Abstract
Background
Prospective data regarding risk factors for peripheral artery disease (PAD) are sparse, especially among women; the relative contribution of systolic versus diastolic blood pressure control for incident PAD has not been well-studied. We evaluated the association of self-reported blood pressure control with incident symptomatic PAD in middle-aged and older women.
Methods
We examined the relationship between reported hypertension and incident confirmed symptomatic PAD (n=178) in 39,260 female health professionals aged ≥45 years without known vascular disease at baseline. Median follow-up was 13.3 years. Women were grouped according to presence of reported isolated diastolic (IDH), isolated systolic (ISH), or combined systolic-diastolic hypertension (SDH) using cut-points of 90 and 140 mmHg for diastolic and systolic blood pressure, respectively. SBP and DBP were modeled as continuous and categorical exposures. Multivariable-adjusted hazard ratios (HRs), including adjustment for CV risk factors, were derived from Cox proportional hazards models.
Results
Adjusted HRs compared to women without reported hypertension were 1.0 (0.4–2.8) for IDH, 2.0 (1.3–3.1) for ISH, and 2.8 (1.8–4.5) for SDH. There was a 43% increased adjusted risk per 10 mmHg of reported SBP (95% CI 27–62%) and a gradient in risk according to SBP category (<120, 120–139, 140–159, and ≥160 mmHg); HRs were 1.0, 2.3, 4.3, and 6.6 (p-trend<0.001), respectively. Reported DBP, while individually predictive in models excluding SBP, was not predictive after adjustment for SBP.
Conclusions
These prospective data suggest a strong prognostic role for uncontrolled blood pressure and, particularly, uncontrolled systolic blood pressure in the development of peripheral atherosclerosis in women.
Keywords: hypertension, peripheral artery disease, women
Introduction
Lower extremity peripheral artery disease (PAD) is an under-diagnosed condition, currently estimated to affect 8 to 10 million Americans.1–2 Functional impairment due to leg symptoms and limb threatening ischemia are the main clinical manifestations.3 Cardiovascular morbidity is high with an estimated annual major cardiovascular event rate (myocardial infarction, ischemic stroke, or vascular death) of approximately 5–7% with symptomatic patients having the worst prognosis.2, 4 Although the main underlying mechanism is progressive atherosclerotic vascular occlusion, this process may differ in the peripheral vasculature where key features of arterial mechanics including pulsatile characteristics of flow, rheologic effects imposed by arterial branching, and tissue-specific metabolic demands differ from other vascular beds.5
Despite these observations, risk factors for PAD have been less extensively studied than for other manifestations of atherosclerosis. In particular, while hypertension is commonly present at the time of PAD diagnosis, to what degree hypertension contributes to PAD development has not been well-established since most published data derive from cross-sectional reports. Prior prospective studies have been limited, being largely exclusive of women and have generally considered hypertension among a broad range of traditional cardiovascular risk factors.6–16 Furthermore, findings from these prospective investigations are conflicting with many,6–7, 9, 11–15 but not all8, 10, 16 having demonstrated a positive association and few studies7, 9 having evaluated the relative importance of systolic and diastolic blood pressure control.
In light of these issues, we examined whether self-reported blood pressure control is associated with incident symptomatic PAD (intermittent claudication and/or lower extremity revascularization) in a large population of relatively healthy middle-aged and older American women followed on average for 13.3 years. We first evaluated hypertension subtypes according to presence of reported diastolic, systolic, or combined systolic-diastolic hypertension. We then assessed the individual associations of reported systolic and diastolic blood pressure control with future PAD and compared their relative importance in multivariable models.
Methods
Study Population
The Women's Health Study (WHS) is a recently completed randomized trial of low-dose aspirin and vitamin E in primary prevention of cardiovascular disease (CVD) and cancer.17–19 Between November 1992 and July 1995, a total of 39,876 US female health professionals aged ≥ 45 years without prior cancer or cardiovascular disease (including myocardial infarction, stroke, coronary and peripheral arterial revascularization) were enrolled and randomized into the study. Registered nurses comprised 75% of the study population. Additionally, roughly 3% were physicians, dentists, or veterinarians and 15% were licensed practical or vocational nurses. Information on baseline variables was collected using mailed questionnaires. Participants provided their height and weight for body mass index (BMI, kg/m2) estimation and answered questions regarding the presence of physician diagnosed diabetes (yes/no), physician diagnosed hyperlipidemia (yes/no cholesterol ≥ 240 mg/dl), smoking (never/past/current), frequency of physical activity (yes/no vigorous activity ≥ 1× per week), and use of menopausal hormone replacement therapy (HRT) (yes/no). Morbidity and mortality data are available on 99% and 100% of participants, respectively.
