Cancer immunotherapy relies on the ability of the immune system to identify and destroy tumor cells and to elicit a long-lasting memory of this interaction. Under ordinary circumstances, however, the ability of tumor cells to trigger an effective immune response is limited. The nominal poor immunogenicity of tumor cells results in part from their weak expression of MHC antigens, adhesion molecules, and costimulatory signals that could allow complete T-cell activation. Tumors may also secrete immunosuppressive molecules, such as IL-10, TGF-β, and PGE2, and they often fail to express cytokines that activate local immune responses (1). These evasive strategies can be overcome by introducing immunomodulatory molecules or genes into the tumor milieu. Over the last 2 decades, researchers have sought to identify cytokines and chemokines that induce the maturation, activation, and migration of inflammatory cells and have used these factors to activate the immune system against tumor cells. Most of this work has been conducted in animals, but results have been encouraging enough that several human trials have been initiated.
Use of cytokines and cytokine genes to boost antitumor responses
The concept that cytokines, such as IFNs and ILs, can enhance immunogenicity and promote tumor regression has lead to increasing interest in their study and clinical application. Clinical trials using IFN or IL-2 have indicated that objective antitumor response could be elicited by systemic administration of exogenous cytokines. For instance, Panelli and Marincola recently analyzed the records of 283 patients with cancer who were treated with high doses of IL-2, and they showed that IL-2 induced a complete response in 9% of patients with renal cell carcinoma and 7% of those with melanoma (2). Other studies have confirmed the observation that systemic and repeated administration of high doses of IL-2 result in often dramatic tumor regression. Cytokines profoundly affect inflammatory cells, and they activate immune responses by multiple mechanisms, but their systemic use is limited by substantial toxicity and effectiveness of circulating cytokines is blunted by rapid degradation and elimination. Local delivery of cytokines was proposed to address these drawbacks. These factors are well suited to local delivery because their biologic activities generally arise from paracrine effects. So delivered, cytokines can promote T-cell responses against weak immunogenic tumor antigens, activate nonspecific killing by natural killer (NK) cells, lymphokine-activated killer cells, monocytes, and macrophages and can enhance presentation of tumor antigens. Indeed, repeated local administration of exogenous cytokines directly into the tumor or in the vicinity of draining lymph nodes appears to promote tumor rejection (3). However, to elicit continuous local secretion of cytokines, gene immunotherapy seems more promising than repeated injections of the protein.
Introduction of cytokine genes into tumor cells, an approach described as ex vivo gene therapy, allows the sustained local release of cytokines capable of enhancing the intensity and quality of the immune response to tumor. Studies in various mouse tumor models have established that administration into syngenic hosts of tumor cells engineered to secrete IL-1, -2, -4, -6, -7, 12, -18, as well as TNF-α, G-CSF, GM-CSF, or IFN-γ, can lead to tumor rejection by stimulating both specific and nonspecific antitumor responses. Rejection depends on a high level of cytokine production by the gene-modified cells and is due in part to stimulation of host antitumor effector response (4, 5). In some circumstances, altering the immunological environment of the tumor allowed the complete rejection of a tumor inoculum and even protected the host against subsequent challenge with unmodified tumor cells (6).
Acute rejection of modified tumor cells probably involves a combination of specific and nonspecific immune mechanisms (3). The induction of antitumor immunity is not completely understood, but it is likely that the various cytokines selectively elicit recruitment of granulocytes, macrophages, dendritic cells, and NK or T cells. T cells are the most potent effectors in the host antitumor response, and systemic antitumor immunity induced by cytokines depends for the most part on CD8+ or CD4+ T cells. Cytokine gene therapy focuses on the harvesting and activation of these cells, especially CD8+ cytotoxic T lymphocytes, which mediate specific responses to tumor cells and can persist even after the initial tumor inoculum is cleared. The importance of nonspecific immune responses in the genesis of an effective adoptive immune response is most clearly demonstrated by the efficacy of IL-2 for stimulating NK cells to become lymphokine-activated killer cells, which can nonspecifically lyse NK-resistant cell lines (7).
