Abstract
Purinergic receptors activate diverse signaling cascades and regulate the activity of cell volume-sensitive ion transporters. However, the effects of ATP and other agonists of P2 receptors on cell volume dynamics are only scarcely studied. In the present work, we used the recently developed dual-image surface reconstruction technique to explore the influence of purinergic agonists on cell volume in the C11-Madin-Darby canine kidney cell line resembling intercalated cells from kidney collecting ducts. Unexpectedly, we found that ATP and UTP triggered very robust (55–60%) cell shrinkage that lasted up to 2 h after agonist washout. Purinergic regulation of cell volume required increases in intracellular Ca2+ and could be partially mimicked by the Ca2+-ionophore ionomycin or activation of protein kinase C by 4β-phorbol 12-myristate 13-acetate. Cell shrinkage was accompanied by strong reductions in intracellular K+ and Cl− content measured using steady-state 86Rb+ and 36Cl− distribution. Both shrinkage and ion efflux in ATP-treated cells were prevented by the anion channel blocker 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) and by the BKCa channel inhibitors charybdotoxin, iberiotoxin, and paxilline. To evaluate the significance of cell-volume changes in purinergic signaling, we measured the impact of ATP on the expression of the immediate-early gene c-Fos. Thirty-minute treatment with ATP increased c-Fos immunoreactivity by approximately fivefold, an effect that was strongly inhibited by charybdotoxin and completely prevented by NPPB. Overall, our findings suggest that ATP-induced cell-volume changes are partially responsible for the physiological actions of purinergic agonists.
Keywords: renal epithelium, intracellular Ca2+, BKCa, anion channels, Madin-Darby canine kidney
animal cells encounter frequent changes in transmembrane osmotic gradients that arise from metabolic processes consuming or producing osmotically active substances, or as a result of variations in the transmembrane transport of ions and nutrients. Because water permeability of the plasma membrane is high in the majority of animal cell types, water moves passively toward the compartment with higher osmolarity, causing cellular swelling or shrinkage. If uncompensated, cell volume alterations disrupt the equilibrium of intracellular enzymatic reactions and, in extreme forms, jeopardize cellular function and integrity. Therefore, all higher animals, including mammals, possess several cellular and systemic mechanisms of homeostatic volume control. At the systemic level, the volume and osmolarity of extracellular fluids are tightly regulated via sensing of [Cl−] in renal tubular fluid and of [Na+] and osmolality in cerebrospinal fluid (7, 37). At the cellular level, cell volume is maintained constant within a 2–5% margin via the coordinated work of several volume-sensitive ion channels and ion/osmolyte transporters (for comprehensive review, see Refs. 21, 25, 31, 34, 58).
It is well documented that pronounced hyperosmotic shrinkage can lead to apoptosis, whereas cell swelling in hyposmotic medium is frequently associated with necrotic cell death (4, 27, 35, 38). It should be stressed, however, that the impact of cell-volume perturbations on cellular physiology is not exclusively negative. Thus cell volume serves as a physiological modulator of cellular functions in hepatocytes, epithelial cells, brain glial cells, and a variety of migrating cells (20, 21, 23–25, 29). In the kidneys, the transepithelial transport of electrolytes across the basolateral and apical membranes of secretory cells is thought to be coordinated by changes in cellular volume that strongly regulate the activity of volume-sensitive K+ and Cl− channels as well as ion carriers, including the Na+, K+, 2Cl− cotransporter (21, 25).
Accumulating evidence indicates that cell-volume regulation and volume-regulatory ion transport pathways are potently modulated by agonists of G protein-coupled receptors (13, 14, 55). A special role in cell-volume regulation likely belongs to G protein-coupled purinergic receptors of the P2Y family, which are activated by ATP, UTP, and several other purine nucleotides (9). For example, in two Madin-Darby canine kidney (MDCK) cell lines, C7 and C11, which respectively resemble principal and intercalated cells of the collecting ducts, ATP transiently stimulates the ubiquitous volume-sensitive Na+, K+, 2Cl− cotransporter NKCC1. In C11-MDCK cells, initial purinergic activation of NKCC1 is followed by the paradoxical, complete inhibition of its activity (1). In brain glial cells, astrocytes, ATP potently modulates the opening of volume-regulated anion channels (VRAC) and, in this way, controls glial release of the excitatory neurotransmitter glutamate (30). In hepatocytes, autocrine ATP release and P2Y receptor activation are thought to be indispensable steps in the activation of volume-sensitive Cl− channels and regulatory volume decrease (RVD) (57). However, the impact of purinergic signaling on cell volume has scarcely been investigated.
The dearth of systematic studies on cell-volume dynamics can be explained by the absence of reliable methods for monitoring cell volume in adherent cells. Cell size is frequently measured electronically by a Coulter counter but only in cell suspensions and after the trypsinization of substrate-attached cells that might be sufficient per se for cell-volume perturbation (3). Indeed, detachment of epithelial and endothelial cells results in anoikis, i.e., cell death accompanied by extensive shrinkage (2). Alternative techniques with membrane-permeable radiotracer compounds, such as [14C] urea and methyl-d-[14C] glucose, require prolonged preincubation, have limited accuracy, and cannot be used for investigations into rapid volume perturbations. Some of these limitations can be overcome in cell-volume measurements on the basis of registration of isosbestic fluorescence of dyes, such as fluo-2 and calcein. It should be underlined that such methods assume the homogenous distribution of optically active molecules inside the cells, their negligible leakage, and photobleaching, as well as minimal side effects that may be caused by the production of reactive oxygen species (19, 51).
In the present study, we took advantage of our recently developed dual-image surface reconstruction (DISUR) technique for simultaneous measurements of cell height, total surface area, and volume (5), which has several clear advantages. In particular, this method allows accurate cell-volume measurement with temporal resolution of ∼100 ms in attached cells whose volume perturbations are mainly caused by alterations of cell height rather than planar area (5, 12, 17). Unexpectedly, we found that the activation of P2Y receptors triggers very robust and long-lasting shrinkage in C11-MDCK cells. We explored the mechanisms of this novel phenomenon and its relative contribution to purinergic signaling.
MATERIALS AND METHODS
Cell culture.
C11-MDCK cells, a cell line subcloned from MDCK cells, are peanut-lectin positive, maintain pHi at 7.16, and have large Cl− and H+ conductances and low transepithelial electrical resistance (16). Thus they resemble intercalated cells of collecting ducts. They were grown in DMEM supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin and used at passages 60 to 80. For more details, see Ref. 1.
Cell-volume measurements.
Cell volumes were measured in substrate-attached cells by an improved version of the DISUR technique, as described in detail in our previous work (5, 12, 17). Briefly, the method involves the 3D reconstruction of cell shape on the basis of two conventional microscopy cell images acquired in two perpendicular directions. Side-view and top-view cell images were recorded for 100 ms with 0.40 NA and 0.25 NA objective lenses, two independent, miniature, charged-coupled cameras (Moticam 350; Motic Instruments, Richmond, BC, Canada), and Motic software at 0.14 μm/pixel and 0.18 μm/pixel resolutions for side-view and top-view images, respectively. These images served to generate a set of topographical curves of the cell surface from its digitized side-view profile and base outline. Cell volume and surface were calculated from the reconstructed cell topographical model. All calculations were carried out with MATLAB (Math Works, Natick, MA). Although the DISUR technique provides absolute cell-volume values, in the present study, data plots show relative volume normalized to the initial volume of intact cells in physiological solution. Cells were briefly trypsinized, plated at low density on glass coverslips, and allowed to recover in serum-containing medium for 12 to 24 h. The coverslips were then mounted in a custom-made 2-ml flow-through imaging chamber perfused at the rate of 1.3 ml/min with HEPES-buffered isotonic medium A at 37°C. The composition of medium A was as follows (in mM): 135 NaCl, 3.8 KCl, 1.2 MgSO4, 1.3 CaCl2, 1.2 KH2PO4, 10 d-glucose, 10 HEPES (pH = 7.4, adjusted with NaOH). In some experiments, medium osmolarity was reduced by ≤30% by decreasing [NaCl] to 85 mM or increased by ∼50% by adding up to 150 mM mannitol. In Ca2+-free medium, CaCl2 was omitted and 0.2 mM EGTA was added.
