forskolin on electrical activity and calcium signaling. In contrast, we expected that basal and stimulated PKA activity is normalized in cells from Prkar1a+/−Prkaca+/− mice, and the stimulatory action of forskolin on electrical activity and Ca2+ influx is not affected. In the second part of this study, we investigated the nature of forskolin-stimulated Na+ conductance. Specifically, we studied effects of replacement of bath Na+ with Li+ and organic cations and effects of several nonselective cation channel blockers on basal and forskolin-stimulated electrical activity and Ca2+ transients.
MATERIALS AND METHODS
Chemicals.
TTX, NNC 05-2090 {1-[3-(9H-carbazol-9-yl)propyl]-4-(2-methoxyphenyl)-4-piperidinol hydrochloride}, and ZD7288 (4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride) were obtained from Tocris (Ellisville, MO). Fura 2-AM was from Invitrogen (Carlsbad, CA). SKF96365 {1-[2-(4-methoxyphenyl)-2-]3-[(4-methoxyphenyl)propoxy-ethyl]-1H-imidazole, monohydrochloride} was from Cayman Chemical (Ann Arbor, MI). All other drugs and chemicals were purchased from Sigma (St. Louis, MO).
Cell cultures.
Experiments were performed on anterior pituitary cells from normal postpubertal female Sprague-Dawley rats obtained from Taconic Farms (Germantown, NY). Mice used in the experiments were from an (CD-1 × C57BL/6) F1 hybrid background. Prkar1a+/− mice have been described previously (15). Prkar1a+/− mice were crossed with mice that were heterozygous for catalytic subunit Cα (Prkaca+/−) to generate a mouse model with double heterozygosity (Prka1a+/−Prkaca+/−), as described previously (37). Euthanasia was performed by asphyxiation with CO2, and the anterior pituitary glands were removed after decapitation. Experiments were approved by the National Institute of Child Health and Human Development (NICHD; Bethesda, MD) Animal Care and Use Committee. Anterior pituitary cells were mechanically dispersed after treatment with trypsin and cultured as mixed cells or enriched fractions in medium 199 containing Earle's salts, sodium bicarbonate, 10% heat-inactivated horse serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). Enriched rat gonadotroph, lactotroph, and somatotroph fractions were obtained as described previously (16). Rat gonadotrophs were identified further by the addition of GnRH, lactotrophs by the addition of dopamine and TRH, and somatotrophs by response to GHRH and the lack of response to dopamine application. Because of the small number of cells, no purification of mouse pituitary cells was done.
Cyclic nucleotide measurements.
Cyclic nucleotide production was monitored using static cultures of pituitary cells. Briefly, cells (1,000,000/well) were plated in poly-l-lysine-treated 24-well plates in Earle's salts medium and incubated overnight at 37°C under 5% CO2-air and saturated humidity. The following day the medium was removed, and cells were washed and then stimulated at 37°C under 5% CO2-air and saturated humidity for 30 min in the presence (experimental groups) and absence of forskolin (controls). The bath medium was supplemented with 1 mM isobutylmethylxantine. Cyclic nucleotides were measured in incubation medium and in cell extracts using specific antiserum provided by Albert Baukal (NICHD). 125cAMP and 125I-3′,5′-cyclic monophosphate (cGMP) tracers were purchased from PerkinElmer Life Sciences (Boston, MA).
PKA assay.
Mouse pituitary glands were homogenized in 0.5 ml of ice-cold buffer containing 10 mM Tris·HCl (pH 7.5), 1 mM EDTA, 1 mM dithiothreitol, and protease inhibitor cocktail I (EMD Biosciences, La Jolla, CA). The lysates were centrifuged at 10,000 g for 10 min. The protein concentration of the supernatant was determined with a BCA Protein Assay Kit (Pierce) and used in a PKA assay. PKA enzymatic activity was measured using a previously described method (23). The assays were carried out in a total volume of 50 μl for 15 min at 37°C in the reaction mixture containing 50 mM Tris·HCl (pH 7.5), 10 mM MgCl2, 1 mM dithiothreitol, 25 μM kemptide, and 25 μM [γ-32P]ATP (0.1 μCi/nmol) with or without 5 μM cAMP and 10 μl of cell extract. After incubation, the reaction mixtures were spotted onto 0.23-mm phosphocellulose (Whatman P81) discs and washed three times in 0.5% phosphoric acid. Filters were air-dried and counted in a liquid scintillation counter. Statistical analysis of comparisons between groups was undertaken using a two-sample t-test; differences were considered significant when P < 0.05.
Immunoprecipitation and Western blot analysis.
Freshly prepared rat and mouse pituitary tissue was washed three times with cold PBS to remove blood residue. The tissue was then homogenized on ice using a glass homogenizer with immunoprecipitation buffer (50 mM Tris·HCl, pH 7.4, 300 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS) containing a protease inhibitor cocktail (Calbiochem). Cell lysates were kept on ice for 30 min and centrifuged at 250,000 g for 35 min at 4°C. The supernatant was collected and precleared with 2 μg of normal rabbit serum and 20 μl of Protein A/G Plus-Agarose (Santa Cruz Biotechnology) at 4°C for 1 h on a rotary shaker. After centrifugation at 40,000 g for 1 min, supernatants were incubated with 2 μg of rabbit anti-AC V/VI antibody (Santa Cruz Biotechnology) overnight at 4°C. A 20-μl aliquot of Protein A/G Plus-Agarose was added to the reaction, followed by 2 h of incubation. The beads were washed four times with 1 ml of immunoprecipitation buffer each, boiled in 2× SDS-PAGE sample buffer with dithiothreitol, and centrifuged. The elutions were subjected to Tris-glycine SDS-PAGE and transferred onto PVDF membranes. The membrane was blocked for 1 h at room temperature with PBS supplemented with 0.1% Tween 20 and 5% nonfat milk and then incubated overnight at 4°C with 1:500 diluted anti-AC V/VI antibody. After washing four times with PBS containing Tween 20, positive signals of individual blots were visualized by incubating the membrane with peroxidase-conjugated goat anti-rabbit secondary antibody (1:10,000; Kirkegaard & Perry Laboratories), followed by subsequent treatment with SuperSignal West Pico luminol/enhanced solution (Pierce) and exposure to X-ray film (Kodak).