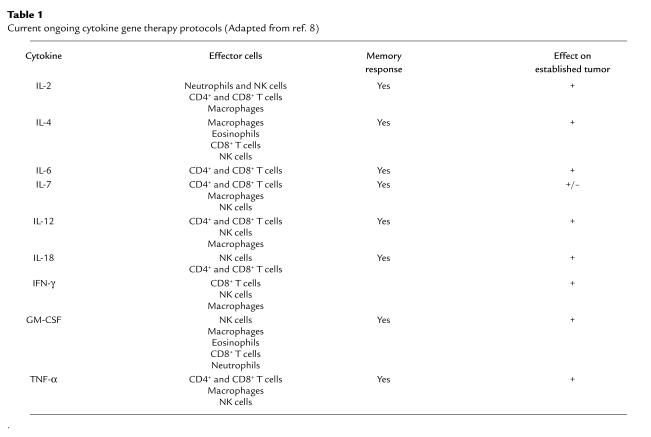
Table 1 summarizes the reported results of the efficacy of various cytokine secreting tumor vaccines in animal models (8), as assessed by studying the cellular infiltrates in regressing tumors, assaying cytotoxic T lymphocytes, and monitoring long-term resistance to tumor growth. The first experimental models and clinical applications of cytokine gene therapy used IL-2 (9). In most experimental systems, the expression of IL-2 by weakly immunogenic tumor cells resulted in growth inhibition of tumor mass. The inhibitory effect is dose dependent, and the degree of suppression of growth correlates directly with the amount of IL-2 produced by the tumor cells (10). Transgenes encoding several other ILs (IL-1, IL-4, IL-6, IL-7, IL-12, and IL-13), IFNs (IFN-γ and IFN-α), hematopoietic growth factors (GM-CSF, G-CSF, and M-CSF), and TNF have also shown promising tumoricidal effects, apparently by different effector mechanisms than IL-2. Although rejection of established tumors occurred in only some of these experimental systems (11), these promising results established the basic biologic value of gene therapy with cytokine-secreting cells that are now applied in clinical trials. More than 60% of recent cytokine-supported clinical cancer gene therapy trials use IL-2; in others, IL-4, -7, and -12; IFN-γ; GM-CSF; or TNF are administered individually or in combination (12).
Table 1.
Current ongoing cytokine gene therapy protocols (Adapted from ref. 8)
Limitations of ex vivo cytokine gene therapy
Despite the considerable interest in this approach, ex vivo engineering of autologous cells suffers from several major drawbacks. Isolation of primary autologous cells that stably express high levels of the therapeutic gene is not only cumbersome and expensive, but also poorly reproducible. Batch-to-batch variation of engineered cells complicates analysis of the biologic effects observed in each patient. Furthermore, implantation of mouse tumor cells into syngenic hosts only imperfectly mimics the biology of spontaneous human cancers (13). In fact, the therapeutic efficacy of cytokine gene-transduced tumor cells is low. In most cases, only a minority of tumor-bearing mice can be cured by administration of cytokine gene transduced tumor cells, especially under conditions that mimic the way these agents would be used in the clinic. The limited efficacy of these vaccines was completely lost if they were not administered in the first few days after implantation of tumor cells. Therapeutic immunization has shown no consistent effect on growth of established tumors beyond the period of concomitant immunity. The situation is slightly more encouraging in the case of micrometastases; a significant reduction in tumor metastasis has been documented with application of several cytokines. Once again, however, only a minority of the mice are cured. A similar picture is emerging from phase I studies of vaccination of patients with cancer with transduced human tumor cells. Even though the approach itself is safe, the available results show that only about 10% of patients displayed an objective response (14–16).
Some of the limitations of ex vivo gene therapy may be addressed by use of viral vectors for intralesional in vivo gene transfer. A number of studies have demonstrated the effectiveness of directly transducing established tumors with cytokine genes using retroviral vectors (17). Similarly, adenovirus-mediated in vivo transduction of tumors with cytokine genes has stimulated significant systemic antitumor response (18).
Strategies to enhance cancer immunomodulation
Combination cytokine therapy.
In a physiological immune response, cytokines are not produced singly, but in succession, and multiple cytokines may be required to ensure an effective response. Several recent studies have reported greater therapeutic activity using vaccines consisting of tumor cells transduced with multiple genes when compared with single-gene vaccines. Combinations of GM-CSF and IFN-γ (19); IL-2 and IL-4 (20); GM-CSF and IL-4 (21); IFN-γ, IL-4, and IL-6 (22); IL-2 and IL-12 (23); and IL-12, pro–IL-18, and IL-1β–converting enzyme (24) have been shown to significantly augment antitumor effects. The objective of such “multiple” gene therapies is to orchestrate an effective multicellular response. Hence, the order, the type, the dose, and the duration of produced cytokines can have distinct biologic effects in antitumor gene therapy, reflecting in part the complexities of the underlying immune response.