Intracellular Ca2+ concentration.
These experiments were performed in accordance with a previously described method (53). Briefly, C11-MDCK cells grown on glass coverslips were incubated for 30–40 min in medium A containing 5–10 μM fura-2 AM. The cells were then washed and mounted in a diagonal position in a custom-made 1 × 1-cm cuvette perfused at the rate of 1.3 ml/min, and fluorescence was measured under permanent stirring at 37°C (λex = 340 and 380 nm, slit 4 nm; λem = 510 nm, slit 12 nm), in a SPEX FluoroMax spectrofluorometer (Edison, NJ). Free intracellular Ca2+ concentration ([Ca2+]i) was quantified as [Ca2+]i = Kd(R − Rmin)/(Rmax − R), where Kd is the dissociation constant of the Ca2+-fura 2 complex (224 nM at 37°C), and R = F340/F380 is the ratio of fluorescence at λex = 340 and 380 nm. To evaluate Fmax, the cells were treated with 0.5 μM ionomycin in the presence of 1 mM CaCl2. To determine Fmin, MnCl2 was added to a final concentration of 2 mM.
Intracellular content of monovalent ions.
C11-MDCK cells were seeded on 12-well plates and grown to confluency in serum-containing DMEM, as described above. The cells were washed with phosphate-buffered saline and incubated for 2 h in 2 ml of medium A containing 0.5 μCi/ml 86Rb, 2–3 μCi/ml 22Na, or 1–2 μCi/ml 36Cl. ATP (100 μM) was then added for 1 h with or without ion transport inhibitors. To prevent the rapid degradation of ATP by ecto-ATPases, in these experiments as well as in the study of c-Fos expression (see below) performed in the absence of the flow-through chamber, medium A contained 100 μM 6,N,N′-diethyl-β,γ-dibromomethylene-d-adenosine-5-triphosphate (ARL 67156). In previous experiments, we observed that this compound strongly suppressed the activity of ecto-ATPases in C11-MDCK cells (1). The cells were transferred on ice, washed four times with 4 ml of ice-cold medium containing 100 mM MgCl2 and 10 mM HEPES-Tris (pH 7.4), and lysed with 1 ml of 1% SDS/4 mM EDTA mixture. Radioactivity of the cell lysates was measured with a liquid scintillation analyzer, and intracellular exchangeable K+, Na+, and Cl− content was calculated as V = A/am, where A was the radioactivity in the sample (counts/min, cpm), a was the specific radioactivity of 86Rb (K+), 36Cl, or 22Na (cpm/nmol) in the incubation medium, and m was protein content in the sample (mg).
Western blotting.
Cells grown in six-well plates and treated as indicated in results were washed with ice-cold medium containing 150 mM NaCl and 10 mM HEPES-Tris (pH 7.4), scraped with a rubber cell scraper, centrifuged (500 g, 5 min), and lysed with buffer containing 150 mM NaCl, 25 mM HEPES-Tris (pH 7.5), 0.1% SDS, 0.25% Na-deoxycholate, 2 mM EGTA, 2 mM EDTA, 1 mM Na3VO4, 10 mM NaF, 200 μM PMSF, 1 μg/ml leupeptin, and 1 μg/ml aprotinin. Equal portions of cell lysate (20 μg/lane) were electrically separated on 10% SDS-polyacrylamide gel, transferred onto nitrocellulose membranes, washed with phosphate-buffered saline containing 0.05% Tween 20 (PBS-Tween) and 0.5% skim milk, and incubated overnight at 4°C with anti-c-Fos antibody. After incubation, the membranes were washed three times with PBS-Tween and incubated for 1 h with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were then washed with PBS-Tween, and the protein bands were visualized with an enhanced chemiluminescence detection kit (Santa Cruz Biotechnology) and exposed to X-ray film.
Chemicals.
Bumetanide, ATP, UTP, forskolin, charybdotoxin, paxilline, clotrimazole and ARL 67156, suramin, 2′-deoxy-N6-methyladenosine-3′,5′-biphosphate (MRS2179), and iberiotoxin were purchased from Sigma-Aldrich (St. Louis, MO); BAPTA-AM, fura 2-AM, ionomycin, 4β-phorbol 12-myristate 13-acetate (4β-PMA), 4α-phorbol-12,13-didecanoate (4α-PDD), and 5-nitro-2-(3-phenylpropylamino-benzoic) acid (NPPB) were procured from EMD-Calbiochem (La Jolla, CA); N,N′′-1,4-butanediylbis[N′-(3-isothiocyanatophenyl)]thio urea (MRS2578) was obtained from Tocris Chemicals, Ellisville, MO. d-glucose, all salts, and buffers were from Sigma-Aldrich or Anachemia (Montreal, PQ). 86RbCl, H36Cl, and 22NaCl were provided by Isotope (St. Petersburg, Russia). Polyclonal antibodies for c-Fos were obtained from Santa Cruz Biotechnology.
Statistical analysis.
All data in this study are presented as means ± SE. Statistical differences between groups were analyzed by Student's t-test using Origin 6.0 software (OriginLab, Northampton, MA). Post hoc Bonferroni correction was applied to all multiple comparisons.
RESULTS
Effects of ATP, UTP, and anisosmotic media on C11-MDCK cell volume.
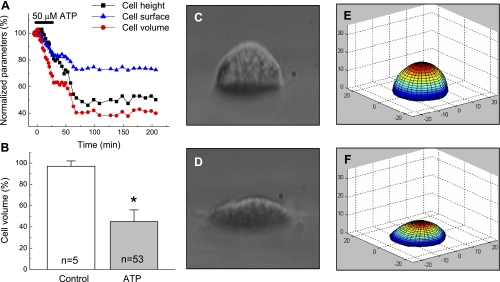
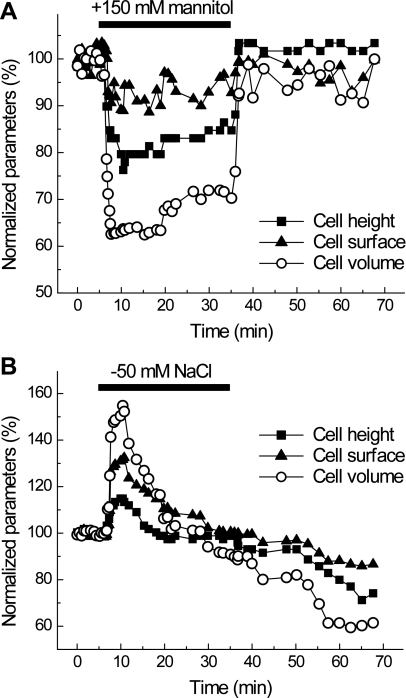
As seen in Fig. 1A, 30-min application of 50 μM ATP resulted in progressive, time-dependent shrinkage of C11-MDCK cells after an initial ∼5-min delay. In all cells tested in four different preparations, this initial ATP-induced cell shrinkage developed further, even after ATP washout, and was essentially irreversible for 2–3 h. On average, at the 60-min time point (30-min treatment with ATP followed by 30-min incubation in ATP-free medium), the volume of C11-MDCK cells was decreased by 55 ± 11% (Fig. 1B). Importantly, ATP-induced cell shrinkage was more pronounced compared with rapid cell shrinkage by 38 ± 8% (n = 4) in cells superfused with hyperosmotic medium in which osmolarity was increased by ∼50% by adding 150 mM mannitol (Fig. 2A). Hyperosmotically shrunken cells showed very limited or no regulatory volume increase (RVI) with complete restoration of their volume upon return to isotonic conditions (Fig. 2A). In contrast, C11-MDCK cells exposed to hyposmotic media, in which osmolarity was decreased by ∼30%, rapidly swelled by 54 ± 13% (n = 5), completely restored their volume within 10–15 min, and then shrank additionally upon return to isosmotic medium (Fig. 2B). This secondary shrinkage can be explained by the reduction in intracellular osmolyte content that made the isosmotic medium hyperosmotic relative to new cytosolic osmolarity. Overall, these results are consistent with rapid RVD and negligible RVI documented in suspensions of MDCK cells with the Coulter counter technique (45, 46).