Subjects found to have confirmed pre-randomization CVD (n=8) or PAD (n=7) were excluded from the analysis. Participants missing baseline data on self-reported systolic or diastolic blood pressure (n=559), diagnosis of hypertension (n=13), or treatment for hypertension (n=29), were also excluded. The final study population thereby comprised 39,260 women without clinical CVD or PAD and with complete baseline data on blood pressure variables. The median follow-up was 13.3 years. Less than 3% of data were missing for other major vascular risk factors including age, history of diabetes, history of hyperlipidemia, smoking status, BMI, and HRT. Written informed consent was obtained from all women enrolled, and the institutional review board of Brigham and Women's Hospital approved the trial.
Ascertainment of Baseline Blood Pressure
Self-reported blood pressure was obtained from baseline questionnaires. Women were asked to report their current level of systolic and diastolic blood pressure if checked within the prior two years. Blood pressure was reported within categories. For SBP, there were 9 categories in 10 mmHg increments from <110 mmHg to ≥ 180 mmHg; for DBP, 7 categories ranging from <65 mmHg to ≥ 105 mmHg. Blood pressure at baseline was self-reported by WHS participants, all of whom were female health professionals. Self-report of blood pressure has proven highly accurate among health professionals and as validated against directly measured values.20–23
Ascertainment of Incident PAD Events
Participants are surveyed annually for several health outcomes including PAD events, defined as intermittent claudication and/or peripheral arterial (lower extremity) revascularization. Specifically, women were queried on an annual basis: “Since you last returned a questionnaire (approximately 1 year ago), have you been NEWLY DIAGNOSED with intermittent claudication? ”. A similar question was asked regarding peripheral arterial revascularization. As of November 23, 2007, 690 such events were reported among 39,260 women included in this analysis. Case confirmation occurred by telephone interview conducted by a cardiovascular physician during which the Edinburgh Claudication questionnaire was utilized to determine the presence of vascular claudication among women reporting intermittent claudication.24 In addition, medical records were obtained to assess concordance of reported symptoms with diagnostic testing when available. Revascularization procedures were confirmed after review of operative notes or procedural reports in the case of peripheral angioplasty or stenting. A final determination of presence of PAD was made by the physician interviewer after review of claudication symptoms, medical record documentation of diagnostic testing, and procedural reports. Of 690 self-reported PAD events, 178 were confirmed comprising 86 cases of IC only, 7 revascularization only, 85 IC plus revascularization. Among disconfirmed events, venous disease, lower extremity arthritis, lumbar disc disease, and peripheral neuropathy were the main causes of nonischemic leg pain. Only confirmed events were considered in the primary analysis. Additionally, unconfirmed events (n=130) were included in sensitivity analyses conducted to assess for consistency of risk associations.
Statistical Analysis
Women were first grouped according to hypertension subtypes based on presence or absence of reported systolic and diastolic hypertension. Categories were normotensive (SBP <140 and DBP < 90 mmHg), isolated diastolic hypertension (SBP <140 and DBP ≥90 mmHg), isolated systolic hypertension (SBP ≥140 and DBP < 90 mmHg), and systolic-diastolic hypertension (SBP ≥140 and DBP ≥90 mmHg). Subjects who reported being normotensive with concurrent use of anti-hypertensive medications were included in the normotensive category as subsequent PAD risk estimates were similar in this group compared to normotensive subjects without treatment. Differences in baseline characteristics were then compared according to self-reported hypertension subtype. Continuous variables are summarized as mean and standard deviation or median and interquartile range as appropriate based on normality of their distributions. Categorical variables are expressed as proportions. Comparisons of continuous variables across reported hypertension subtypes were performed by ANOVA for difference in means or the Kruskall-Wallis test for difference in medians. Between-group differences were assessed by the Wilcoxon rank sum test. Categorical variables were analyzed using the ×2 test.
Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for incident PAD, according to reported hypertension subtype. Multivariable estimates were derived from models adjusting for age (after natural log transformation), smoking (never, past, current), baseline history of diabetes (yes/no), BMI (linear continuous), history of hyperlipidemia (yes/no cholesterol ≥ 240 mg/dl), randomized treatment with aspirin and/or Vitamin E, and menopausal HRT (yes/no). In secondary analyses, models were additionally adjusted for baseline use of anti-hypertensive and lipid-lowering therapy.