Use of replicating viruses for delivery of cytokines.
For most cytokines, dosage seems to be crucial for eliciting an immune response, and both IL-2 or TNF-α show a more potent antitumor effect when expressed at higher concentrations (25, 26). Likewise, repeated intratumoral injections of IL-2-expressing adenovirus increase the efficiency with which implanted tumor cells are rejected (1). Thus, direct cytokine gene transfer to tumor cells in vivo may have a therapeutic potential, provided that a high-enough transduction efficiency and production can be achieved. Poor tumor penetration has been an intractable problem for other systemically administered macromolecules (27), so nonreplicating vectors may not be suitable for efficient tumor cell gene delivery or high-level expression of transgenes. All other conditions being equal (e.g., dose and route of administration), a replicating vector should deliver genes more efficiently into tumors than a nonreplicating vector owing to local spread from initially transduced cells. As Heise and Kirn (this Perspective Series, ref. 28) discuss, the replication-competent adenovirus-derivative ONYX-015 was developed to specifically replicate in tumor cells that lack functional p53 protein. ONYX-015 achieves a higher transduction efficiency and oncolysis of transduced p53 mutant tumor cells (29) and can also confer a dramatically higher expression of a transgene in transduced cells, relative to a replication-defective version (30).
Despite their promise, ONYX-015 and related adenovirus derivatives are limited by several factors. First, as with other adenoviral vectors, robust immune responses make it impractical to readminister a given viral serotype after an initial infection. Within 24 hours after intravenous administration, 90% of the adenovirus vector is eliminated from the mouse liver, indicating the involvement of innate immune mechanisms (31, 32). A rapid CD8+ cytotoxic T lymphocyte (CTL) response to viral proteins eliminates virus-infected cells, and a long-lasting humoral response to viral epitopes, further limit the readministration of the virus. Initial studies with adenoviral vectors have established an in vivo correlation between serum levels of antiviral antibodies and inhibition of viral transduction (31–33). The immune response to intratumoral delivery is less well defined, but the efficient replication of the ONYX-015 adenoviruses within tumor cells results in increased intratumoral viral titers by 100- to 1,000-fold over the course of 72 hours after intravenous injection (34). This effective replication is expected to elicit both a cellular and humoral response against the viral genes. Unlike the humoral response, which may preclude repeated treatment regimens, CTL-mediated responses to tumor-infected cells may play an important additional role in tumor destruction.
Other potential limitations to the use of ONYX-015 relate to its limited ability to replicate and its restricted range of host cells. This virus appears to spread inefficiently within a tumor mass, relative to a wild-type adenovirus. For the virus to destroy a tumor mass effectively, at least 5% of the tumor cells have to be transduced (34). These observations are confirmed by phase I clinical trials of head and neck cancer, wherein patients receiving five daily doses of ONYX-015 intratumorally were more likely to experience significant tumor necrosis than those receiving a single injection (29). Furthermore, by design, ONYX-015 adenovirus replicates selectively in tumor cells lacking functional p53, yet many human tumors show intratumoral heterogeneity with respect to p53 status (35). As Heise and Kirn (this Perspective Series, ref. 28) note, some of this heterogeneity may be more apparent than real, as there are many epigenetic and genetic changes that can diminish p53 function. Nevertheless, the possibility remains that this virus will not eradicate tumors but merely select for the emergence of p53+ cells
To address the concerns about the limited efficacy of this treatment, it may be helpful to combine the oncolytic ability of the virus with other antitumor modalities. ONYX-015 has been used successfully to deliver suicide genes. The recombinant virus causes tumor regression not only by inducing oncolysis but also by promoting a cytotoxic bystander effect (ref. 36; see also Springer and Niculescu-Duvaz, this Perspective Series, ref. 36). If used to deliver cytokine genes to tumors, this vector should allow high expression of the desired cytokines and should elicit an effective and potent immune response against the virus and the lysed tumor cells. However, at high concentrations, cytokines such as IL-2 and TNF-α are clearly associated with systemic toxicity and fatality (25, 26). These effects do not preclude the use of replication-competent viruses for cytokine gene therapy, but they may necessitate the use of regulatable systems to control cytokine gene expression. We turn in the accompanying article (Agha-Mohammadi and Lotze, this Perspective Series, ref. 37) to the challenge of designing regulated transgene expression for gene therapy vectors. Such regulation may be a key feature of a later generation of replication-competent viruses that will be used for gene delivery into cancer cells.