Fig. 1.
ATP causes persistent shrinkage of C11-Madin-Darby canine kidney (MDCK) cells. A: representative traces showing the time course of changes in height, surface area, and volume in cell superfused with 50 μM ATP for 30 min under isosmotic conditions. Initial height, surface, and volume values were taken as 100%. B: quantitative cell-volume changes in cells subjected to 60-min superfusion with isosmotic medium (control), or for 30 min with isosmotic medium containing 50 μM ATP, followed by 30 min with ATP-free medium (ATP). Initial cell volume values were measured before the addition of ATP or vehicle and varied in the 4,268 ± 246 μm3 range (n = 53). In all cases, individual initial cell volumes were taken as 100%. *P < 0.05 vs. control. C and D: light-microscope side-view images of the same cell immediately before ATP addition (C) and after 30-min treatment with 50 μM ATP, followed by 30-min incubation in ATP-free medium (D). E and F: perspective view of 3D models corresponding to the images in C and D, which were produced by the dual-image surface reconstruction (DISUR) technique, as described in materials and methods.
Fig. 2.
Cell-volume dynamics in C11-MDCK cell exposed to hyperosmotic (A) or hyposmotic (B) media. A: representative time course of changes in height, surface area, and volume of cell superfused for 30 min with hypertonic medium (+150 mM mannitol). Initial values were taken as 100%. B: representative time course of changes in height, surface area, and volume of C11-MDCK cell superfused for 30 min with hypotonic medium ([NaCl]o reduced from 135 to 85 mM). Initial values were taken as 100%.
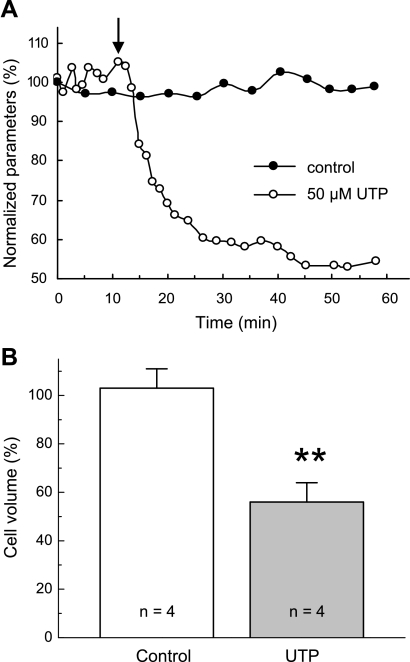
Similarly to ATP, 30-min perfusion with 50 μM UTP induced sustained shrinkage of C11-MDCK cells (Fig. 3A). In four independent preparations, the average reduction in cell volume was ∼45% (Fig. 3B). Control cells showed no statistically significant volume changes during the comparable time period (Fig. 3A). These data strongly indicated that cell shrinkage is triggered by activation of ATP- and UTP-sensitive P2Y receptors rather than by ATP-sensitive, UTP-resistant P2X receptors.
Fig. 3.
Effect of the P2Y agonist UTP on C11-MDCK cell volume. A: representative time course of changes in the volume of control cells (●) and cell superfused for 30 min with isosmotic medium containing 50 μM UTP (○). The moment of UTP addition is shown by arrow. Initial values were taken as 100%. B: average cell-volume changes in cells subjected to 60-min perfusion with isosmotic medium (control) or superfused for 30 min with 50 μM UTP and followed by 30 min of UTP-free medium (UTP). UTP was added in 5 min of initial perfusion with isosmotic medium as shown in A. Initial values of cell volume measured before the addition of UTP or vehicle were taken as 100%. Data represent mean values ± SE obtained in 4 independent experiments. **P < 0.01 vs. control.
Role of Ca2+i and protein kinase A and C.
In previous studies, we found that P2Y receptors in C11-MDCK cells trigger diverse signaling cascades, including PKC activation, [Ca2+]i elevation, and cAMP production (1, 36). Keeping this in mind, we explored the role of these signaling systems in ATP-induced cell shrinkage.
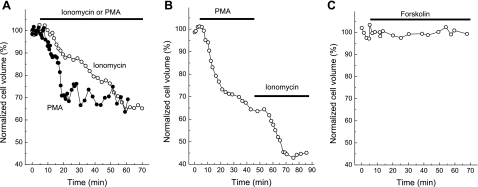
One-hour exposure to the Ca2+ ionophore ionomycin and the activator of Ca2+ and phospholipid-sensitive PKC isoforms, 4β-PMA, caused time-dependent shrinkage of C11-MDCK cells (Fig. 4A) that resulted in decreases of cell volume by ∼30% compared with untreated cells (Table 1). These results are similar to ∼20% volume reduction in ionomycin-treated MDCK-F cells, as quantified by Schneider and coworkers with atomic force microscopy (49). When 4β-PMA and ionomycin were applied sequentially, their effects on cell volume were additive (Fig. 4B), and, after 1 h of combined incubation with these compounds, cell volume was decreased by almost 60% (Table 1). In contrast, treatment of C11-MDCK cells with inactive analog of 4β-PMA, 4α-PDD, as well as with the adenylate cyclase activator forskolin, that in our previous experiments has been shown to drastically elevate cAMP production in C11-MDCK cells (36), did not affect cell volume (Fig. 4C, Table 1).
Fig. 4.
Representative kinetics of actions of the Ca2+ ionophore ionomycin, the PKC activator 4β-phorbol 12-myristate 13-acetate (4β-PMA), and the adenyl cyclase activator forskolin on single C11-MDCK cell volume. 1 μM ionomycin (A), 1 μM 4β-PMA (A), combination of 4β-PMA and ionomycin (B), or 10 μM forskolin (C) were added as indicated. Initial cell volume was taken as 100%.
Table 1.
Effect of ionomycin, 4β-PMA, 4α-PDD, and forskolin on volume of C11-MDCK cells
| Additions, μM | n | Cell Volume, % |
|---|---|---|
| None (control) | 3 | 97 ± 5 |
| Ionomycin, 1 | 4 | 70 ± 4 (P < 0.01) |
| 4β-PMA, 1 | 4 | 73 ± 4 (P < 0.05) |
| 4α-PDD, 1 | 3 | 105 ± 8 (NS) |
| Forskolin, 10 | 3 | 103 ± 7 (NS) |
| Ionomycin + 4β-PMA | 4 | 43 ± 6 (P < 0.002) |
Means ± SE in n experiments and P values are shown. Cells were incubated for 1 h in medium A with compounds listed in the left column. Initial cell volume was taken as 100%. MDCK, Madin-Darby canine kidney; 4β-PMA, 4β-phorbol 12-myristate 13-acetate; 4α-PDD, 4α-phorbol-12,13-didecanoate; NS, not significant.