Analyses of self-reported systolic and diastolic blood pressure values were then performed using several approaches. First, to determine the shape of the relationship of SBP and DBP with PAD risk, we fitted models using natural cubic splines to the data after each individual was assigned the mid-point value for their reported blood pressure category. Participants reporting an SBP <110 or ≥ 180 mmHg were given a value of 95 or 190 mmHg, respectively; those with a DBP < 65 or ≥ 110 mmHg were assigned the values 55 or 110 mmHg, respectively. This method allows for a flexible shape in the association of reported SBP and DBP with PAD and has the attractive feature that the shape of the relationship at one extreme of the data (e.g. for SBP < 110 mmHg or SBP ≥ 180 mmHg) has little influence on the shape in other parts of the curve.
Individuals were then grouped according to cut-points most closely approximating Joint National Committee (JNC) VII categories.25 For SBP, these were <120, 120–139, 140–159, and ≥ 160 mm Hg and for DBP were <75, 75–84, 85–89, ≥ 90 mmHg. Cox proportional hazards models were then constructed first adjusted for age and then multiple variables as indicated above. Trends across categories were tested using the mid-point for each category, 110 and 180 for extreme SBP categories, and 65 and 100 for extreme DBP categories. PAD risk per 10 mmHg increment in reported blood pressure was also assessed.
To compare the predictive value of reported systolic and diastolic blood pressures, two methods were used. Likelihood ratio statistics were first used to assess the added value of each blood pressure variable to models including age and other cardiovascular risk factors. Models inclusive of either variable were then ranked and compared following the principle that a larger model likelihood ratio statistic corresponds to enhanced prediction and better model fit.26
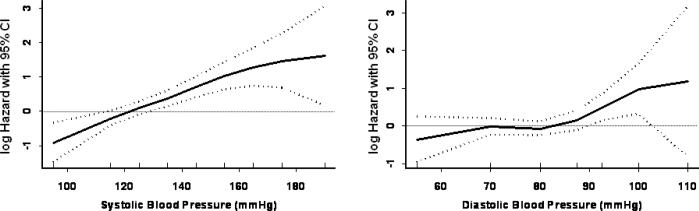
The proportionality assumption of constant hazards over time was tested using interaction terms of reported hypertension categories with the logarithm of time. This was done for regression models incorporating both hypertension subtypes and categories based on continuous measures. No violation of the proportional hazards assumption was detected. All CIs are 2-tailed and calculated at p=0.05 level of significance. All analyses were conducted using SAS statistical software version 9.1 (SAS Institute, Cary, NC). S-Plus 6.2 software was used to obtain cubic spline fits for Figure 1.
Figure 1.
SBP, DBP, and hazard of peripheral artery disease by use of cubic splines in a Cox model adjusting for covariates in Table 1.
Results
Table 1 demonstrates the baseline characteristics according to four self-reported blood pressure categories: normotensive (SBP <140 and DBP <90 mmHg), isolated diastolic hypertension (SBP <140 and DBP ≥ 90 mmHg), isolated systolic hypertension (SBP ≥ 140 and DBP <90 mmHg), and systolic-diastolic hypertension (SBP ≥ 140 and DBP ≥ 90 mmHg). A number of risk factors including age, BMI, dyslipidemia, diabetes, and physical inactivity increased in severity or prevalence across groups. Use of anti-hypertensive medications also increased across groups. Median SBP was incrementally higher across categories (p-trend < 0.001). PAD incidence rates were similar in the normotensive and IDH groups (0.28 events per 1000 person-years in both groups) but 3 to 4-fold higher in women with ISH (0.82 events per 1000 person-years) and SDH (0.93 events per 1000 person-years) (Table 2). In multivariable analyses adjusting for differences in baseline risk factors, we found similar results; adjusted HRs were 1.0, 1.03, 2.03, and 2.83, respectively (p-trend <0.001). Additional adjustment for anti-hypertensive or lipid-lowering therapy only slightly attenuated this effect.
Table 1.
