Abstract
Cold-inducible RNA-binding protein (RBM3) is suggested to be involved in the regulation of skeletal muscle mass. Cell death pathways are implicated in the loss of muscle mass and therefore the role of RBM3 in muscle apoptosis in C2C12 myoblasts was investigated in this study. RBM3 overexpression was induced by either cold shock (32°C exposure for 6 h) or transient transfection with a myc-tagged RBM3 expression vector. Cell death was induced by H2O2 (1,000 μM) or staurosporine (StSp, 5 μM), and it was shown that cold shock and RBM3 transfection were associated with attenuation of morphological changes and an increase in cell viability compared with normal temperature or empty vector, respectively. No changes in proliferation were observed with either cold shock or RBM3 transfection. DNA fragmentation was not increased in response to H2O2, and a cell permeability assay indicated that cell death in response to H2O2 is more similar to necrosis than apoptosis. RBM3 overexpression reduced apoptosis and the collapse of the membrane potential in response to StSp. Moreover, the increase in caspase-3, -8, and -9 activities in response to StSp was returned to control levels with RBM3 overexpression. These results indicate that increased RBM3 expression decreases muscle cell necrosis as well as apoptosis and therefore RBM3 could potentially serve as an intervention for the loss of muscle cell viability during muscle atrophy and muscle diseases.
Keywords: muscle atrophy, hibernation, RNA-binding proteins, apoptosis, necrosis
skeletal muscle has a remarkable capacity to adapt to changes in mechanical and metabolic environment. Whereas increased use in the form of resistance exercise leads to hypertrophy, reduced muscle activity, as well as certain physiological and pathological processes, can lead to a severe loss in muscle mass (for review see Ref. 25). Skeletal muscle atrophy is often associated with diseases such as cancer, acquired immunodeficiency syndrome, diabetes, and congestive heart failure, and when present carries a poor prognosis (6, 44). In addition, age-related loss of muscle mass (sarcopenia) is an important predictor of mortality in the aged (40, 48, 49, 63) and has been suggested as a main contributor to the loss of independence in elderly (40, 50). Muscle atrophy is also a common symptom in neuromuscular diseases, even though the etiology of the disorders is often quite different. Cell death-related signaling events are common in muscle cells undergoing atrophy, but the exact role of apoptosis, autophagy, and necrosis (three common forms of cell death) remains to be determined.
In a previous study, we identified the cold-inducible RNA-binding motif protein 3 (RBM3) as a possible regulator of skeletal muscle mass under disuse-induced atrophy (23). RBM3 gene expression and protein abundance were increased in muscles atrophied in response to hindlimb suspension (HS), particularly in old rats (23). RBM3 is a small RNA-binding protein (17 kDa) identified because of its similarities to other RNA-binding proteins (16), and it is part of the family of cold shock proteins (28, 43, 60). RBM3 belongs to the glycine-rich RNA-binding protein family, which also includes cold-inducible RNA-binding protein (CIRP) (14). Proteins from this family have been found in all three domains of life (41) and are induced by various environmental stresses, such as hypoxia (67), and particularly by cold (3, 14, 60). The proposed function of cold shock proteins is thought to be related to their action as RNA chaperones that maintain RNA in the correct secondary structure during periods of cold stress, preventing RNA degradation (3, 28, 29) and facilitating translation (43, 60). Previous studies in a neuronal cell line showed that RBM3 enhanced global protein synthesis and the formation of active polysomes while reducing the levels of ribonucleoprotein complexes containing microRNAs (18, 58).
Besides its effect on protein synthesis, RBM3 may serve to prevent the loss of muscle mass by its ability to decrease cell death. Apoptosis (programmed cell death type I) has been proposed to be involved in skeletal muscle atrophy (4, 5, 20, 21, 36, 47), and we have shown previously that apoptotic loss of myonuclei occurs with disuse- and age-associated muscle atrophy (22, 24, 37). RBM3 suppressed cell death in both neuronal and nonneuronal cell lines (34) and was shown to be responsible for the neuroprotective effect of hypothermia through a decrease in apoptosis (12). Conversely, inhibition of RBM3 was associated with attenuated cell survival, an increase in susceptibility to chemotherapy (72), as well as an increase in apoptosis (62, 68). Recently, it has been shown that RBM3 was required for cell proliferation and mitosis (62, 68) and all these findings taken together have prompted the idea that RBM3 and other cold-inducible RNA-binding proteins could be proto-oncogenes (38). The increase in RBM3 mRNA abundance during muscle atrophy occurred at a point in time that apoptosis is already elevated (24) and may therefore be a compensatory response to the loss of nuclei. Siu et al. (57) proposed a similar scenario for antiapoptotic regulatory factors which were increased in old muscle undergoing atrophy and suggested that there may be an “apoptosis-braking” mechanism to counter the apoptotic elimination of myonuclei (57).