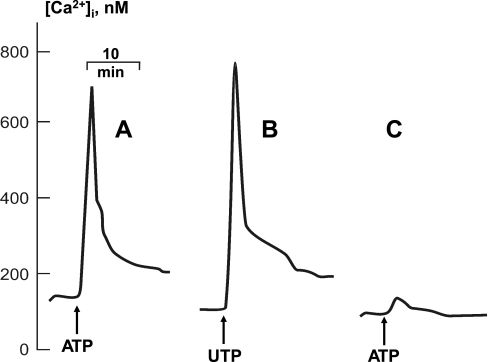
As seen in Fig. 5, both ATP and UTP triggered rapid elevation of [Ca2+]i with average maximal values of 775 ± 102 (n = 4) and 783 ± 121 nM (n = 3) for ATP and UTP, respectively. We did not observe any significant action of 4β-PMA on baseline [Ca2+]i (data not shown). To further explore the role of Ca2+ in ATP-induced cell shrinkage, we loaded C11-MDCK cells with the Ca2+i chelator BAPTA-AM in Ca2+-free medium containing the extracellular Ca2+ chelator EGTA. This procedure blocked the ATP-induced elevation of [Ca2+]i in C11-MDCK cells [Fig. 5C, Δ[Ca2+]i = 673 ± 91 (n = 4) and 67 ± 33 nM (n = 3) in control and in the presence of Ca2+ chelators, respectively, P < 0.001] and completely prevented cell shrinkage induced by ATP, UTP and 4β-PMA (Table 2). Overall, these results point to a key role of [Ca2+]i and Ca2+-dependent PKC isoforms in cell shrinkage triggered by P2Y receptors.
Fig. 5.
Representative kinetics showing the actions of 50 μM ATP (A, C) and UTP (B) on [Ca2+]i concentration in C11-MDCK cells. A, B: cells were perfused with control medium A. C: cells were perfused for 30 min with 20 μM BAPTA-AM in Ca2+-free medium A containing 0.2 mM EGTA, and then ATP was added.
Table 2.
Chelation of extracellular and intracellular Ca2+ completely abolishes ATP-, UTP-, and 4β-PMA-induced cell shrinkage
| Additions, μM | n | Cell Volume, % |
|---|---|---|
| 1. None (control) | 3 | 103 ± 6 |
| 2. ATP, 50 | 5 | 55 ± 5 (P < 0.001) |
| 3. UTP, 50 | 5 | 58 ± 6 (P < 0.005) |
| 4. 4β-PMA, 1 | 4 | 68 ± 5 (P < 0.01) |
| 5. BAPTA-AM, 20 | 3 | 95 ± 6 (NS) |
| 6. BAPTA-AM + ATP | 3 | 98 ± 7 (NS) |
| 7. BAPTA-AM + UTP | 3 | 105 ± 6 (NS) |
| 8. BAPTA-AM + 4β-PMA | 3 | 104 ± 8 (NS) |
Means ± SE and P values in n experiments are shown. In experiments 1–4, C11-MDCK cells were incubated in medium A for 30 min, and the compounds listed in the left column were added for the next 1 h. In experiments 5–8, cells were perfused for 30 min with BAPTA-AM in Ca2+-free medium A containing 0.2 mM EGTA, and then ATP, UTP, and 4β-PMA were added for 1 h. Initial cell volume was taken as 100%.
Effect of P2Y receptor antagonist.
With an exception of P2Y14, ATP activates all cloned purinergic receptors, whereas UTP is a potent and selective agonist of P2Y2, P2Y4, and P2Y6 receptors (10). Previously, we reported that C11-MDCK cells express mRNA messages encoding for P2Y1, P2Y2, P2Y11, and P2Y12 receptors (1). Keeping these data in mind, we examined actions of selective antagonists P2Y1 and P2Y6 receptors, compounds MRS2179 and MRS2578, respectively, and suramin, a potent antagonist of P2X2, P2X5, P2Y2, P2Y4, and P2Y11 receptors (10), on cell-volume modulation and Ca2+i signaling triggered by ATP and UTP. Table 3 shows that suramin completely abolished cell shrinkage and sharply diminished increments of [Ca2+]i triggered by ATP or UTP. In contrast, neither MRS2179 nor MRS2578 affected these parameters in ATP-treated C11-MDCK cells.
Table 3.
Effect of antagonists of purinergic receptors on cell shrinkage and [Ca2+]i elevation triggered by ATP and UTP
| Additions, μM | Cell Volume, % | Maximal Increments of [Ca2+]i evoked by ATP or UTP, nM |
|---|---|---|
| 1. ATP, 100 (n = 4) | 52 ± 8 | 673 ± 91 |
| 2. ATP + MRS2179, 10 (n = 3) | 59 ± 6 | 703 ± 82 |
| 3. ATP + MRS2578, 10 (n = 3) | 49 ± 7 | 651 ± 88 |
| 4. ATP + suramin, 100 (n = 5) | 99 ± 11 | 93 ± 67 |
| 5. UTP, 100 (n = 3) | 58 ± 8 | 781 ± 132 |
| 6. UTP + suramin (n = 5) | 104 ± 12 | 118 ± 56 |
| P1,4 | <0.02 | <0.005 |
| P5,6 | <0.02 | <0.005 |
Means ± SE for n experiments and P values <0.05 are shown. C11-MDCK cells were perfused during 10 min in medium A with or without antagonists of purinergic receptors listed in the left column. ATP or UTP were then added for 60 min (cell-volume measurements) or 10 min ([Ca2+]i measurements). Initial cell volume was taken as 100%. MRS2179, 2′-deoxy-N6-methyladenosine-3′,5′-biphosphate; MRS2578, N,N′′-1,4-butanediylbis[N′-(3-isothiocyanatophenyl)]thio urea.
Effect of ion transport inhibitors on ATP-induced cell-volume changes.
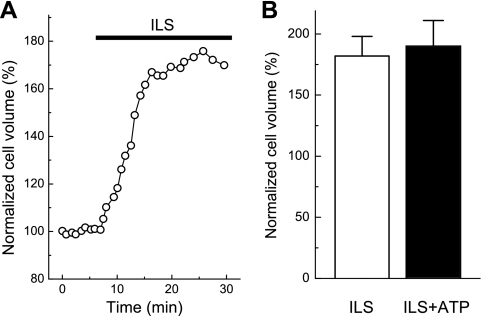
We recently found that elevation of medium osmolality decreases the volume of lung carcinoma A549 cells after dissipation of transmembrane gradients of Na+ and K+ and even after plasma membrane permeabilization (12). To examine the role of transmembrane ion gradients in the ATP-induced shrinkage of C11-MDCK cells, we exposed these cells to intracellular-like, high-K+/low-Na+ medium. As observed in previous studies (34), this medium led to pronounced cell swelling (Fig. 6A). We also found that incubation of cells in intracellular-like medium completely prevented ATP-induced cell shrinkage (Fig. 6B). These results show that the shrinkage of ATP-treated cells is caused by the activation of outwardly directed and/or by the inhibition of inwardly directed fluxes of intracellular inorganic osmolytes and osmotically obliged water rather than by events independent of transmembrane ion movements.
Fig. 6.
ATP-induced C11-MDCK cell shrinkage requires transmembrane K+ and Na+ gradients. A: representative time course of changes in cell volume induced by superfusion with high-K+, low-Na+ intracellular-like solution (ILS) containing (in mM) 10 NaCl, 110 KCl, 1 MgCl2, 1 CaCl2, 5 glucose, and 10 HEPES (pH 7.2, 37°C). B: average volumes of C11-MDCK cells subjected for 60 min to ILS medium or for 30 min to ILS medium followed by 30 min in the same medium containing 50 μM ATP. Initial cell volume measured before application of ILS medium was taken as 100%. Data represent mean values ± SE obtained in 3 independent experiments.
Previously, we demonstrated that sustained application of ATP and UTP resulted in almost complete inhibition of Na+, K+, 2Cl− cotransport in C11-MDCK cells (8, 36). Table 3 shows that 30-min inhibition of Na+, K+, 2Cl− cotransport by 10 μM bumetanide resulted in small ∼15–20% decreases in C11-MDCK cell volume. However, robust shrinkage induced by ATP was preserved in the presence of bumetanide. These results indicate that, although Na+, K+, 2Cl− cotransport inhibition may partially contribute to ATP-induced cell shrinkage, on its own it is insufficient to explain the effects of ATP.