RT-PCR analysis.
Total RNA from the primary pituitary cells was extracted using the RNeasy Mini Kit (Sigma). Subsequently, 1 μg of total RNA was treated with DNAse I (Invitrogen) and reverse transcribed with SuperScript III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen). Quantitative RT-PCR was performed using predesigned TaqMan Gene Expression Assays (Applied Biosystems) with LightCycler TaqMan Master Mix and LightCycler 2.0 Real-Time PCR system (Roche Applied Science). Gene expression levels of the target genes were determined by the comparative 2−ΔΔCT quantification method, using GAPDH as a reference gene, where (ΔΔCT) = (CT, target − CT, reference) sample − (CT, target − CT, reference) control. To compare the relative expression level of individual transient receptor potential C (TRPC) channels, results were expressed as means ± SE relative to TRPC1 gene expression (set to 100%). The Applied Biosystems predesigned TaqMan Gene Expression Assays were used: TRPC1-Rn00585625_m1, TRPC2-Rn00575304_m1, TRPC3-Rn00572928_m1, TRPC4-Rn00584835_m1, TRPC5-Rn00590142_m1, TRPC6-Rn00677564_m1, TRPC7-Rn01448763_m1, and GAPDH-Rn01462662_g1.
Single-cell intracellular calcium measurements.
For measurements of [Ca2+]i, cells were incubated in Krebs Ringer buffer with 2 μM fura-2 AM at room temperature for 60 min. For sodium-free experiments, NaCl was replaced by N-methyl-d-glucamine (NMDG), and pH was brought to 7.4 by adding HCl. Coverslips with cells were then washed with Krebs-Ringer buffer and mounted on the stage of an Observer-D1 microscope (Carl Zeiss, Oberkochen, Germany) attached to an ORCA-ER camera (Hamamatsu Photonics, Hamamatsu City, Japan) and a Lambda 10-B filter wheel (Sutter, Novato, CA). Hardware control and image analysis was performed using Metafluor software (Molecular Devices, Downingtown, PA). Cells were examined under an oil immersion objective during exposure to alternating 340- and 380-nm light beams, and the intensity of light emission at 520 nm was measured. The ratio of light intensities, F340/F380, which reflects changes in [Ca2+]i, was followed in several single cells simultaneously at the rate of one point per second.
Electrophysiological recordings.
Membrane potentials and whole cell currents were measured using the amphotericin perforated patch-clamp technique. During the experiments, the dishes with cell cultures were perfused continuously with an extracellular solution containing the following (in mM): 150 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, 10 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid, and 10 glucose. The pH was adjusted to 7.3 with NaOH. Patch pipettes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL) and heat-polished to a tip resistance of 5–7 MΩ. The pipette solution contained the following (in mM): 90 K-aspartate, 50 KCl, 3 MgCl2, and 10 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid. The pH was adjusted to 7.2 with KOH. Prior to measurement, amphotericin B was added to the pipette solution from a stock solution (20 mg/ml in dimethyl sulfoxide, always freshly prepared) to obtain a final concentration of 200 μg/ml. Current clamp and voltage clamp recordings were performed using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Recordings were started when the series resistance dropped <100 MΩ for current clamp or 40 MΩ for voltage clamp recordings. Series resistance was compensated to >50%. Membrane potentials were corrected online for a liquid junction potential of 9.9 mV. Drugs dissolved to final concentration in extracellular solution were delivered to the recording chamber by a gravity-driven microperfusion system (ALA Scientific Instruments, Westbury, NY) with a common outlet.
RESULTS
Forskolin stimulates cyclic nucleotide production and Ca2+ influx.
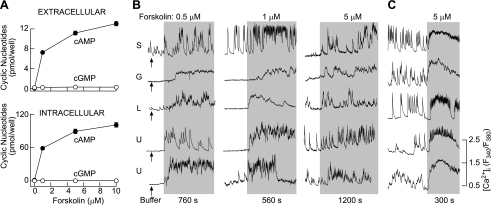
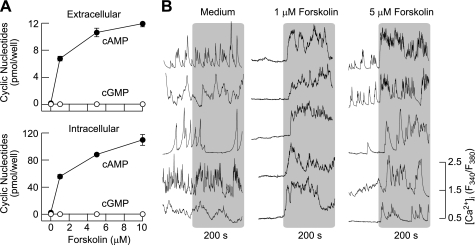
In rat pituitary cells bathed in medium containing 1 mM isobutylmethylxantine, a common inhibitor of phosphodiesterases, forskolin-stimulated cAMP intracellular accumulation and release occurred in a concentration-dependent manner, whereas the effects on cGMP accumulation were modest (Fig. 1A). Most of the cAMP produced de novo was intracellular, and residual cAMP was released into the bath medium. At a 10 μM concentration of forskolin, there was a 780-fold increase in intracellular cAMP accumulation and a 150-fold increase in cAMP released into the bath medium compared with levels of cAMP in the absence of forskolin. In contrast, there was only a two- to threefold increase in cGMP intracellular accumulation and release. Forskolin also stimulated cAMP accumulation and release in mouse anterior pituitary cells, with the profile highly comparable with that observed in rat pituitary cells (Fig. 2A).
Fig. 1.
Concentration-dependent effect of forskolin on cyclic nucleotide production and calcium signaling in normal (A and B) and GH3 immortalized (C) rat anterior pituitary cells. A: forskolin-stimulated cAMP and 3′,5′-cyclic monophosphate (cGMP) intracellular accumulation and release in cells with inhibited phosphodiesterases in the presence of 1 mM isobutylmethylxanthine. Cells were stimulated for 30 min at 37°C. Data shown are means ± SE from 6 determinations. B: forskolin-stimulated calcium transients in pituitary cells. The addition of medium was used as a control (arrows at left). C: forskolin-stimulated calcium influx in GH3 cells. In B and C, traces shown are representative for 5–7 experiments with ≥15 single-cell recordings/experiment. Gray areas indicate the duration of forskolin stimulation. S, somatotrophs; G, gonadotrophs; L, lactotrophs; U, unidentified cells. Cells were identified as described in materials and methods.