Another line of evidence suggesting a role for RBM3 in the decrease in muscle atrophy is its potential function during hibernation. For example, gene expression profiling identified RBM3 as the only gene to be highly induced during hibernation in liver, brain, and cardiac tissue in the golden-mantled ground squirrel (69) and to be elevated in all tissues studied, including skeletal muscle, of the hibernating arctic ground squirrel (71). RBM3 was also the most highly elevated gene in liver and muscle of hibernating black bears in concordance with genes mostly involved in protein synthesis (26). Interestingly, during mammalian hibernation (as well as amphibian aestivation) muscle mass in bears, squirrels, prairie dogs, bats, and hamsters does not decrease to the extent expected from the level of inactivity (30, 32, 35, 39, 51, 55). Similarities between these results and our observations of increased RBM3 expression in response to conditions inducing muscle atrophy may indicate a common role of RBM3 (23). We have shown that RBM3 is elevated 2 wk after HS (23), indicating that hibernation and disuse atrophy may have a common response, as was also suggested by Yan et al. (71). Given that RBM3 was increased relatively late during atrophy, i.e., after apoptosis was already evident (24), we propose that its elevation may serve as a compensatory mechanism that limits losses in muscle size under atrophy-inducing conditions. Since cell death signaling pathways are known to be involved in atrophy, we concentrated on those pathways in this study.
Therefore, we hypothesized that RBM3 decreases cell death and related mechanisms in skeletal muscle cells. To test this hypothesis, we used the C2C12 myoblast cell line to investigate the response to cell death-inducing agents staurosporine (StSp) and H2O2 in the presence and absence of cold shock and RBM3 overexpression through transient transfection. In this study, myoblasts were used instead of myotubes because induction of apoptosis in myotubes in vitro is challenging caused by their high antiapoptotic environment. Even though there are differences in the cell death mechanisms between myoblasts and myotubes, this study will begin to investigate the effects of RBM3 and cold shock on muscle cells and will form a starting point for future investigations in myotubes and whole muscle in vivo.
METHODS
Cell culture.
Monolayer cultures of C2C12 mouse myoblast cell line were grown in Dulbecco's modified Eagle's medium (DMEM) (Mediatech, Manasses, VA) supplemented with 20% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) and 50 μg/ml penicillin and streptomycin (Invitrogen, Carlsbad, CA) at 37°C in a humidified 10% CO2-90% air atmosphere incubator as described previously (64).
To induce cold shock, cells were plated in six-well plates at ∼60% confluency and were placed at 32°C for 6 h, followed by 16 h at 37°C.
Heat shock was induced in cells plated at 60% confluency by incubating at 42°C for 6 h followed by 16 h at 37°C.
Transient transfections.
Transient transfections were performed using Lipofectamine LTX (Invitrogen) according to the manufacturer's instructions. Specifically, C2C12 cells were plated at 40,000 cells per well using six-well plates the day before transfection; 6.25 μl Lipofectamine LTX was incubated with 2.5 μg of total DNA and 2.5 μl Plus reagent in 500 μl serum-free DMEM for 2 h. The mixture was then added to the cells and incubated for 18 h after which the assays were performed. Cells were transfected with c-myc-RBM3-pcDNA3.1 construct (58) or with the empty vector pcDNA3.1 (Invitrogen). Transfection efficiency under these circumstances was 50–60%.
Induction of cell death.
After cold shock or transfection, the susceptibility of the cells to cell death inducing agents was determined. To induce cell death, C2C12 myoblast cells were treated with either 1,000 μM H2O2 or 5 μM StSp for 3 h. These concentrations and this time point were chosen after determination of the optimal response in myoblasts (70). Control plates were treated with vehicle only for the same amount of time and were always performed at the same time as the experimental plates. All quantitative data were obtained from three to eight duplicate plates, and the number of actual plates is specifically listed in the figure legends.
Cell viability.