Because K+ is the main intracellular osmolyte, we tested the effects of K+ channel blockers in subsequent experiments. Paxilline and iberiotoxin are known to be selective inhibitors of high-conductance; Ca2+-activated K+ channels (BKCa), charybdotoxin is a potent inhibitor of BKCa and intermediate-conductance Ca2+-activated K+ channels (IKCa), whereas clotrimazole blocks IKCa (41, 48, 59). We observed that 1-h incubation with charybdotoxin and paxilline caused modest swelling of C11-MDCK cells (Table 4) that was consistent with the data on charybdotoxin-treated MDCK-F cells (49). Importantly, charybdotoxin, iberiotoxin, and paxilline completely abolished the cell shrinkage evoked by ATP. Neither baseline cell volume nor ATP-induced cell shrinkage was affected by clotrimazole.
Table 4.
Effect of ion transport inhibitors on ATP-induced cell shrinkage
| Additions, μM | n | Cell Volume, % |
|---|---|---|
| 1. None (control) | 5 | 99 ± 3 |
| 2. Bumetanide, 10 | 5 | 79 ± 4 |
| 3. Charybdotoxin, 0.1 | 3 | 111 ± 5 |
| 4. Paxilline, 2 | 3 | 112 ± 6 |
| 5. Clotrimazole, 2 | 3 | 95 ± 6 |
| 6. Iberiotoxin, 0.1 | 3 | 104 ± 7 |
| 7. NPPB, 100 | 3 | 97 ± 6 |
| 8. ATP, 50 | 6 | 47 ± 6 |
| 9. Bumetanide + ATP | 6 | 52 ± 5 |
| 10. Charybotoxin + ATP | 4 | 96 ± 4 |
| 11. Paxilline + ATP | 4 | 101 ± 6 |
| 12. Iberiotoxin + ATP | 4 | 90 ± 7 |
| 13. Clotrimazole + ATP | 6 | 67 ± 9 |
| 14. NPPB + ATP | 6 | 92 ± 9 |
| P1,2 | <0.01 | |
| P1,8 | <0.001 | |
| P1,9 | <0.001 | |
| P2,9 | <0.005 | |
| P1,13 | <0.005 | |
| P8,10 | <0.001 | |
| P8,11 | <0.001 | |
| P8,12 | <0.005 | |
| P8,13 | =0.092 | |
| P8,14 | <0.002 |
Means ± SE for n experiments and P values <0.05 are shown. C11-MDCK cells were perfused in medium A for 60 min with or without the ion transport inhibitors listed in the left column. In experiments 8–14, ATP was added during the last 30 min of perfusion. Initial cell volume was taken as 100%. NPPB, 5-nitro-2-(3-phenylpropylamino-benzoic) acid.
NPPB, the agent that blocks almost all known anion channels, including Ca2+-activated Cl− channels and VRAC (33), completely inhibited cell-volume modulation by ATP (Table 4).
Effects of ATP on intracellular content of monovalent ions.
To determine the loss of which of the intracellular osmolytes is associated with cell shrinkage, we measured intracellular K+, Na+, and Cl− contents as steady-state distribution of 86Rb+, 22Na+, and 36Cl−. As seen in Table 5, 1-h incubation of C11-MDCK cells with ATP decreased the intracellular content of exchangeable K+ and Cl− by ∼20%. Inhibition of ecto-ATPase by addition of 100 μM ARL 67156 did not affect intracellular content of these monovalent ions but augmented the loss of K+ and Cl− in ATP-treated cells up to 33% (P < 0.02) and 41% (and P < 0.05), respectively. Considering this, we examined the action of inhibitors of K+ and Cl− channels in ATP-treated cells in the presence of ARL 67156. We found that inhibition of Ca2+-activated K+ channels by paxilline, iberiotoxin, or charybdotoxin completely prevented the loss of K+ and Cl− evoked by ATP. ATP effects on intracellular ion content were also suppressed by the potent inhibitor of anion channels, NPPB.
Table 5.
Effect of ATP and ion transport inhibitors on intracellular content of monovalent cations
| Additions, μM | K+ | Na+ | Cl− |
|---|---|---|---|
| None (control) | 210 ± 12 | 49 ± 10 | 146 ± 19 |
| ATP, 100 | 161 ± 12* | ND | 114 ± 17 |
| ARL 67156, 100 | 221 ± 18 | ND | 152 ± 21 |
| ATP + ARL 67156 | 141 ± 13** | 32 ± 8 | 86 ± 10* |
| ATP + ARL 67156+ charybdotoxin, 0.1 | 205 ± 15 | 35 ± 12 | 138 ± 17 |
| ATP + ARL 67156+ paxilline, 2 | 218 ± 8 | 43 ± 10 | 150 ± 14 |
| ATP + ARL 67156+ iberiotoxin, 0.1 | 197 ± 11 | 40 ± 8 | 130 ± 12 |
| ATP + ARL 67156+ NPPB, 100 | 189 ± 15 | 50 ± 13 | 139 ± 16 |
Means ± SE values (in nmol/mg of protein) from experiments performed in triplicate are shown. Cells were incubated for 1 h in medium A with the compounds listed in the left column.
P < 0.05 and
P< 0.02 compared with control. ND, these parameters were not measured; ARL 67156, 6,N,N′-diethyl-β,γ-dibromomethylene-d-adenosine-5-triphosphate.
Charybdotoxin and NPPB suppress ATP-induced c-Fos expression.
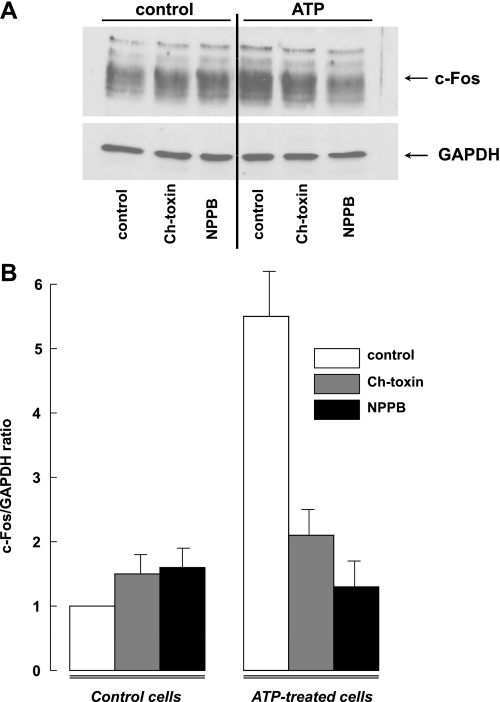
Because the c-Fos promoter contains serum and (Ca2+ + cAMP) response elements, its expression is regulated by diverse stimuli eliciting [Ca2+] elevation (53) and protein kinase activation, including agonists of P2Y receptors (32). Keeping this in mind, we compared the action of ATP on c-Fos expression in the absence and presence of charybdotoxin and NPPB. Figure 7 demonstrates that 30-min incubation of C11-MDCK cells with ATP increased c-Fos protein content by approximately fivefold. Similar to their effects on the ATP-induced cell shrinkage (Table 4), charybdotoxin sharply attenuated and NPPB completely inhibited the ATP-induced c-Fos immunoreactivity.
Fig. 7.
c-Fos expression in C11-MDCK cells. A: representative Western blot showing the effects of ATP, charybdotoxin, and 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) on c-Fos and GAPDH content in C11-MDCK cells. B: effect of charybdotoxin and NPPB on c-Fos/GAPDH ratio in control and ATP-treated cells. Cells were incubated in medium A containing 100 μM 6,N,N′-diethyl-β,γ-dibromomethylene-d-adenosine-5-triphosphate (ARL 67156) ± 0.1 μM charybdotoxin or 100 μM NPPB for 10 min, and then 50 μM ATP or vehicle were added for the next 30 min. c-Fos/GAPDH ratio in the absence of ATP and other tested compounds was taken as 1.0. Shown are the means ± SE obtained in 4 experiments.