Baseline Characteristics According to Hypertension Category*
| Characteristic | Normotensive† (N=32,978) | IDH (N=1,141) | ISH (N=3,158) | SDH (N=1,983) | P-value |
|---|---|---|---|---|---|
| Cardiovascular Risk Factors | |||||
| Age, mean ± SD (yrs) | 54.0 ± 6.7 | 54.8 ± 6.7 | 59.4 ± 8.2 | 56.4 ± 7.2 | <0.0001 |
| BMI, mean ± SD(kg/m2) | 25.5 ± 4.7 | 28.0 ± 5.6 | 28.7 ± 5.9 | 29.5 ± 6.1 | <0.0001 |
| Non-Hispanic White Ethnicity (%) | 94.4 | 89.8 | 93.4 | 90.8 | <0.0001 |
| History of High Cholesterol (%) | 27.1 | 37.7 | 43.0 | 45.0 | <0.0001 |
| History of Diabetes (%) | 2.1 | 4.7 | 9.0 | 7.6 | <0.0001 |
| Current Smoker (%) | 13.3 | 12.2 | 12.1 | 12.5 | 0.18 |
| Family history of MI (%) | 12.8 | 14.2 | 13.4 | 14.2 | 0.16 |
| Vigorous Activity < 1× per wk (%) | 56.5 | 63.6 | 66.1 | 69.9 | <0.001 |
| HRT User (%) | 42.4 | 42.1 | 40.1 | 39.4 | 0.008 |
| Anti-Hypertensive Therapy (%) | 8.1 | 35.3 | 42.0 | 53.5 | <0.001 |
| Blood Pressure Measures | |||||
| SBP, median (IQR) (mmHg) | 115 (115,125) | 135 (125,135) | 145 (145,145) | 145 (145,155) | <0.0001 |
| DBP, median (IQR) (mmHg) | 80 (70,80) | 93 (93,93) | 88 (80,88) | 93 (93,93) | <0.0001 |
BMI-body-mass index; MI-myocardial infarction, HRT-hormone replacement therapy; SBP -systolic blood pressure; IQR-interquartile range; DBP-diastolic blood pressure.
Blood pressure categories are based on self-report.
The normotensive category includes participants with a history of hypertension who report taking anti-hypertensive medications.
Table 2.
PAD Incidence Rates and Multivariable Hazard Ratios According to Hypertension Subtype*
| Normotensive† (N=32,978) | IDH (N=1,141) | ISH (N=3,158) | SDH (N=1,983) | |
|---|---|---|---|---|
| Number of Events | 119 | 4 | 32 | 23 |
| Follow-Up (1000 person-years) | 422.3 | 14.3 | 39.2 | 24.7 |
| Incidence (per 1000 person-years) | 0.28 | 0.28 | 0.82 | 0.93 |
| Model 1 HR‡ | 1.0 | 1.03 (0.38–2.80) | 2.03 (1.33–3.09) | 2.83 (1.76–4.54) |
| Model 2 HR§ | 1.0 | 0.98 (0.36–2.68) | 1.89 (1.22–2.93) | 2.55 (1.53–4.22) |
Blood pressure categories are based on self-report.
The normotensive category includes participants with a history of hypertension who report taking anti-hypertensive medications.
Model 1 adjusted for age (logage), BMI (continuous), non-Hispanic White ethnicity (yes/no), history of hyperlipidemia (yes/no cholesterol ≥ 240 mg/dl), history of diabetes (yes/no), smoking (never/past/current), family history of premature CAD (yes/no), physical activity (vigorous activity < 1 × per week yes/no), use of postmenopausal hormone therapy (yes/no), and randomized treatment with aspirin and/or vitamin E.
Model 2 additionally adjusted for pharmacologic treatment for hyperlipidemia or hypertension.
Figure 1 shows the continuous association between self-reported SBP and DBP controlling for potential confounding variables. Women with lower SBP levels had lower PAD risk with no apparent risk threshold. DBP was associated with a slight increase in risk at roughly 85 to 90 mmHg. Age-adjusted and multivariable-adjusted HRs for PAD according to clinically defined categories of blood pressure and per 10 mmHg increments are shown in Table 3. Compared to women with a reported SBP <120 mmHg, those reporting pre-hypertension (SBP 120–139 mmHg) had a nearly 2-fold increase in multivariable adjusted risk (HR 1.84; 95% CI 1.24–2.74), those with stage 1 hypertension (SBP 140–159 mmHg) a 3-fold increase in risk (HR 3.30; 95% CI 2.07–5.28), and stage 2 hypertension (SBP ≥160 mmHg) a 5.5-fold increase in risk (HR 5.51; 95% CI 2.50–12.13). A more modest association was noted for reported DBP with significant effects noted only in the highest category (≥ 90 mmHg; HR 1.98; 95% CI 1.22–3.22). Accounting for baseline use of anti-hypertensive and lipid-lowering therapy had minimal effect; the multivariable hazard ratios across categories of reported SBP were 1.00, 1.80, 3.09, and 5.07 (p-trend <0.001) and for DBP were 1.00, 0.96, 1.06, and 1.67 (p-trend =0.11). In comparison, HRs associated with current smoking, past smoking, diabetes, and family history of premature coronary disease were 13.31(95% CI 8.64–20.51), 3.01 (95% CI 1.94–4.69), 2.44 (95% CI 1.40–4.26), and 0.94 (0.6–1.5), respectively.
Table 3.