References
- 1.Leroy P, et al. Cancer immunotherapy by direct in vivo transfer of immunomodulatory genes. Res Immunol. 1998;149:681–684. doi: 10.1016/s0923-2494(99)80038-8. [DOI] [PubMed] [Google Scholar]
- 2.Panelli, M.C., and Marincola, F.M. 1998. Immunotherapy update: from interleukin-2 to antigen-specific therapy. In ASCO educational book. M.C. Perry, editor. American Society of Clinical Oncology. Chicago, Illinois, USA. 467–473.
- 3.Colombo MP, Modesti A, Parmiani G, Forni G. Local cytokine availability elicits tumor rejection and systemic immunity through granulocyte-T-lymphocyte cross-talk. Cancer Res. 1992;52:4853–4857. [PubMed] [Google Scholar]
- 4.Fearon ER, et al. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990;60:397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- 5.Viret C, Lindemann A. Tumor immunotherapy by vaccination with cytokine gene transfected cells. Int Rev Immunol. 1997;14:193–212. doi: 10.3109/08830189709116852. [DOI] [PubMed] [Google Scholar]
- 6.Golumbek PT, et al. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science. 1991;254:713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- 7.Lotze MT, Grimm EA, Mazumder A, Strausser JL, Rosenberg SA. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res. 1981;1:4420–4425. [PubMed] [Google Scholar]
- 8.Peron, J.-M., Shurin, M.R., and Lotze, M.T. 1999. Cytokine gene therapy of cancer. In Gene therapy of cancer. E.C. Lattime and S.L. Gerson, editors. Academic Press. San Diego, California, USA. 359–371.
- 9.Bubenik J, et al. Local administration of cells containing an inserted IL-2 gene and producing IL-2 inhibits growth of human tumours in nu/nu mice. Immunol Lett. 1988;19:279–282. doi: 10.1016/0165-2478(88)90155-1. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt W, et al. Cancer vaccines: the interleukin 2 dosage effect. Proc Natl Acad Sci USA. 1995;92:4711–4714. doi: 10.1073/pnas.92.10.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tahara H, et al. Effective eradication of established murine tumors with IL-12 gene therapy using a polycistronic retroviral vector. J Immunol. 1995;154:6466–6474. [PubMed] [Google Scholar]
- 12.Rosenberg SA, et al. Human gene marker/therapy clinical protocols. Hum Gene Ther. 1999;10:3067–3123. doi: 10.1089/10430349950016465. [DOI] [PubMed] [Google Scholar]
- 13.Haddada H, Ragot T, Cordier L, Duffour MT, Perricaudet M. Adenoviral interleukin-2 gene transfer into P815 tumor cells abrogates tumorigenicity and induces antitumoral immunity in mice. Hum Gene Ther. 1993;4:703–711. doi: 10.1089/hum.1993.4.6-703. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Wahab Z, et al. A phase I clinical trial of immunotherapy with interferon-gamma gene-modified autologous melanoma cells: monitoring the humoral immune response. Cancer. 1997;80:401–412. doi: 10.1002/(sici)1097-0142(19970801)80:3<401::aid-cncr8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 15.Roth JA, Cristiano RJ. Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst. 1997;89:21–39. doi: 10.1093/jnci/89.1.21. [DOI] [PubMed] [Google Scholar]
- 16.Belli F, et al. Active immunization of metastatic melanoma patients with interleukin-2-transduced allogeneic melanoma cells: evaluation of efficacy and tolerability. Cancer Immunol Immunother. 1997;44:197–203. doi: 10.1007/s002620050373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurford RK, Dranoff G, Mulligan RC, Tepper RI. Gene therapy of metastatic cancer by in vivo retroviral gene targeting. Nat Genet. 1995;10:430–435. doi: 10.1038/ng0895-430. [DOI] [PubMed] [Google Scholar]
- 18.Addison CL, et al. Intratumoral injection of an adenovirus expressing interleukin 2 induces regression and immunity in a murine breast cancer model. Proc Natl Acad Sci USA. 1995;92:8522–8526. doi: 10.1073/pnas.92.18.8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bausero MA, Panoskaltsis-Mortari A, Blazar BR, Katsanis E. Effective immunization against neuroblastoma using double-transduced tumor cells secreting GM-CSF and interferon-gamma. J Immunother Emphasis Tumor Immunol. 1996;19:113–124. doi: 10.1097/00002371-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Ohe Y, et al. Combination effect of vaccination with IL2 and IL4 cDNA transfected cells on the induction of a therapeutic immune response against Lewis lung carcinoma cells. Int J Cancer. 1993;53:432–437. doi: 10.1002/ijc.2910530314. [DOI] [PubMed] [Google Scholar]