DISCUSSION
We report here that prolonged activation of P2Y receptors produces strong and persistent shrinkage of intercalated-like C11-MDCK cells derived from distal tubules of the canine kidney. ATP-induced cell-volume changes are mediated by the activation of charybdotoxin-, iberiotoxin-, and paxilline-sensitive, Ca2+-activated high-conductance K+ channels and NPPB-sensitive anion channels. Importantly, our data indicate that cell shrinkage per se is responsible for at least some effects of purinergic agonists. In our hands, ATP-induced c-Fos expression was completely prevented by blockers of K+ and anion channels, which mediate cell shrinkage. Therefore, cell volume may serve as a previously unrecognized signaling/transducing factor in the purinergic regulation of cellular functions.
Robust and persistent shrinkage of ATP/UTP-treated C11-MDCK cells.
The existing literature suggests that P2Y receptor agonists can modulate cell volume in a cell type-specific manner. Thus ATP was found to produce cell shrinkage in hepatocytes and Ehrlich ascites tumor cells (42, 56) but swelling in cultured brain astrocytes (52). Although changes in cell volume may be of physiological significance, particularly for renal epithelial cells, which are exposed to frequent cell-volume changes in anisotonic environments, detailed studies on cell-volume dynamics in response to ATP are lacking. The absence of such information is in part due to the lack of reliable noninvasive techniques for monitoring cell volume over prolonged periods of time. In this study, we took advantage of the newly developed DISUR conventional microscopy technique to close some of the existing gaps in our knowledge. Very unexpectedly, we observed that ATP causes robust and long-lasting shrinkage in kidney C11-MDCK cells resembling intercalated cells from collecting ducts (Fig. 1). UTP was equipotent to ATP in inducing cell shrinkage (Fig. 3), pointing to the involvement of ATP/UTP-sensitive, G protein-coupled P2Y receptors.
The role of G protein-coupled receptors in cell-volume regulation has recently received much attention (13, 14). It has become increasingly clear that agonists of these receptors, and ATP in particular, may accelerate cell-volume recovery under anisosmotic conditions and potently modulate volume-sensitive channels as well as transporters in cells, even in the absence of cell-volume changes (15, 28, 30, 54). It has also been established that signaling linked to G protein-coupled receptors may impact cell volume, as, for example, in mouse fibroblasts with bradykinin (44), rat hepatocytes with arginine vasopressin (AVP), insulin, glucagon, adenosine, and ATP (18, 56), rat astrocytes with AVP and ATP (11, 52), rat vascular smooth muscle cells with isoproterenol (39), and rat L6 myoblasts with acetylcholine (50). However, in essentially all the above-mentioned cases, changes in cell volume were rather modest, not exceeding ±10–15% of initial baseline values. Therefore, the ∼50% cell shrinkage that we observed in C11-MDCK renal epithelial cells is unexpected and unprecedented. Such extensive shrinkage was comparable or exceeded cell shrinkage triggered by hypertonic solution with as large as a 50% increase in extracellular osmolarity (Fig. 2A). If a similar phenomenon is found in vivo, it would undoubtedly have significant implications for renal physiology, as discussed below.
Cellular mechanisms mediating ATP-induced cell shrinkage.
Our previous work established that C11-MDCK cells express mRNA messages encoding for P2Y1, P2Y2, P2Y11, and P2Y12 receptors (1). On the basis of the nearly equal potency of ATP and UTP in the signaling cascade evoked by these nucleotides in C11-MDCK cells seen in previous experiments (1) and their similar effects on cell volume in the present work (Figs. 1 and 3), it is very likely that ATP/UTP-induced cell shrinkage is triggered by the Gq/G11-coupled P2Y2 receptors possessing equal affinity for these nucleotides (9). Indeed, neither MRS2179 nor MRS2578, selective antagonists of UTP-sensitive P2Y1 and P2Y6 receptors, affected cell shrinkage of ATP-treated cells, whereas the broad-spectrum antagonist of purinergic receptors (including P2Y2, P2Y4, and P2Y11) suramin completely abolished Ca2+i signaling and cell shrinkage triggered by ATP and UTP (Table 3).
Activation of Gq/G11-coupled P2Y receptors, including P2Y2, leads to stimulation of phospholipase C-β, augmented production of inositol-1,4,5-trisphosphate (IP3), and diacylglycerol (DAG) that, in turn, evoke Ca2+ release from IP3-sensitive intracellular stores and activation of Ca2+- and DAG-sensitive PKC isoforms. In our experiments, chelation of [Ca2+]i with BAPTA-AM (Table 2) completely abolished the effect of ATP on cell volume. Similar to ATP and UTP, shrinkage of C11-MDCK cells could be triggered by the Ca2+ ionophore ionomycin and by activation of DAG-dependent isoform(s) of PKC by 4β-PMA (Fig. 4A). The actions of 4β-PMA and Ca2+ ionophore on C11-MDCK cell volume were additive (Fig. 4B), and their combination evoked similar decreases in cell volume as in the presence of ATP/UTP (Table 1). The effects of 4β-PMA on cell volume were Ca2+i sensitive (Table 2), indicating involvement of conventional Ca2+-sensitive, DAG-dependent PKC isoform(s). A similar mechanism involving conventional PKC-α and -βI was reported for ATP-induced activation of VRACs in astrocytes (47).
The ATP-induced cell shrinkage has to be associated with massive Ca2+-dependent loss of intracellular osmolytes. Consistent with this idea, disruption of transmembrane ionic gradients by transferring C11-MDCK cells to intracellular-like ionic medium completely abolished the effect of ATP on cell volume (Fig. 6). We, furthermore, found that ATP triggered statistically significant loss of 86Rb/K+ (∼35%) and 36Cl− (∼40%) and showed a trend for reductions in the intracellular 22Na+ (Table 5). The observed degrees of the ATP-induced loss of intracellular K+ and Cl− seem insufficient to completely explain 55–60% cell shrinkage. Therefore, it is possible that such shrinkage is also associated with loss of compatible organic osmolytes, as seen in many cell types (21, 25).
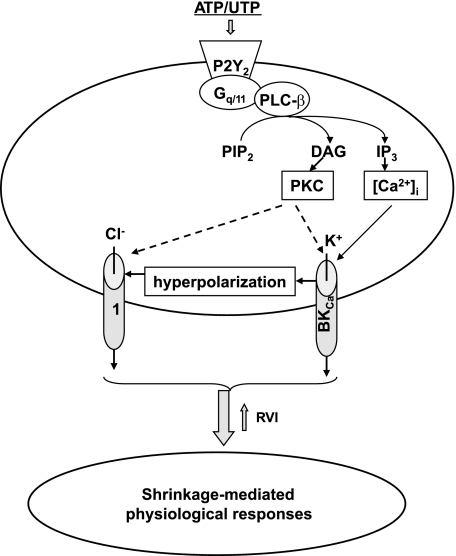
MDCK cells express a number of Ca2+-activated channels, including BKCa and IKCa, as well as Cl− channels inhibited by NPPB (6, 26). Pacha and coworkers (40) observed that the apical membranes of intercalated cells are highly abundant with BKCa compared with principal cells from collecting ducts. In the present study, we found that shrinkage of ATP-treated intercalated-like C11-MDCK cells was completely abolished by the BKCa inhibitors iberiotoxin and paxilline as well as by NPPB and the nonselective BKCa/IKCa blocker charybdotoxin. The IKCa inhibitor clotrimazole showed only minor effect on the ATP-induced volume changes (Table 4). Paxilline, iberiotoxin, charybdotoxin, and NPPB also prevented loss of intracellular K+ and Cl− (Table 5). These results strongly suggest that BKCa and NPPB-sensitive anion channels play a key role in the loss of K+ and Cl− and shrinkage of ATP-treated cells (Fig. 8). It should be underlined, however, that, because of the poorly explored pharmacology of ion channels in canine cells, distinct rank order of potencies and possible side effects of above listed compounds are expected. Thus detailed dose-dependent studies and knockdown of potential targets with siRNA should be performed to draw out the final conclusion on the relative impact of ion channels and other osmolytes transporters in the shrinkage of C11-MDCK cells triggered by P2Y2 receptors.