Comparison of Multivariable Hazard Ratios and 95% CIs for PAD According to Reported Blood Pressure Level
| SBP Only | DBP Only | Both SBP and DBP | ||||
|---|---|---|---|---|---|---|
| Variables/Tests | Age Adjusted | Multivariable Adjusted* | Age Adjusted | Multivariable Adjusted* | Age Adjusted | Multivariable Adjusted* |
| Categorical Variables | ||||||
| SBP Categories | ||||||
| <120 mmHg | 1.00 (reference) | 1.00 (reference) | … | … | 1.00 (reference) | 1.00 (reference) |
| 120–139 mmHg | 1.73 (1.18–2.53) | 1.84 (1.24–2.74) | … | … | 2.20 (1.42–3.39) | 2.31 (1.48–3.63) |
| 140–159 mmHg | 3.11 (2.01–4.80) | 3.30 (2.07–5.28) | … | … | 4.17 (2.41–7.21) | 4.26 (2.41–7.56) |
| ≥ 160 mmHg | 5.11 (2.37–11.00) | 5.51 (2.50–12.13) | … | … | 6.49 (2.70–15.62) | 6.63 (2.70–16.31) |
| P, linear trend | <0.0001 | <0.0001 | … | … | <0.0001 | <0.0001 |
| DBP categories | ||||||
| < 75 mmHg | … | … | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 75–84 mmHg | … | … | 1.04 (0.73–1.48) | 1.00 (0.69–1.44) | 0.68 (0.46–1.01) | 0.65 (0.43–0.99) |
| 85–89 mmHg | … | … | 1.15 (0.73–1.80) | 1.20 (0.75–1.91) | 0.56 (0.34–0.95) | 0.61 (0.36–1.03) |
| ≥ 90 mmHg | … | … | 2.03 (1.29–3.20) | 1.98 (1.22–3.22) | 0.76 (0.43–1.35) | 0.78 (0.43–1.41) |
| P, linear trend | … | … | 0.01 | 0.10 | 0.09 | 0.12 |
| LRT 1† (df) | 32.255 (3 df) | 30.854 (3 df) | 9.055 (3 df) | 8.324 (3 df) | 37.978 (6 df) | 35.927 (3 df) |
| LRT 2‡ (df) | 96.66 (4 df) | 281.61 (16 df) | 73.46 (4 df) | 259.08 (16 df) | 102.38 (7 df) | 286.68 (19 df) |
| Continuous Variables | ||||||
| SBP (per 10 mmHg) | 1.33 (1.22–1.46) | 1.35 (1.23–1.48) | … | … | 1.43 (1.28–1.61) | 1.43 (1.27–1.62) |
| DBP (per 10 mmHg) | … | … | 1.23 (1.05–1.44) | 1.23 (1.04–1.45) | 0.83 (0.68–1.01) | 0.84 (0.69–1.03) |
| LRT 1† (df) | 36.744 (1 df) | 35.699 (1 df) | 6.698 (1 df) | 6.014 (1 df) | 40.257 (2 df) | 38.376 (2 df) |
| LRT 2‡ (df) | 101.15 (2 df) | 286.45 (14 df) | 71.10 (2 df) | 256.76 (14 df) | 104.66 (3 df) | 289.13 (15 df) |
MV Model adjusted for age (logage), BMI (continuous), non-Hispanic White ethnicity (yes/no), history of hyperlipidemia (yes/no cholesterol ≥ 240 mg/dl, history of diabetes (yes/no), smoking (never/past/current), family history of MI (yes/no), physical activity (vigorous activity < 1 × per week yes/no), use of postmenopausal hormone therapy (yes/no), and randomized treatment with aspirin and/or vitamin E. Ranges in parentheses are 95% confidence intervals.
LRT 1 indicates likelihood ratio statistic comparing the model with blood pressure variable compared to model without the blood pressure variable for both age-adjusted and multivariable-adjusted analyses. Higher values indicate greater contribution to model fit.
LRT 2 indicates likelihood ratio statistic for all variables compared to no variables in model. Higher values indicate improved global fit.