Fig. 2.
Concentration-dependent effect of forskolin on cyclic nucleotide production and Ca2+ influx in mouse anterior pituitary cells. A: concentration dependence of forskolin on cAMP and cGMP intracellular accumulation and release in normal mouse pituitary cells. Data shown are means ± SE from 6 determinations. Experimental conditions were as described in Fig. 1. B: stimulatory effect of forskolin on Ca2+ influx in non-lineage-specific pituitary cells. Five representative traces are shown for 5–7 experiments, each with ≥10 single-cell recordings.
The effects of forskolin on Ca2+ signaling were studied in Fura 2-loaded single rat (Fig. 1B) and mouse (Fig. 2B) pituitary cells. About 55% of the rat pituitary cells (240 of 436) exhibited the spontaneous Ca2+ transients, whereas the residual cells were quiescent and ∼87% of rat GH3 immortalized pituitary cells (183 of 210) were spontaneously active. The addition of Krebs-Ringer buffer did not obviously change the signaling pattern (Fig. 1B, arrows at left, and Fig. 2B, left), in contrast to forskolin. In quiescent cells forskolin initiated [Ca2+]i transients, and in spontaneously active cells it increased the amplitude and/or frequency of the transients. Forskolin-stimulated [Ca2+]i transients were observed in identified gonadotrophs, lactotrophs, and somatotrophs as well as in other unidentified rat pituitary cells in culture (Fig. 1B) and GH3 cells (Fig. 1C). Both spontaneous and forskolin-stimulated [Ca2+]i transients were abolished by the removal of bath Ca2+ (data not shown), indicating that forskolin promotes Ca2+ influx. The stimulatory effect of forskolin on Ca2+ influx was observed at low (0.5 μM) concentrations (18 of 109 cells), and further increases in the forskolin concentration did not obviously change the pattern of Ca2+ signaling but did increase the percentage of responding cells (5 μM forskolin, 71 of 241 cells). In mouse pituitary cells, forskolin also stimulated Ca2+ influx in the same manner (Fig. 2B). These results indicate that the facilitation of Ca2+ influx by forskolin is a common feature of secretory pituitary cells and occurs in ∼30% of cells at higher forskolin concentrations.
Forskolin stimulates Ca2+ influx by activating PKA.
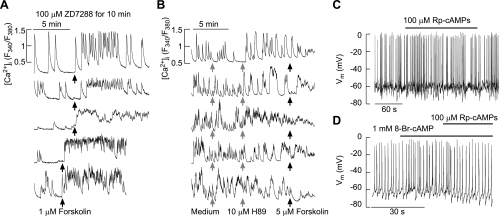
In general, cAMP can stimulate the excitability of pituitary cells directly by activating HCN channels and indirectly by PKA-dependent phosphorylation of some other channels. HCN channels are nonselective cation-conducting channels and are expressed in immortalized pituitary cells (17, 30, 35). However, when expressed in cultured rat pituitary cells, these channels were not critical for spontaneous and forskolin-stimulated Ca2+ influx. Figure 3A shows that spontaneous [Ca2+]i transients were present in cells treated with 100 μM ZD7288, a blocker of these channels. In a fraction of cells ZD7288 had instantaneous stimulatory effects on [Ca2+]i, and in both responders and nonresponders forskolin stimulated Ca2+ influx (35 of 155 cells). Forskolin was also able to stimulate Ca2+ influx in cells bathed in medium containing 1 mM Cs+, another blocker of these channels (76 of 275 cells, traces not shown).
Fig. 3.
Effects of hyperpolarization-activated/cyclic nucleotide-modulated (HCN) channel and PKA inhibitors on forskolin-stimulated calcium influx in non-lineage-specific rat anterior pituitary cells. A: the stimulation of Ca2+ influx by forskolin in cells with HCN channels inhibited by pretreatment with ZD7288, which was applied for 10 min prior to recording. B: the lack of an effect of forskolin on Ca2+ influx after treatment of cells by 10 μM H89, an inhibitor of PKA. Notice the change in the pattern of calcium signaling after addition of H89 (2 top traces). Traces shown are representative of 159 records. C and D: PKA inhibitor Rp-cAMPs did not alter the pattern of spontaneous firing of action potentials (9 of 9 cells; C) but did abolish the 8-Br-cAMP-induced increase in the firing frequency (4 of 4 cells; D). Vm, membrane potential.
To study whether forskolin/cAMP stimulates Ca2+ influx through PKA, we used two inhibitors of these enzymes, H89 and Rp-cAMPs, in cultured rat pituitary cells. At a 10 μM concentration, H89 inhibits PKA to ∼2% of the level observed in controls (6). In 20% of cells (32 of 159), 10 μM H89 stimulated Ca2+ influx, which demonstrates the PKA-independent effects of this compound. The stimulatory effect of H89 on Ca2+ influx was observed in spontaneously active cells (Fig. 3B, left) and quiescent cells (data not shown). In the presence of H89, forskolin was able to facilitate Ca2+ influx in only two of 159 cells. In cells maintained in medium containing 10 μM H89 for ≥30 min, forskolin was also ineffective (25 of 25 cells). Inhibition of PKA by Rp-cAMPs did not affect the basal firing of APs (9 of 9 cells; Fig. 3C) but did inhibit the 8-Br-cAMP-induced increase in the frequency of APs (4 of 4 cells; Fig. 3D).
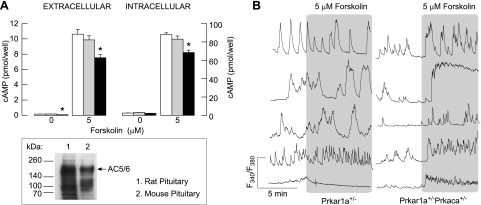
To address the potential role of PKA on Ca2+ influx in pituitary cells more directly, we used Prkar1a+/− and Prkar1a+/−Prkaca+/− mice. In pituitary cells from Prkar1a+/− and Prkar1a+/−Prkaca+/− animals, forskolin also stimulated cAMP intracellular accumulation and release. Forskolin-induced cAMP production was comparable in controls and Prkar1a+/−Prkaca+/− mice but was significantly lower in cells from Prkar1a+/− animals (Fig. 4A, top). The basal PKA activity was also comparable between controls (9,931 ± 1,980 counts·min−1·mg−1 protein) and Prkar1a+/−Prkaca+/− cells (11,462 ± 1,885 counts·min−1·mg−1 protein) but was higher in Prkar1a+/− cells (14,020 ± 2,059 counts·min−1·mg−1 protein; 41% increase). Mouse and rat pituitary cells express AC5/6 (Fig. 4A, bottom), and the phosphorylation of these enzymes by PKA leads to an inhibition of their catalytic activity (40). Thus, it is reasonable to conclude that increased basal PKA activity in Prkar1a+/− cells accounted for the inhibition of cAMP production.