Fig. 8.
Intermediates of intracellular signaling involved in shrinkage of ATP/UTP-treated cells. Broken arrows indicate unknown targets; 1: nonidentified NPPB-sensitive ion channels. DAG, diacylglycerol; IP3, inositol-1,4,5-trisphosphate; RVI, regulatory volume increase; PIP2 phosphatidylinositol 4,5-bisphosphate.
Physiological implications of ATP-induced cell-volume changes.
To address potential physiological significance of the ATP-induced cell shrinkage, we explored its connection to activation of c-Fos, which belongs to the superfamily of early response genes whose expression is regulated by diverse stimuli, including [Ca2+]i elevation (53). We found that 30-min incubation with ATP led to an approximately fivefold increase in the c-Fos immunoreactivity (Fig. 7). This effect was sharply attenuated by charybdotoxin and completely suppressed by NPPB. These data strongly suggest a key role of cell shrinkage in excitation-transcription coupling and intracellular signaling pathways mediated by diverse c-Fos-sensitive late-response genes. Such coupling may occur in the kidneys, for example, during the exposure of intercalated cells, protruding further into the lumen of the renal tubular epithelium than principal cells, to mechanical stimuli, such as shear stress and transepithelial pressure pulses, which have been shown to cause massive ATP and UTP release from both the apical and basolateral surfaces of epithelial monolayers (9). Recently, using calcein-loaded cells, Holtzclaw and coworkers (22) reported that 30-min exposure to shear stress led to shrinkage of C11-MDCK cells that was abolished by Ca2+i depletion and inhibition of K+ channels and was sharply diminished in cells treated with BKCa-β4 siRNA (22). These data and the results of our study prompt us to hypothesize that purinergic signaling evoked by autocrine ATP/UTP release contributes to the shrinkage of intercalated cells and the elevation of intraluminal diameter triggered by mechanical stimuli. This hypothesis is consistent with the inhibition of Ca2+i signaling provoked by transepithelial pressure pulse in monolayers of polarized MDCK cells by the ATPase apyrase and suramin, a nonspecific antagonist of P2X and P2Y receptors (43). With a mind toward key role of P2Y2 receptors in ATP/UTP-induced signaling in intercalated cells (1), P2Y2-null mice should be studied to examine the involvement of purinergic signaling in shear stress-evoked shrinkage of intercalated cells in vivo.
In conclusion, our work characterized unusually strong and persistent shrinkage of C11-MDCK cells resembling intercalated cells from collecting ducts, in response to purinergic stimulation. Cell shrinkage is mediated by elevation of [Ca2+]i and activation of conventional PKCs that, in turn, trigger massive [Ca2+]i-dependent efflux of K+ and Cl− via BKCa and NPPB-sensitive anion channels, respectively. We also report here that cell shrinkage governs purinergic regulation of the early response gene c-Fos. Therefore, cell volume decrease may serve as a previously unrecognized signaling factor in the purinergic regulation of cellular functions.
GRANTS
This study was supported in part by grants from the Kidney Foundation of Canada (to S. Orlov), the Heart and Stroke Foundation of Canada (to S. Orlov), the Natural Sciences and Engineering Council of Canada (to R. Grygorczyk and S. Orlov), the National Institutes of Health (NS061953 to A. Mongin), and the Russian Foundation for Fundamental Research (09-04-00646A to S. Orlov).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Dr. Michel Gekle (Julius-Bernstein Institute of Physiology, Halle-Wittenberg University, Germany) for the gift of C11-MDCK cells and Mr. Ovid Da Silva for editing this manuscript.
REFERENCES
- 1. Akimova OA, Grygorzcyk A, Bundey RA, Bourcier N, Gekle M, Insel PA, Orlov SN. Transient activation and delayed inhibition of Na+, K+, Cl− cotransport in ATP-treated C11-MDCK cells involve distinct P2Y receptor subtypes and signaling mechanisms. J Biol Chem 281: 31317–31325, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Akimova OA, Poirier M, Kotelevtsev SV, Hamet P, Orlov SN. The death of ouabain-treated renal epithelial cells: evidence against anoikis occurrence. Apoptosis 13: 670–680, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Andersen LK, Contera SA, Justesen J, Duch M, Hansen O, Chevallier J, Foss M, Pedersen FS, Besenbacher F. Cell volume increase in murine MC3T3-E1 pre-osteoblasts attaching onto biocompatible tantalum observed by magnetic AC mode atomic force microscopy. Eur Cell Mater 10: 61–68, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bortner CD, Cidlowski JA. Absence of volume regulatory mechanisms contributes to the rapid activation of apoptosis in thymocytes. Am J Physiol Cell Physiol 271: C950–C961, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Boudreault F, Grygorczyk R. Evaluation of rapid volume changes of substrate-adherent cells by conventional microscopy 3D imaging. J Microsc 215: 302–312, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bourcier N, Grygorczyk R, Gekle M, Berthiaume Y, Orlov SN. Purinergic-induced ion current in monolayers of C7-MDCK cells: role of basolateral and apical ion transporters. J Membr Biol 186: 131–143, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Bourque CW, Oliet SH, Richard D. Osmoreceptors, osmoreception, and osmoregulation. Front Neuroendocrinol 15: 231–274, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Brindikova TA, Bourcier N, Torres B, Pchejetski D, Gekle M, Maximov GV, Montminy V, Insel PA, Orlov SN, Isenring P. Purinergic-induced signaling in C11-MDCK cells inhibits the secretory Na-K-Cl cotransporter. Am J Physiol Cell Physiol 285: C1445–C1453, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87: 659–797, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci 64: 1471–1483, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Del Bigio MR, Fedoroff S, Qualtiere LF. Morphology of astroglia in colony cultures following transient exposure to potassium ion, hypoosmolarity and vasopressin. J Neurocytol 21: 7–18, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Fels J, Orlov SN, Grygorczyk R. The hydrogel nature of mammalian cytoplasm contributes to osmosensing and extracellular pH sensing. Biophys J 96: 4276–4285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fisher SK, Cheema TA, Foster DJ, Heacock AM. Volume-dependent osmolyte efflux from neuronal tissues: regulation by G-protein-coupled receptors. J Neurochem 106: 1998–2014, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franco R, Panayiotidis MI, de La Paz LD. Autocrine signaling involved in cell volume regulation: the role of released transmitters and plasma membrane receptors. J Cell Physiol 216: 14–28, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Franco R, Rodriquez R, Pasantes-Morales H. Mechanisms of the ATP potentiation of hyposmotic taurine release in Swiss 3T3 fibroblasts. Pflügers Arch 449: 159–169, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Gekle M, Wunsch S, Oberleithner H, Silbernagl S. Characterization of two MDCK-cell subtypes as a model system to study principal cell and intercalated cell properties. Pflügers Arch 428: 157–162, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Groulx N, Boudreault F, Orlov SN, Grygorczyk R. Membrane reserves and hypotonic cell swelling. J Membr Biol 214: 43–56, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Hallbrucker C, vom Dahl S, Lang F, Gerok W, Haussinger D. Modification of liver cell volume by insulin and glucagon. Pflügers Arch 418: 519–521, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Hamann S, Kiilgaard JF, Litman T, Alvarez-Leefmans FJ, Winther BR, Zeuthen T. Measurement of cell volume changes by fluorescence self-quenching. J Fluoresc 12: 139–145, 2002 [Google Scholar]