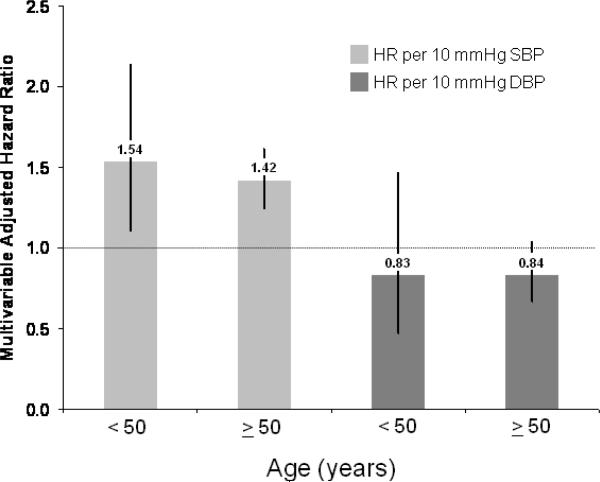
An increase in SBP by 10 mmHg conferred a 35% increase in PAD risk (95% CI 23– 48%) and for DBP a 23% (95% CI 4–45%) increase in risk. In models including both reported SBP and DBP both as categorical or continuous measures, DBP was no longer significant and, if anything, appeared inversely related to PAD [HR per 10 mmHg: 0.84 (95% CI 0.69–1.03), p=0.10] whereas SBP remained strongly associated with incident PAD [HR per 10 mmHg: 1.43 (95% CI 1.27–1.62), p<0.001]. Models with both SBP and DBP were no better than models with SBP alone. The likelihood ratio statistic for difference between models was not statistically significant (BP categories: LR X2=5.07, 3 df; p=0.17 and per 10 mmHg: LR X2=2.68, 1 df; p=0.10). Based on all criteria used, SBP was the better predictor of future PAD than DBP. Our findings persisted in sensitivity analyses in which women with unconfirmed PAD (n=130) were included among events. Adjusted HR for reported IDH, ISH, and SDH compared to normotensive women were 1.03 (95% CI 0.51–2.11), 1.70 (95% CI 1.22–2.39), and 2.31 (95% CI 1.57–3.39) and SBP remained a stronger predictor of future PAD than DBP. Given the marked association of systolic hypertension with aging, we also stratified the cohort into two groups based on age < or ≥ 50 years (Figure 2). In these analyses, there was a persistent association of reported systolic but not diastolic blood pressure in both age groups without evidence for effect modification (both p-values for interaction of age with SBP and DBP >0.2).
Figure 2.
Multivariable adjusted hazard ratio (HR) for peripheral artery disease according to 10 mmHg increment in SBP and DBP stratified by age (< 50 versus ≥ 50 years) adjusting for covariates in Table 1.
Discussion
In this large-scale study of otherwise healthy middle-aged and older American women, we found a strong association between participant-reported systolic hypertension and incident symptomatic PAD. IDH was not associated with future PAD whereas both ISH and SDH conferred a 2–3 fold increase in risk. We also found a linear increase in PAD risk with increasing SBP throughout the range of baseline values. A prominent association was evident even among women with reported SBP levels below current JNC thresholds for diagnosis of hypertension. While reported DBP was individually predictive of future PAD, this relationship was no longer significant after accounting for association with elevated SBP. Furthermore, baseline SBP was the more informative blood pressure variable in all risk prediction models evaluated. Importantly, a 10 mmHg difference in reported SBP was associated with incident PAD among both younger (HR 1.54; 95% CI 1.11–2.14) and older women (HR 1.42; 95% CI 1.25–1.62).
These prospective data among a large cohort of women add to prior information regarding hypertension and development of PAD. The first prospective evaluation of this issue derives from the Framingham Heart Study.6 Gender-adjusted multivariable results demonstrated a gradient in risk for intermittent claudication with a roughly 2-fold increase in the highest blood pressure category (SBP ≥160 mmHg or DBP ≥100 mmHg),12 while gender-specific risk estimates suggested a stronger association in women (standardized regression coefficients 0.356 for women and 0.178 for men; risks in BP categories were not provided).6–7 Similar to our results, the age-adjusted incidence rate was shown to increase over the continuum of SBP while a threshold effect for DBP occurred at >85 mmHg for women (>95 mmHg in men).7.
Among studies confined to men, hypertension is either not associated with subsequent PAD or findings are limited by methodological concerns. In French-Canadian men,9 while an increase in risk in the highest quintile of both SBP (RR 2.7; 95% CI 1.8–4.2) and DBP (1.5; 95% CI 1.0–2.1) were observed, results were not adjusted for baseline diabetes. In the Honolulu Heart Study11 of Japanese American men, hypertension was associated with a roughly 2-fold increased risk, however the analysis was restricted to men who survived 25 years from baseline to PAD assessment [ankle-brachial index (ABI) < 0.9]. Three other prospective cohort studies8, 10, 16 in men did not share these methodological issues and no effect of hypertension on incident PAD was seen. In other longitudinal population-based studies inclusive of women, a history of hypertension was associated with a significant 1.6–1.8 fold increase in risk.13–15 In general, it should be noted that in all but one of these reports, hypertension was considered amongst a broad range of traditional cardiovascular risk factors.9 Consequently, assessment for linearity of associations, blood pressure categories, and comparison of systolic versus diastolic hypertension was rarely performed.