Cell viability was measured using the Trypan blue exclusion assay. This assay is based on the fact that dead cells take up Trypan blue dye while viable cells exclude the dye. C2C12 cells were plated with 40,000 cells/well on six-well plates; cells were then treated with H2O2 or StSp as described above or treated as control. After incubation, cells were trypsinized, spun down, and resuspended in serum-free DMEM after which 0.4% Trypan blue was added for 2 min and the number of viable cells was counted with a hemocytometer. The number of viable cells was expressed as a percentage of controls.
Cell proliferation determination.
Cell proliferation was assessed by measuring 5-bromo-2-deoxyuridine (BrdU) incorporation according to the manufacturer's instructions. In brief, cells were incubated with BrdU labeling reagent (Invitrogen) diluted 1:100 in media for 3 h. Cells that had undergone cold shock were stained after their return to 37°C. After the incubation with BrdU labeling reagent, cells were fixed in 70% ethanol for 30 min at 4°C and then blocked with 3% H2O2 in 100% methanol for 10 min, followed by 30 min of incubation in denaturing solution and 10 min in blocking buffer (BrdU staining kit, Invitrogen). Biotinylated mouse anti-BrdU (Invitrogen) was added for 1 h after which cells were rinsed in PBS and incubated in streptavidin-peroxidase for 10 min. Plates were then treated with 1:200 dilution of fluorescein tyramide signal amplification (TSA) buffer for 30 min (Perkin Elmer, Boston, MA). To stain all nuclei, plates were treated with Hoechst at 2.4 μg/ml and incubated for 20 min. Cells were counted using a fluorescent microscope (Zeiss Axio Observer, Thornwood, NY), and the ratio of BrdU-positive cells to total cell number was calculated.
ELISA assay.
DNA fragmentation in response to cell death inducers was determined by measuring mono- and oligonucleosomal fragments in C2C12 cells using a cell death ELISA kit (Roche Biochemicals) per manufacturer's instructions. Briefly, cells and media containing cells were harvested in the buffer supplied by the kit, centrifuged, and resuspended in equal volumes between samples. Colorimetric changes were measured using Spectra Max M2 Microplate reader (Molecular Devices). Apoptotic index is expressed as arbitrary density units per μg protein.
Terminal transferase dUTP nick end labeling assay.
Cells exhibiting DNA fragmentation were identified by terminal transferase dUTP nick end labeling assay (TUNEL) assay according to the manufacturer's recommendations with minor revision (Roche Molecular Biochemicals, Pleasanton, CA). Briefly, after 3 h of incubation with cell death inducers, myoblasts were rinsed with PBS and fixed in 2% paraformaldehyde at room temperature for 20 min, then blocked in 3% H2O2 for 20 min and permeabilized with 0.1% Triton X-100 and 0.1% sodium citrate. Cells were incubated with TUNEL labeling mix diluted (1:3) with dilution buffer in a humidified chamber at 37°C for 1 h, followed by incubation with streptavidin-peroxidase enzyme conjugate at 37°C for 30 min, and the signal was amplified with TSA (PerkinElmer) at a dilution of 1:200 for 30 min. Cells were then incubated with Hoechst dye at 2.4 μg/ml for 20 min. Cells were visualized using a Zeiss Axio Observer microscope and TUNEL-positive (green) as well as total nuclei (blue) were counted in five separate fields. Apoptotic susceptibility was presented as the percentage of TUNEL-positive nuclei per total counted nuclei.
Membrane permeability/dead cell apoptosis assay.
To distinguish between apoptotic and necrotic cells, the membrane permeability/dead cell apoptosis assay was used according to the manufacturer's instructions (Invitrogen). C2C12 myoblast cells were seeded in six-well plates, where the plates were grown to ∼60% confluency before being treated with either 1,000 μM H2O2 or 5 μM StSp. After cell death induction, the media of the cells were changed to serum-free DMEM and the two separate dyes, YO-PRO-1 and propidium iodide (PI), contained in the kit, were added to the cells at 1 μl/ml and incubated at 37°C for 20 min. YO-PRO, a green fluorescent dye (530 nm emission wavelength), can enter the more permeant cytoplasmic membrane of apoptotic cells, whereas PI, a red fluorescent dye (610 emission wavelength), cannot, but enters necrotic cells. Therefore, apoptotic cells show only green fluorescence, necrotic cells show red and green fluorescence, and live cells show no fluorescence. Ethanol-treated cells were used as a positive control for necrosis because they show only red fluorescence and no green fluorescence. Cells were photographed using a fluorescent Zeiss Axio Observer microscope, and Axio Vision software (Zeiss) was used to quantify optical density from the fluorescent signal.
Assessment of mitochondrial membrane potential.