- 21.Wakimoto H, et al. Intensified antitumor immunity by a cancer vaccine that produces granulocyte-macrophage colony-stimulating factor plus interleukin 4. Cancer Res. 1996;56:1828–1833. [PubMed] [Google Scholar]
- 22.Nishihara K, et al. Increased in vitro and in vivo tumoricidal activity of a macrophage cell line genetically engineered to express IFN-gamma, IL-4, IL-6, or TNF-alpha. Cancer Gene Ther. 1995;2:113–124. [PubMed] [Google Scholar]
- 23.Addison CL, et al. Intratumoral coinjection of adenoviral vectors expressing IL-2 and IL-12 results in enhanced frequency of regression of injected and untreated distal tumors. Gene Ther. 1998;5:1400–1409. doi: 10.1038/sj.gt.3300731. [DOI] [PubMed] [Google Scholar]
- 24.Oshikawa K, et al. Synergistic inhibition of tumor growth in a murine mammary adenocarcinoma model by combinational gene therapy using IL-12, pro-IL-18, and IL-1beta converting enzyme cDNA. Proc Natl Acad Sci USA. 1999;96:13351–13356. doi: 10.1073/pnas.96.23.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Malley BW, Jr, et al. Limitations of adenovirus-mediated interleukin-2 gene therapy for oral cancer. Laryngoscope. 1999;109:389–395. doi: 10.1097/00005537-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Marr RA, Hitt M, Muller WJ, Gauldie J, Graham FL. Tumour therapy in mice using adenovirus vectors expressing human TNFa. Int J Oncol. 1998;12:509–515. doi: 10.3892/ijo.12.3.509. [DOI] [PubMed] [Google Scholar]
- 27.Dvorak HF, Nagy A, Dvorak JT, Dvorak AM. Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am J Pathol. 1988;133:95–109. [PMC free article] [PubMed] [Google Scholar]
- 28.Heise C, Kirn DH. Replication-sensitive adenoviruses as oncolytic agents. J Clin Invest. 2000;105:847–851. doi: 10.1172/JCI9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirn, D. 1999. Selectively replicating viruses as therapeutic agents against cancer. In Gene therapy of cancer. E.C. Lattime and S.L. Gerson, editors. Academic Press. San Diego, California, USA. 235–248.
- 30.Freytag SO, Rogulski KR, Paielli DL, Gilbert JD, Kim JH. A novel three-pronged approach to kill cancer cells selectively: concomitant viral, double suicide gene, and radiotherapy. Hum Gene Ther. 1998;9:1323–1333. doi: 10.1089/hum.1998.9.9-1323. [DOI] [PubMed] [Google Scholar]
- 31.Worgall S, Wolff G, Falck-Pedersen E, Crystal RG. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther. 1997;8:37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Ertl HC, Wilson JM. MHC class I–restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 33.Juillard V, et al. Long-term humoral and cellular immunity induced by a single immunization with replication-defective adenovirus recombinant vector. Eur J Immunol. 1995;25:3467–3473. doi: 10.1002/eji.1830251239. [DOI] [PubMed] [Google Scholar]
- 34.Heise CC, Williams A, Olesch J, Kirn DH. Efficacy of a replication-competent adenovirus (ONYX-015) following intratumoral injection: intratumoral spread and distribution effects. Cancer Gene Ther. 1999;6:499–504. doi: 10.1038/sj.cgt.7700071. [DOI] [PubMed] [Google Scholar]
- 35.Mirchandani D, et al. Heterogeneity in intratumoral distribution of p53 mutations in human prostate cancer. Am J Pathol. 1995;147:92–101. [PMC free article] [PubMed] [Google Scholar]
- 36.Springer CJ, Niculescu-Duvaz I. Prodrug-activating systems in suicide gene therapy. J Clin Invest. 2000;105:1161–1167. doi: 10.1172/JCI10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agha-Mohammadi S, Lotze MT. Regulatable systems: applications in gene therapy and replicating viruses. J Clin Invest. 2000;105:1177–1182. doi: 10.1172/JCI10027. [DOI] [PMC free article] [PubMed] [Google Scholar]