The influence of aging is an important confounder in the pathophysiologic link between hypertension and PAD as the age-associated degenerative changes in vascular structure and function distinct from those attributable to elevated blood pressure alone are not easily discerned. While our data do not allow characterization of the underlying mechanisms, our findings do demonstrate that in otherwise healthy women at low risk for cardiovascular disease, a relationship between reported systolic hypertension and future peripheral artery events persisting even among younger women in the cohort. Our findings in this population of relatively healthy women are discordant with null findings from prior longitudinal studies exclusively comprised of men8, 10, 16 and thereby suggest heightened susceptibility to the effects of hypertension among women. Our results may also indicate an important role of central arterial stiffness in the development of peripheral atherosclerosis in women. Arterial stiffening results in higher transmission velocities of both forward and reflected pulse waves causing premature return of reflected waves in the central aorta during late systole rather than diastole as normally occurs. The resulting systolic augmentation of blood pressure is further amplified with progressive loss of aortic elasticity. For instance, Khaleghi et al. demonstrated that the degree of systolic augmentation significantly correlates with lower ankle-brachial index independent of other cardiovascular risk factors.27 Whether abnormalities in ABI precede arterial stiffening is not clear from this community-based, cross-sectional study27 and whether this association is stronger in women has not been evaluated.
Our findings should be interpreted in the context of both strengths and potential limitations. The major strengths of this study are the large sample size, the prospective design, long-term follow-up and annual assessment with validation of symptomatic PAD events. However, the study included predominantly non-Hispanic white women with higher levels of education and income, and our findings should not be generalized to other racial/ethnic groups, to populations of lower socioeconomic status, or to men. In addition, these findings are observational, and we cannot rule out the possibility that these associations are related to unmeasured, healthier behaviors among participants who report lower blood pressures. Perhaps more importantly, WHS participants do not undergo health examination. Therefore, the use of symptomatic PAD as our primary a priori endpoint by definition excludes subclinical disease, which may have otherwise been detected by physical examination through abnormal pulse exam or ABI. It should be noted that women in particular may be less symptomatic relative to men.1 Although potential misclassification resulting from atypical or occult disease may have occurred, this, if anything, would have biased our results toward the null by inclusion of women with PAD among the control population. Our event rate (0.03 annualized percent) is similar to other large prospective cohorts such as the Women's Health Initiative (WHI) which defined incident PAD using hospitalizations and revascularizations. In the WHI, the annualized percentage ranged from 0.03 to 0.08.28–29 The use of self-reported blood pressure may also have led to exposure misclassification; however, in this population of female health professionals a high proportion of subjects underwent surveillance for hypertension (92.3% reported blood pressure evaluation within the first year of follow-up) and good correlation between self-reported and measured values in cohorts of medical professionals has been previously demonstrated.20 Furthermore, self-reported blood pressure in the WHS has been shown to have similar prognostic value for cardiovascular events as compared to measured blood pressure in other population-based cohort studies.23, 30
Thus, in assessing the association between participant-reported blood pressure control with incident PAD, our data demonstrate a strong prognostic role of uncontrolled blood pressure, in particular, uncontrolled systolic blood pressure in the clinical development of PAD in women. Our findings highlight the need for additional longitudinal investigations evaluating the proatherogenic effects of hypertension from other large cohorts inclusive of women.
Acknowledgements
None
Funding Acknowledgement This work was supported by the National Heart, Lung, and Blood Institute (Bethesda, MD) [HL43851, HL58755, HL75771, and HL 082740], the National Cancer Institute (Bethesda, MD) [CA47988], and the Donald W. Reynolds Foundation (Las Vegas, NV).
Footnotes
Conflict of Interest Statement The Authors declare that there is no conflict of interest.