After treatment with H2O2 and StSp, mitochondrial membrane potential (Δψm) was assessed using the cationic dye JC-1 (5,5′, 6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolylcarbocyanine iodide), according to the manufacturer's protocol (Biotium, Hayward, CA) and as described previously (70). In healthy cells the dye stains the cells bright red due to accumulation of the dye within the mitochondria. In cells in which the mitochondrial membrane potential has been changed due to the induction of apoptosis the dye is unable to accumulate within the mitochondria and the dye stains the cells green (for examples of pictures see Ref. 70). The ratio of red divided by green fluorescence is calculated for all cells in a particular field of view combined, and a decrease in this ratio indicates a decrease in mitochondrial membrane potential. C2C12 cells were plated at ∼60% confluency on six-well plates and were exposed to 1,000 μM H2O2, 5 μM StSp or control conditions for 3 h, rinsed with PBS, and followed by incubation with JC-1 reagent 1:100 at 37°C for 30 min. After incubation, cells were again rinsed twice with PBS and images were obtained using TRITC (red, 610 nm) and FITC (green, 535 nm) filters on a fluorescent microscope (Zeiss Axio Observer). The total fluorescence of all the cells within one field was determined in both the red and the green filters. Care was taken to obtain pictures with identical exposure times, and pictures were analyzed using Axio Vision software (Zeiss). The automated measurement program with identical user-defined parameters for densitometric and geometric variables was used to determine fluorescence intensity for both filters. The ratio of the sum of intensities of red divided by green fluorescence was determined.
Caspase activity measurements.
Whole cell lysates of myoblasts were obtained using RIPA buffer (Santa Cruz Biotechnology, Santa Cruz, CA) along with 1 μg/ml leupeptin and 1 mM PMSF. Caspase activities for caspase-3, -8, and -9 were measured in the protein homogenate using fluorometric substrates as described previously (37, 70). The following substrates were used for caspase-3, -8, and -9, respectively: Ac-DEVD-AMC, Ac-IETD-AMC, and Ac-LEHD-AMC (Peptides International, Louisville, KY). The substrates were cleaved proteolytically by the corresponding caspases, and the fluorescence of free AMC was measured and compared with a standard curve for free AMC (Sigma, St. Louis, MO). For determination of caspase activities 100 μg of total protein were incubated for 3 h in caspase buffer (100 mM HEPES, 10% sucrose, 10 mM DTT, 0.1% CHAPS, 1 μg/ml leupeptin, and 1 mM PMSF) with 100 mM substrate. Fluorescence was determined with an excitation wavelength of 380 nm and an emission wavelength of 460 nm for AMC using a Spectra Max M2 Fluorescent Microplate reader (Molecular Devices, Sunnyvale, CA). Values are expressed as nmol AMC per μg of protein.
WESTERN BLOT ANALYSIS.
Whole cell lysates of myoblasts were obtained using RIPA buffer (Santa Cruz Biotechnology) along with 1 μg/ml leupeptin, 1 mM PMSF, 1 μg/ml pepstatin, and 1 μg/ml aprotinin. Protein concentration of the supernatants was determined using the bicinchoninic acid assay (Pierce Biotechnology). A total of 10–30 μg of protein per sample were separated on a 4–15% acrylamide gradient gel (Bio-Rad, Hercules, CA) and transferred to PVDF-FL membranes (Millipore, Billerica, MA). Membranes were placed in Odyssey blocking buffer (Li-Cor, Lincoln, NE) for 1 h at room temperature, followed by incubation with the following primary antibodies: heat shock protein 70 (HSP-70) and HSP-25 1:1,000 (Assay Designs, Ann Harbor, MI), receptor interacting protein (RIP)-1 1:1,000 (Cell Signaling), RIP-3 1:200 (ProSci), and RBM3 1:1,000 (18). Primary antibody incubation was performed overnight at room temperature after which membranes were rinsed in PBS and further incubated with the corresponding IRdye 680 or 800-conjugated secondary antibody (Li-Cor). Blots were scanned with the Odyssey Infrared Imaging System (Li-Cor), and the density of resulting bands was quantified using Odyssey software (Li-Cor). Subsequently, membranes were incubated in Ponceau S solution (Sigma) for 5 min for visualization of the protein and assurance of equal loading in all the lanes.
Statistical analysis.
Statistical computations were performed using SigmaStat (SSI, Richmond, CA). For comparisons of means between treatments, a one-way- or two way-ANOVA was applied where appropriate. In case of significant differences, the Holm-Sidak multiple comparisons test was used. Values are listed as means ± SE. Statistical significance was set at P < 0.05.