- 20. Haussinger D. The role of cellular hydration in the regulation of cell function. Biochem J 313: 697–710, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev 89: 193–277, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Holtzclaw JD, Liu L, Grimm PR, Sansom SC. Shear stress-induced volume decrease of C11-MDCK cells by BK-α4/β4. Am J Physiol Renal Physiol 299: F507–F516, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hussy N, Deleuze C, Desarmenien MG, Moos FC. Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog Neurobiol 62: 113–134, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Kuchkina NV, Orlov SN, Pokudin NI, Chuchalin AG. Volume-dependent regulation of respiratory burst of activated human neutrophils. Experientia 49: 995–997, 1993 [DOI] [PubMed] [Google Scholar]
- 25. Lang F, Busch G, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev 78: 247–306, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Lang F, Paulmichl M. Properties and regulation of ion channels in MDCK cells. Kidney Int 48: 1200–1205, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Lang F, Ritter M, Gamper N, Huber S, Fillon S, Tanneur V, Lepple-Wienhues A, Szabo I, Gulbins E. Cell volume in the regulation of cell proliferation and apoptotic cell death. Cell Physiol Biochem 10: 417–428, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Loveday D, Heacock AM, Fisher SK. Activation of muscarinic cholinergic receptors enhances the volume-sensitive efflux of myo-inositol from SH-SY5Y neuroblastoma cells. J Neurochem 87: 476–486, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Mongin AA, Kimelberg HK. ATP potently modulates anion channel-mediated excitatory amino acid release from cultured astrocytes. Am J Physiol Cell Physiol 283: C569–C578, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Mongin AA, Kimelberg HK. ATP regulates anion channel-mediated organic osmolyte release from cultured rat astrocytes via multiple Ca2+-sensitive mechanisms. Am J Physiol Cell Physiol 288: C204–C213, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Mongin AA, Orlov SN. Mechanisms of cell volume regulation and possible nature of the cell volume sensor. Pathophysiology 8: 77–88, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Muscella A, Elia MG, Greco S, Storelli C, Marsigliante S. Activation of P2Y receptor induces c-FOS protein through a pathway involving mitogen-activated protein kinases and phosphoinositide 3-kinases in HeLa cells. J Cell Physiol 195: 234–240, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev 81: 1415–1459, 2001 [DOI] [PubMed] [Google Scholar]
- 34. O'Neill WC. Physiological significance of volume-regulated transporters. Am J Physiol Cell Physiol 276: C995–C1011, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J Physiol 532: 3–16, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Orlov SN, Dulin NO, Gagnon F, Gekle M, Douglas JG, Schwartz JH, Hamet P. Purinergic regulation of Na+,K+,Cl− cotransport and MAP kinases is limited to C11-MDCK cells resembling intercalated cells from collecting ducts. J Membr Biol 172: 225–234, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Orlov SN, Mongin AA. Salt sensing mechanisms in blood pressure regulation and hypertension. Am J Physiol Heart Circ Physiol 293: H2039–H2053, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Orlov SN, Pchejetski D, Taurin S, Thorin-Trescases N, Maximov GV, Pshezhetsky AV, Rubin AB, Hamet P. Apoptosis in serum-deprived vascular smooth muscle cells: evidence for cell volume-independent mechanism. Apoptosis 9: 55–66, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Orlov SN, Tremblay J, Hamet P. Cell volume in vascular smooth muscle is regulated by bumetanide-sensitive ion transport. Am J Physiol Cell Physiol 270: C1388–C1397, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Pacha J, Frindt G, Sackin H, Palmer LG. Apical maxi K channels in intercalated cells of CCT. Am J Physiol Renal Fluid Electrolyte Physiol 261: F696–F705, 1991 [DOI] [PubMed] [Google Scholar]
- 41. Pedarzani P, Stocker M. Molecular and cellular basis of small- and intermediate-conductance calcium-activated potassium channel function in the brain. Cell Mol Life Sci 65: 3196–3217, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pedersen SF, Pedersen S, Lambert IH, Hoffmann EK. P2 receptor-mediated signal transduction in Ehrlich ascites tumor cells. Biochim Biophys Acta 1374: 94–106, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Praetorius HA, Frokier J, Leipziger J. Transepithelial pressure pulses induce release in polarized MDCK cells. Am J Physiol Renal Physiol 288: F133–F141, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Ritter M, Woll E, Haussinger D, Lang F. Effect of bradykinin on cell volume and intracellular pH in NIH 3T3 fibroblasts expressing the ras oncogene. FEBS Lett 307: 367–370, 1992 [DOI] [PubMed] [Google Scholar]
- 45. Rothstein A, Mack E. Volume-activated K+ and Cl- pathways of dissociated epithelial cells (MDCK): role of Ca2+. Am J Physiol Cell Physiol 258: C827–C834, 1990 [DOI] [PubMed] [Google Scholar]
- 46. Roy G, Sauvé R. Effect of anisotonic medium on volume, ion and amino-acid content and membrane potential of kidney cells (MDCK) in culture. J Membr Biol 100: 83–96, 1987 [DOI] [PubMed] [Google Scholar]
- 47. Rudkouskaya A, Chernoguz A, Haskew-Layton RE, Mongin AA. Two conventional protein kinase C isoforms, alpha and beta I, are involved in the ATP-induced activation of volume-regulated anion channel and glutamate release in cultured astrocytes. J Neurochem 105: 2260–2270, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sanchez M, McManus OB. Paxilline inhibition of the α-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology 35: 963–968, 1996 [DOI] [PubMed] [Google Scholar]
- 49. Schneider SW, Pagel P, Rotsch C, Danker T, Oberleithner H, Radmacher M, Schwab A. Volume dynamics in migrating epithelial cells measured with atomic force microscopy. Pflügers Arch 439: 297–303, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Sen CK, Hanninen O, Orlov SN. Unidirectional sodium and potassium flux in myogenic L6 cells: mechanisms and volume-dependent regulation. J Appl Physiol 78: 272–181, 1995 [DOI] [PubMed] [Google Scholar]
- 51. Solenov E, Altamirano J, Huerto L, Alvarez-Leefmans FJ. Sevenfold-reduced osmotic water permeability in primary astrocyte cultures from AQP-4-deficient mice, measured by a fluorescence quenching method. Am J Physiol Cell Physiol 286: C426–C432, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Takano T, Kang J, Jaiswal JK, Simon SM, Lin JH, Yu Y, Li Y, Yang J, Dienel G, Zielke HR. Receptor-mediated glutamate-release from volume-sensitive channels in astrocytes. Proc Natl Acad Sci USA 102: 16466–16471, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Taurin S, Dulin NO, Pchejetski D, Grygorczyk R, Tremblay J, Hamet P, Orlov SN. c-Fos expression in ouabain-treated vascular smooth muscle cells from rat aorta: evidence for an intracellular-sodium-mediated, calcium-independent mechanism. J Physiol 543: 835–847, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tilly BC, Edixhoven MJ, van den Berghe N, Bot AG, de Jonge HR. Ca2+-mobilizing hormones potentiate hypotonically-induced activation of ionic conductances in intestine 407 cells. Am J Physiol Cell Physiol 267: C1271–C1278, 1994 [DOI] [PubMed] [Google Scholar]
- 55. Vazquez-Juarez E, Ramos-Mandujano G, Hernandez-Benitez R, Pasantes-Morales H. On the role of G-protein-coupled receptors in cell volume regulation. Cell Physiol Biochem 21: 1–14, 2008 [DOI] [PubMed] [Google Scholar]
- 56. vom Dahl S, Hallbrucker C, Lang F, Haussinger D. Regulation of cell volume in the perfused rat liver by hormones. Biochem J 280: 105–109, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci USA 93: 12020–12025, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wehner F, Olsen H, Tinel H, Kinne-Saffran E, Kinne RKH. Cell volume regulation: osmolyte transport and signal transduction. Rev Physiol Biochem Pharmacol 148: 1–80, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channels: a potential immunosuppressant. Proc Natl Acad Sci USA 97: 8151–8156, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]