References
- 1.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–24. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292(4):453–61. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 4.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326(6):381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 5.O'Rourke MF. Pressure and flow waves in systemic arteries and the anatomical design of the arterial system. J Appl Physiol. 1967;23(2):139–49. doi: 10.1152/jappl.1967.23.2.139. [DOI] [PubMed] [Google Scholar]
- 6.Gordon T, Kannel WB. Predisposition to atherosclerosis in the head, heart, and legs. The Framingham study. JAMA. 1972;221(7):661–6. [PubMed] [Google Scholar]
- 7.Kannel WB, McGee DL. Update on some epidemiologic features of intermittent claudication: the Framingham Study. J Am Geriatr Soc. 1985;33(1):13–8. doi: 10.1111/j.1532-5415.1985.tb02853.x. [DOI] [PubMed] [Google Scholar]
- 8.Bowlin SJ, Medalie JH, Flocke SA, et al. Epidemiology of intermittent claudication in middle-aged men. Am J Epidemiol. 1994;140(5):418–30. doi: 10.1093/oxfordjournals.aje.a117264. [DOI] [PubMed] [Google Scholar]
- 9.Gagnon F, Dagenais GR, Robitaille NM, et al. Impact of systolic and diastolic blood pressure on ischemic vascular diseases in French-Canadian men from 1974 to 1990. Can J Cardiol. 1994;10(1):97–105. [PubMed] [Google Scholar]
- 10.Ingolfsson IO, Sigurdsson G, Sigvaldason H, et al. A marked decline in the prevalence and incidence of intermittent claudication in Icelandic men 1968–1986: a strong relationship to smoking and serum cholesterol--the Reykjavik Study. J Clin Epidemiol. 1994;47(11):1237–43. doi: 10.1016/0895-4356(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 11.Curb JD, Masaki K, Rodriguez BL, et al. Peripheral artery disease and cardiovascular risk factors in the elderly. The Honolulu Heart Program. Arterioscler Thromb Vasc Biol. 1996;16(12):1495–500. doi: 10.1161/01.atv.16.12.1495. [DOI] [PubMed] [Google Scholar]
- 12.Murabito JM, D'Agostino RB, Silbershatz H, et al. Intermittent claudication. A risk profile from The Framingham Heart Study. Circulation. 1997;96(1):44–9. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
- 13.Hooi JD, Kester AD, Stoffers HE, et al. Incidence of and risk factors for asymptomatic peripheral arterial occlusive disease: a longitudinal study. Am J Epidemiol. 2001;153(7):666–72. doi: 10.1093/aje/153.7.666. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy M, Solomon C, Manolio TA, et al. Risk factors for declining ankle-brachial index in men and women 65 years or older: the Cardiovascular Health Study. Arch Intern Med. 2005;165(16):1896–902. doi: 10.1001/archinte.165.16.1896. [DOI] [PubMed] [Google Scholar]
- 15.Tzoulaki I, Murray GD, Lee AJ, et al. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. Eur Heart J. 2007;28(3):354–62. doi: 10.1093/eurheartj/ehl441. [DOI] [PubMed] [Google Scholar]
- 16.Kollerits B, Heinrich J, Pichler M, et al. Intermittent claudication in the Erfurt Male Cohort (ERFORT) Study: its determinants and the impact on mortality. A population-based prospective cohort study with 30 years of follow-up. Atherosclerosis. 2008;198(1):214–22. doi: 10.1016/j.atherosclerosis.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293–304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 18.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 19.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 20.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123(5):894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 21.Klag MJ, He J, Mead LA, et al. Validity of physicians' self-reports of cardiovascular disease risk factors. Ann Epidemiol. 1993;3(4):442–7. doi: 10.1016/1047-2797(93)90074-e. [DOI] [PubMed] [Google Scholar]
- 22.Hu FB, Willett WC, Colditz GA, et al. Prospective study of snoring and risk of hypertension in women. Am J Epidemiol. 1999;150(8):806–16. doi: 10.1093/oxfordjournals.aje.a010085. [DOI] [PubMed] [Google Scholar]
- 23.Conen D, Ridker PM, Buring JE, et al. Risk of cardiovascular events among women with high normal blood pressure or blood pressure progression: prospective cohort study. BMJ. 2007;335(7617):432. doi: 10.1136/bmj.39269.672188.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leng GC, Fowkes FG. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol. 1992;45(10):1101–9. doi: 10.1016/0895-4356(92)90150-l. [DOI] [PubMed] [Google Scholar]
- 25.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 26.Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Khaleghi M, Kullo IJ. Aortic augmentation index is associated with the ankle-brachial index: a community-based study. Atherosclerosis. 2007;195(2):248–53. doi: 10.1016/j.atherosclerosis.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsia J, Criqui MH, Rodabough RJ, et al. Estrogen plus progestin and the risk of peripheral arterial disease: the Women's Health Initiative. Circulation. 2004;109(5):620–6. doi: 10.1161/01.CIR.0000115309.63979.92. [DOI] [PubMed] [Google Scholar]
- 29.Hsia J, Criqui MH, Herrington DM, et al. Conjugated equine estrogens and peripheral arterial disease risk: the Women's Health Initiative. Am Heart J. 2006;152(1):170–6. doi: 10.1016/j.ahj.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]