Fig. 4.
Effects of forskolin on cyclic nucleotide production and Ca2+ influx in unidentified single anterior pituitary cells from PKA mice models. A, top: basal and forskolin (5 μM)-stimulated cAMP intracellular accumulation (right) and release (left) in pituitary cells from normal (open bars), Prkar1a+/−Prkaca+/− (gray bars), and Prkar1a+/− mice (black bars); means ± SE from 6 determinations. *Significant differences between normal and Prkar1a+/− cells. A, bottom: Western blot analysis of adenylyl cyclase (AC)5/6 expression in mouse and rat pituitary cells. B: forskolin stimulates Ca2+ influx in anterior pituitary cells from Prkar1a+/−Prkaca+/− but not Prkar1a+/− animals. Traces shown are representative of 5 similar experiments with ≥10 recordings/experiment.
Basal [Ca2+]i level in Prkar1a+/− cells was (F340/F380) 0.51 ± 0.05 (n = 91) and in Prkar1a+/−Prkaca+/− cells was 0.52 ± 0.05 (n = 66), highly comparable with that observed in normal mice pituitary cells (0.47 ± 0.05; n = 118). The percentage of cells exhibiting spontaneous [Ca2+]i transients was also comparable in the three groups: Prkar1a+/− 49 of 91, Prkar1a+/−Prkaca+/− 35 of 66, and controls 60 of 118. At a 5 μM concentration, the forskolin-induced cAMP intracellular accumulation in Prkar1a+/− cells (68 ± 3 pmol/well; Fig. 4A) was still significantly higher than in control cells stimulated with 1 μM forskolin (55 ± 3 pmol/well; Fig. 2A). However, the ability of 5 μM forskolin to initiate Ca2+ influx in Prkar1a+/− cells was almost completely abolished (Fig. 4B, left) in contrast to control cells stimulated with 1 μM forskolin (Fig. 2B) and cells from Prkar1a+/−Prkaca+/− mice (Fig. 4B, right). These results indicate that forskolin facilitates Ca2+ influx in pituitary cells through PKA.
Spontaneous and forskolin-stimulated electrical activity depends on Na+ influx.
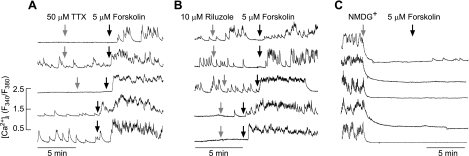
To examine the relevance of Na+ influx in spontaneous and forskolin-stimulated [Ca2+] transients, several experiments were performed. TTX applied for 15 min at a concentration that should block both high- and low-sensitive Nav channels (50 μM) did not change the percentage of cells exhibiting spontaneous [Ca2+]i transients or the pattern of Ca2+ signaling (85 of 85 cells). Furthermore, in both quiescent and spontaneously active cells, 5 μM forskolin increased [Ca2+]i in ∼25% of cells (23 of 85) (Fig. 5A). Riluzole, an inhibitor of the persistent Na+ current, was also unable to inhibit spontaneously in 68 of 68 cells, and forskolin stimulated [Ca2+]i transients in 29 of 108 cells (Fig. 5B). When bath Na+ was substituted with NMDG+, there was a rapid cessation of spontaneous [Ca2+]i transients in pituitary cells (41 of 43 cells), whereas in quiescent cells no obvious change in basal [Ca2+]i was observed (31 of 31 cells). The subsequent application of forskolin was ineffective in the elevation of [Ca2+]i (Fig. 5C).
Fig. 5.
Sodium dependence of forskolin action on Ca2+ influx in rat anterior pituitary cells. A: stimulation of Ca2+ influx by 5 μM forskolin in cells bathed in 50 μM tetrodotoxin (TTX). Gray arrows indicate the moment of TTX application (top 3 traces). In some experiments, TTX was applied for 15 min prior to forskolin application (bottom 2 traces). B: the lack of effects of rilusole, a blocker of leak Na+ channels, on forskolin-stimulated Ca2+ influx. C: inhibition of spontaneous Ca2+ influx by replacing bath Na+ with N-methyl-d-glucamine (NMDG+). Notice the lack of effect of forskolin in cells bathed in NMDG+-containing medium. Traces shown are representative from 4 (A), 4 (B), and 3 experiments (C) with ≥15 recordings/experiment.
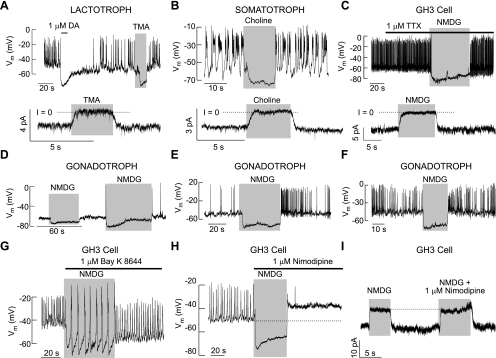
Similarly, the replacement of bath Na+ with organic cations NMDG+, choline+, and tetramethylammonium (TMA+) abolished spontaneous electrical activity caused by cell membrane hyperpolarization. Such an effect was observed in gonadotrophs, lactotrophs, somatotrophs, and other unidentified cells as well as in GH3 cells (in total, 89 of 89 cells; Fig. 6). Consistent with experiments with [Ca2+]i measurements (Fig. 5), TTX did not affect the firing of APs in cultured gonadotrophs, lactotrophs, somatotrophs, or unidentified pituitary cells (in total, 15 of 15 cells). Furthermore, in the presence of TTX, replacement of bath Na+ with NMDG+ also hyperpolarized the cell membrane (Fig. 6C). The hyperpolarizing effect of NMDG+ was also observed in cells with inhibited L-type Cav channels (Fig. 6H). The effect of NMDG+ was not dependent on the status of electrical activity, since it was observed in quiescent cells (Fig. 6D) and spontaneously active cells firing APs with low (Fig. 6E) and high frequencies (Fig. 6F).
Fig. 6.
Dependence of spontaneous electrical activity on the background-depolarizing conductance in single rat pituitary cells. A–C: effects of complete replacement of bath Na+ with organic cations on spontaneous electrical activity (top) and membrane-holding current (bottom) in rat lactotrophs (A), somatotrophs (B), and GH3 cells (C). Traces shown are representative from ≥5 cells/tretament. D–F: independence of the effects of NMDG+ on resting Vm of the electrical status of cells. Experiments were performed in identified gonadotrophs. G–I: the background-depolarizing conductance in GH3 cells is independent of the status of voltage-gated calcium influx. Stimulation of electrical activity by BayK 8644, an L-type of voltage-gated calcium channel (Cav) agonist, in cells bathed in NMDG+-containing medium (G) and the lack of effects of nimodipine on NMDG+-induced hyperpolarization of the cell membrane (H) and the outward-like current (I).
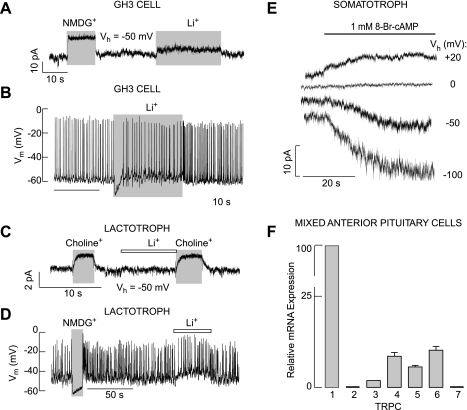
When cells were clamped at −50 mV, close to the resting potentials from which firing of APs occurred, we consistently (54 of 54 cells) observed an outward-like current after replacement of bath Na+ with organic cations, reflecting a decrease in holding membrane current. This response was observed in experiments with TMA+ (Fig. 6A), choline+ (Fig. 6B), and NMDG+ (Fig. 6C) and was not dependent on the status of TTX-sensitive Nav channels (Fig. 6C, bottom), dihydropyridine-sensitive Cav channels (Fig. 6I), or cell type used for studies (Fig. 6, A, B, C, and I). On the other hand, Li+ was able to substitute for Na+ in electrical activity and resting membrane potential. Figure 7A shows only a small change in holding membrane current in GH3 cells after replacement of bath Na+ with Li+ (Fig. 7A) and a transient hyperpolarization (7 of 7 cells; Fig. 7B). In lactotrophs, Li+ was completely able to substitute for Na+ in electrical activity (n = 4; Fig. 7, C and D). A similar effect was observed in other cell types (data not shown).
Fig. 7.
Characterization of the background Na+ conductance. A–D: lithium can substitute for Na+ in the background-depolarizing conductance in GH3 cells (A and B) and lactotrophs (C and D). E: 8-Br-cAMP-stimulated current in voltage-clamped somatotrophs. Horizontal thick line indicates duration of treatments. In A–E, representative traces from 5 similar experiments are shown. F: quantitative RT-PCR analysis of TRPC-mRNA expression in rat pituitary cells. Data shown are means ± SE from 6 experiments expressed as normalized values, using TRPC1-mRNA expression as 100%. Vh, holding potential.
To study the effects of cyclic nucleotides on electrical activity and Ca2+ influx more directly, voltage-clamped rat somatotrophs were stimulated with 8-Br-cAMP. The amplitude of the 8-Br-cAMP-induced current was larger at a more negative potential, and the reversal potential for this current was ∼0 mV (Fig. 7E). These results indicate that a nonselective cation-conducting channel contributes to forskolin-stimulated [Ca2+]i transients, presumably by facilitating Na+ and Ca2+ influx.
TRPC channels are the largest family of cation-conducting channels, and their activation leads to the depolarization of cells due to Na+ and Ca2+ influx (4). To evaluate the expression of TRPC channels in rat pituitary cells, quantitative RT-PCR analysis was performed as described in materials and methods. This analysis revealed the high expression of mRNA transcripts for TRPC1 and the lower expression of TRPC6 in these cells. TRPC3, TRPC4, and TRPC5 mRNA transcripts were also present in pituitary cells (Fig. 7F).
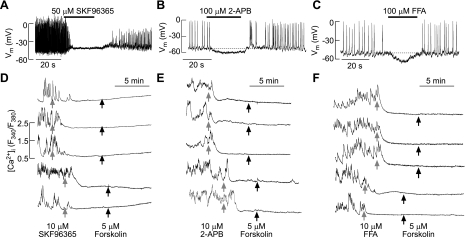
We also used two inhibitors of these channels, SKF96365 and 2-aminoethyl diphenyl-borinate (2-APB). When added to Na+-containing bath medium, these compounds rapidly abolished spontaneous electrical activity in GH3 cells (Fig. 8, A and B) and spontaneous [Ca2+]i transients in normal unidentified rat pituitary cells (Fig. 8, D and E). Furthermore, in SKF96365 and 2-APB-treated cells there was no spontaneous recovery of [Ca2+]i transients, and forskolin treatment was ineffective (94 of 97 cells and 105 of 111 cells; Fig. 8, D and E, respectively). Frequently, both compounds elevated [Ca2+]i levels. In the case of SKF96365, baseline [Ca2+]i levels increased slowly (Fig. 8D), whereas in 2-AP-treated cells, a rapid increase in [Ca2+]i levels was observed, followed by abolition of spiking (Fig. 8E). These effects were also observed in quiescent cells, where forksolin was unable to stimulate [Ca2+]i transients (data not shown). flufenamic acid, a blocker of nonselective cation channels, was also able to abolish spontaneous electrical activity in GH3 cells due to hypeprolarization of the cell membrane (3 of 3 cells; Fig. 8F), and spontaneous [Ca2+]i transients in unidentified pituitary cells (79 of 82 cells; Fig. 8F) and further application of forskolin had no effect. These results indicate that cAMP simulates nonselective cation channels, presumably the TRPC channels, in a PKA-dependent manner.
Fig. 8.
Effects of inhibitors of nonselective cationic channels on spontaneous and forskolin-stimulated electrical activity and Ca2+ influx in single rat anterior pituitary cells. A–C: effects of 1-[2-(4-methoxyphenyl)-2–3-(4-methoxyphenyl)propoxy-ethyl]-1H-imidazole, monohydrochloride (SKF96365; A), 2-aminoethyl diphenyl-borinate (2-APB; B), and flufenamic acid (FFA; C) on spontaneous electrical activity and calcium signaling in GH3 cells. Traces shown are representative form ≥3 recordings. D–F: effects of SKF96365 (D), 2-APB (E), and FFA (F) on spontaneous and forskolin-stimulated Ca2+ influx in unidentified rat pituitary cells. Traces shown are representative from 8 (D), 6 (E), and 5 experiments (F), with ≥15 recordings/experiment.
DISCUSSION
Here, we show that spontaneous Ca2+ influx was observed in about 55% of primary mouse and rat pituitary cells and that forskolin at high concentrations stimulates Ca2+ influx in about 30% of cells. We further show that both spontaneous and forskolin-stimulated electrical activity and Ca2+ influx depend on background Na+ conductance, and such conductance is operative not only in somatotrophs but also in gonadotrophs, lactotrophs, and other unidentified pituitary cell types as well as in immortalized GH3 pituitary cells. The main focus of this work is on the mechanism by which cAMP stimulates electrical activity and Ca2+ influx in endocrine pituitary cells and the nature of the background Na+ conductance.
In general, forskolin-stimulated cAMP production could facilitate electrical activity and Ca2+ influx directly by stimulating HCN channels or indirectly through the cAMP-dependent signaling molecules PKA and/or Epac. Immortalized pituitary cells and rat somatotrophs express HCN channels (17, 30, 35), which conduct Na+ in addition to K+ and Ca2+ (5). The pharmacological identification of these channels is based on the application of several inhibitors, including extracellular Cs+ and ZD7288. However, in our experiments forskolin was also able to stimulate Ca2+ influx in cells treated with ZD7288 for 10–20 min as well as in cells bathed in 1 mM Cs+-containing medium, indicating that cAMP-stimulated HCN channels are not critical for this action.
PKA is a tetramer consisting of two regulatory (R) and two catalytic (C) subunits. Binding of cAMP to regulatory subunits alters its affinity for catalytic subunits, which leads to dissociation into a dimer of regulatory subunits and two active monomeric catalytic subunits responsible for phosphorylation of target proteins (34). To date, four types of regulatory subunits (RIα, RIIα, RIβ, and RIIβ) and four types of catalytic subunits (Cα, Cβ, Cγ, and PRKX) have been identified as separate gene products, and the various configurations of these subunits result in two types of PKA (I and II) based on their patterns of chromatographic elution (2). Both enzymes are inhibited by H89; in vitro, the residual PKA activity is about 2% in the presence of 10 μM H89 (6). When intact pituitary cells were treated with 10 μM H89 for 3–4 min, forskolin-stimulated Ca2+ influx was inhibited, an observation consistent with earlier studies (12, 22). This finding does not necessarily support the hypothesis that PKA is responsible for this effect because H89 in a 10 μM concentration also inhibits S6K1 (100%), ROCK-II (100%), MSK1 (97%), PKBα (83%), AMPK (81%), CHK1 (79%), SGK (75%), and PHK (49%) (6). Here, we have also shown that the application of H89 causes a transient stimulation of Ca2+ influx, a finding that is consistent with observations in other cell types (31) and indicates the nonspecific effect of this compound. Experiments with Rp-cAMPs also showed that spontaneous electrical activity in pituitary cells is not affected by the inhibition of PKA, whereas an 8-Br-cAMP-stimulated increase in the frequency of APs was abolished in the presence of this compound.
The PKA dependence of forskolin-stimulated Ca2+ influx was also analyzed in recently developed PKA mouse models Prkar1a+/− and Prkar1a+/−Prkaca+/− (15, 37). RIα has a nearly ubiquitous distribution, and the downregulation of this subunit by up to 70% leads to a concomitant increase in kinase activity and increased cell proliferation, which causes endocrine and other tumors (8, 9). Mouse Prkar1a haploinsufficiency alone does not cause tumors except in selected tissues, but never in the pituitary gland (15). Here, we showed that basal and forskolin-stimulated cAMP production are significantly reduced in pituitary cells from Prkar1a+/− mice. We further showed that these cells express AC5/6, whose phosphorylation by PKA causes an inhibition of the enzyme activity. This observation indirectly suggests that Prkar1a haploinsufficiency is accompanied by elevated PKA activity, a hypothesis we confirmed by the direct measurement of PKA activity. The Prkar1a haploinsufficiency also caused the loss of stimulatory action of the forskolin on Ca2+ influx. In contrast, basal and stimulated PKA activity were normalized in cells from Prkar1a+/−Prkaca+/− mice, and the stimulatory action of forskolin on Ca2+ influx was restored.
In cells bathed in medium containing organic cations NMDG+, TMA+, or choline+, instead of Na+, forskolin was unable to stimulate Ca2+ influx, indicating that Na+ influx is critical for spontaneous and cAMP-PKA-stimulated Ca2+ influx. We also show that Li+ substitutes for Na+ in electrical activity. In neurons, TTX-sensitive Nav channels are critical for the development of the depolarizing phase of APs. All endocrine pituitary cells also express TTX-sensitive Nav channels (39), but a large fraction of these channels are inactivated at the resting membrane potential (39), and they do not participate in spontaneous electrical activity (32). It is also unlikely that the persistent Na+ current accounts for spontaneous and forskolin-stimulated [Ca2+]i transients because riluzole, a blocker of this current, was ineffective. Others have shown the importance of low TTX-sensitive Nav channels in GHRH-induced electrical activity in somatotrophs, but PKC rather than PKA accounted for the GHRH-induced facilitation of this current (42). Consistent with the finding of this group, we show here that it is unlikely that low TTX-sensitive Nav channels mediate this conductance, because TTX in 50 μM concentrations did not block spontaneous and forskolin-stimulated Ca2+ influx. In accordance with experiments by others (22, 33), we also observed that 8-Br-cAMP stimulated an inward nonselective current with larger amplitude at more negative potentials. The reversal potential of this current was consistent with the operation of a nonselective cation channel.
Our pharmacological studies further indicate that the cation-conducting TRP channels, presumably the TRPC family of channels (4), are good candidates for this conductance. Spontaneous electrical activity and [Ca2+]i transients were abolished by the application of two blockers of these channels, SKF96365 and 2-APB, and in the presence of SKF96365 and 2-APB, forskolin was unable to facilitate Ca2+ influx. Our quantitative RT-PCR analysis revealed that mRNA transcripts for TRPC channels are expressed in anterior pituitary cells in the following order: TRPC1 >> TRPC6 > TRPC4 > TRPC5 > TRPC3. Qualitative RT-PCR analysis by others also showed the presence of TRPC transcripts in pituitary cells (7, 27, 41). All members of this family of channels other than TRPC1 have consensus PKA phosphorylation sites, and TRPC6 is phosphorylated by PKA and associated with other PKA substrates (10).
Consistent with the role of the background Na+ current in the control of resting membrane potential, some TRPC channels are constitutively active (24, 36). Furthermore, in interstitial cells of Cajal, TRPC4 was suggested as a molecular candidate for the nonselective cation channel responsible for the pacemaker activity (14). In the entorhinal cortex, TRPC cation channels mediating persistent muscarinic currents contribute significantly to the firing and mnemonic properties of projection neurons (44). In guinea pig kisspeptin neurons (25) and prooipiomelanocirtin neurons (26), leptin depolarizes plasma membrane via activation of TRPC channels. These channels also contribute to kisspeptin-induced depolarization of gonadotropin-releasing hormone neurons (43).
Together, these results suggest that Na+-conducting nonselective cation channels are constitutively active in pituitary cells and contribute to the control of pacemaking by setting the resting membrane potential. The conductivity of these channels is facilitated by cAMP in a PKA-dependent manner, providing an effective pathway for Gs-coupled receptors to facilitate Ca2+ influx and secretion. These observations do not argue against the role of other channels, such as resistant Nav, HCN, Cav, and K+ channels (3, 11, 19, 20, 30, 35), in cAMP-PKA-mediated facilitation of the excitability of pituitary cells but rather suggest that multiple channels could be used as pathways for the stimulatory action of Gs-coupled receptors on Ca2+ influx and secretion in pituitary cells. Further studies are needed to identify cation-conducting channels, presumably member(s) of the TRPC family of proteins, contributing to background and PKA-stimulated Na+ influx.
GRANTS
This work was supported by the Intramural Research Program of the National Institute of Child Health and Human Development.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We are thankful to Drs. Fred Van Goor and Hana Zemkova for help with some experiments.
REFERENCES
- 1. Almeida MQ, Muchow M, Boikos S, Bauer AJ, Griffin KJ, Tsang KM, Cheadle C, Watkins T, Wen F, Starost MF, Bossis I, Nesterova M, Stratakis CA. Mouse Prkar1a haploinsufficiency leads to an increase in tumors in the Trp53+/− or Rb1+/− backgrounds and chemically induced skin papillomas by dysregulation of the cell cycle and Wnt signaling. Hum Mol Genet 19: 1387–1398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bossis I, Stratakis CA. Minireview: PRKAR1A: normal and abnormal functions. Endocrinology 145: 5452–5458, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Chen C, Clarke IJ. Modulation of Ca2+ influx in the ovine somatotroph by growth hormone-releasing factor. Am J Physiol Endocrinol Metab 268: E204–E212, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev 57: 427–450, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Craven KB, Zagotta WN. CNG and HCN channels: two peas, one pod. Annu Rev Physiol 68: 375–401, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res 26: 159–178, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Griffin KJ, Kirschner LS, Matyakhina L, Stergiopoulos S, Robinson-White A, Lenherr S, Weinberg FD, Claflin E, Meoli E, Cho-Chung YS, Stratakis CA. Down-regulation of regulatory subunit type 1A of protein kinase A leads to endocrine and other tumors. Cancer Res 64: 8811–8815, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Griffin KJ, Kirschner LS, Matyakhina L, Stergiopoulos SG, Robinson-White A, Lenherr SM, Weinberg FD, Claflin ES, Batista D, Bourdeau I, Voutetakis A, Sandrini F, Meoli EM, Bauer AJ, Cho-Chung YS, Bornstein SR, Carney JA, Stratakis CA. A transgenic mouse bearing an antisense construct of regulatory subunit type 1A of protein kinase A develops endocrine and other tumours: comparison with Carney complex and other PRKAR1A induced lesions. J Med Genet 41: 923–931, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hassock SR, Zhu MX, Trost C, Flockerzi V, Authi KS. Expression and role of TRPC proteins in human platelets: evidence that TRPC6 forms the store-independent calcium entry channel. Blood 100: 2801–2811, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Kato M. Growth hormone-releasing hormone augments voltage-gated Na+ current in cultured rat pituitary cells. Am J Physiol Cell Physiol 270: C125–C130, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Kato M, Hattori MA, Suzuki M. Inhibition by extracellular Na+ replacement of GRF-induced GH secretion from rat pituitary cells. Am J Physiol Endocrinol Metab 254: E476–E481, 1988 [DOI] [PubMed] [Google Scholar]
- 13. Kato M, Suzuki M. Growth hormone releasing factor depolarizes rat pituitary cells in Na+-dependent mechanism. Brain Res 476: 145–148, 1989 [DOI] [PubMed] [Google Scholar]
- 14. Kim BJ, So I, Kim KW. The relationship of TRP channels to the pacemaker activity of interstitial cells of Cajal in the gastrointestinal tract. J Smooth Muscle Res 42: 1–7, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Kirschner LS, Kusewitt DF, Matyakhina L, Towns WH, 2nd, Carney JA, Westphal H, Stratakis CA. A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res 65: 4506–4514, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Koshimizu TA, Tomić M, Wong AO, Zivadinovic D, Stojilkovic SS. Characterization of purinergic receptors and receptor-channels expressed in anterior pituitary cells. Endocrinology 141: 4091–4099, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Kretschmannova K, Gonzalez-Iglesias AE, Tomić M, Stojilkovic SS. Dependence of hyperpolarisation-activated cyclic nucleotide-gated channel activity on basal cyclic adenosine monophosphate production in spontaneously firing GH3 cells. J Neuroendocrinol 18: 484–493, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Kucka M, Kretschmannova K, Murano T, Wu CP, Zemkova H, Ambudkar SV, Stojilkovic SS. Dependence of multidrug resistance protein-mediated cyclic nucleotide efflux on the background sodium conductance. Mol Pharmacol 77: 270–279, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuryshev YA, Haak L, Childs GV, Ritchie AK. Corticotropin releasing hormone inhibits an inwardly rectifying potassium current in rat corticotropes. J Physiol 502: 265–279, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee AK, Tse A. Mechanism underlying corticotropin-releasing hormone (CRH) triggered cytosolic Ca2+ rise in identified rat corticotrophs. J Physiol 504: 367–378, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lussier BT, French MB, Moor BC, Kraicer J. Free intracellular Ca2+ concentration and growth hormone (GH) release from purified rat somatotrophs. III. Mechanism of action of GH-releasing factor and somatostatin Endocrinology 128: 592–603, 1991 [DOI] [PubMed] [Google Scholar]
- 22. Naumov AP, Herrington J, Hille B. Actions of growth-hormone-releasing hormone on rat pituitary cells: intracellular calcium and ionic currents. Pflugers Arch 427: 414–421, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Nesterova M, Yokozaki H, McDuffie E, Cho-Chung YS. Overexpression of RII beta regulatory subunit of protein kinase A in human colon carcinoma cell induces growth arrest and phenotypic changes that are abolished by site-directed mutation of RII beta. Eur J Biochem 235: 486–494, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Nichols RA, Dengler AF, Nakagawa EM, Bashkin M, Paul BT, Wu J, Khan GM. A constitutive, transient receptor potential-like Ca2+ influx pathway in presynaptic nerve endings independent of voltage-gated Ca2+ channels and Na+/Ca2+ exchange. J Biol Chem 282: 36102–36111, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Qiu J, Fang Y, Bosch MA, Rønnekleiv OK, Kelly MJ. Guinea pig kisspeptin neurons are depolarized by leptin via activation of TRPC channels. Endocrinology 152: 1503–1514, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci 30: 1560–1565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Riccio A, Mattei C, Kelsell RE, Medhurst AD, Calver AR, Randall AD, Davis JB, Benham CD, Pangalos MN. Cloning and functional expression of human short TRP7, a candidate protein for store-operated Ca2+ influx. J Biol Chem 277: 12302–12309, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Sankaranarayanan S, Simasko SM. A role for a background sodium current in spontaneous action potentials and secretion from rat lactotrophs. Am J Physiol Cell Physiol 271: C1927–C1934, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Simasko SM. A background sodium conductance is necessary for spontaneous depolarizations in rat pituitary cell line GH3. Am J Physiol Cell Physiol 266: C709–C719, 1994 [DOI] [PubMed] [Google Scholar]
- 30. Simasko SM, Sankaranarayanan S. Characterization of a hyperpolarization-activated cation current in rat pituitary cells. Am J Physiol Endocrinol Metab 272: E405–E414, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Song SK, Choi SY, Kim KT. Opposing effects of protein kinase A and C on capacitative calcium entry into HL-60 promyelocytes. Biochem Pharmacol 56: 561–567, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Stojilkovic SS, Tabak J, Bertram R. Ion channels and signaling in the pituitary gland. Endocr Rev 31: 845–915, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takano K, Takei T, Teramoto A, Yamashita N. GHRH activates a nonselective cation current in human GH-secreting adenoma cells. Am J Physiol Endocrinol Metab 270: E1050–E1057, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem 59: 971–1005, 1990 [DOI] [PubMed] [Google Scholar]
- 35. Tian L, Shipston MJ. Characterization of hyperpolarization-activated cation currents in mouse anterior pituitary, AtT20 D16:16 corticotropes. Endocrinology 141: 2930–2937, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Trebak M, Vazquez G, Bird GS, Putney JW., Jr The TRPC3/6/7 subfamily of cation channels. Cell Calcium 33: 451–461, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Tsang KM, Starost MF, Nesterova M, Boikos SA, Watkins T, Almeida MQ, Harran M, Li A, Collins MT, Cheadle C, Mertz EL, Leikin S, Kirschner LS, Robey P, Stratakis CA. Alternate protein kinase A activity identifies a unique population of stromal cells in adult bone. Proc Natl Acad Sci USA 107: 8683–8688, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tse A, Hille B. Role of voltage-gated Na+ and Ca2+ channels in gonadotropin-releasing hormone-induced membrane potential changes in identified rat gonadotropes. Endocrinology 132: 1475–1481, 1993 [DOI] [PubMed] [Google Scholar]
- 39. Van Goor F, Zivadinovic D, Stojilkovic SS. Differential expression of ionic channels in rat anterior pituitary cells. Mol Endocrinol 15: 1222–1236, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev 87: 965–1010, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Yamashita M, Oki Y, Iino K, Hayashi C, Yogo K, Matsushita F, Sasaki S, Nakamura H. The role of store-operated Ca2+ channels in adrenocorticotropin release by rat pituitary cells. Regul Pept 156: 57–64, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Yang SK, Wang K, Parkington H, Chen C. Involvement of tetrodotoxin-resistant Na+ current and protein kinase C in the action of growth hormone (GH)-releasing hormone on primary cultured somatotropes from GH-green fluorescent protein transgenic mice. Endocrinology 149: 4726–4735, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Zhang C, Roepke TA, Kelly MJ, Ronnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 28: 4423–4434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang Z, Reboreda A, Alonso A, Barker PA, Séguéla P. TRPC channels underlie cholinergic plateau potentials and persistent activity in entorhinal cortex. Hippocampus 21: 386–397, 2010 [DOI] [PubMed] [Google Scholar]