RESULTS
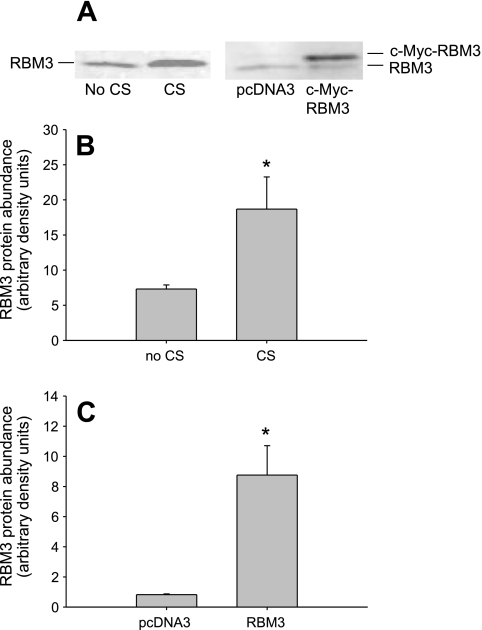
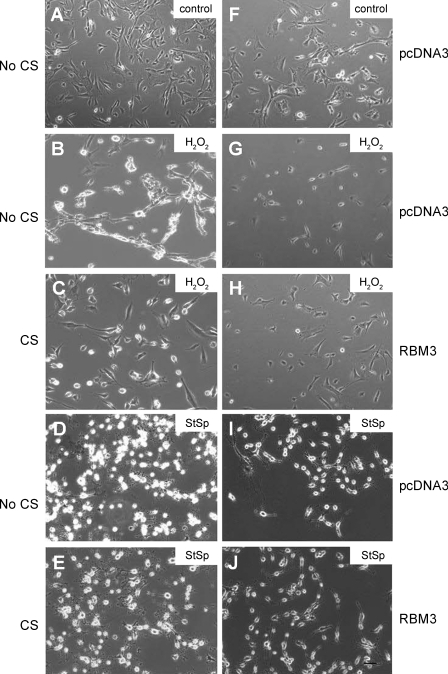
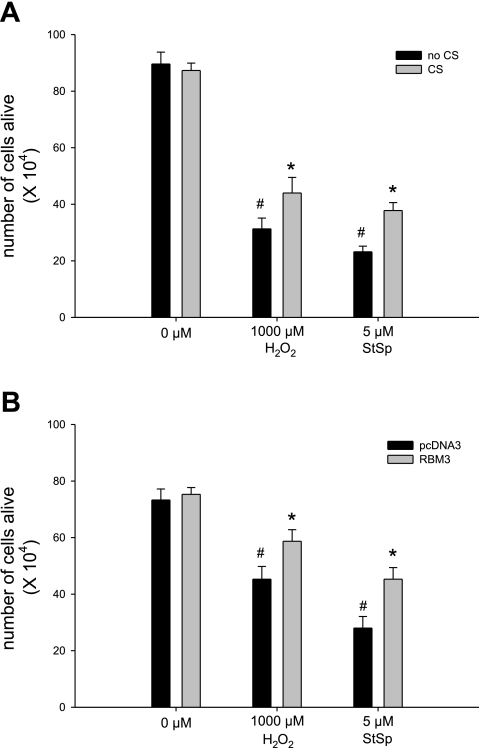
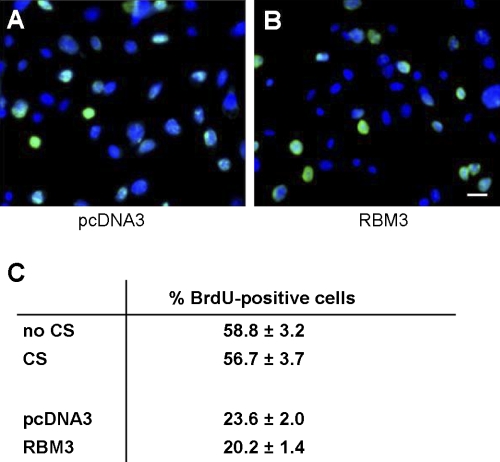
Overexpression of RBM3 protein in C2C12 cells was accomplished by subjecting the cells to cold shock or to transfection with c-myc-RBM3 expression construct. Cold shock increased the protein abundance of RBM3 by 2.5-fold (Fig. 1A, left two lanes, and Fig. 1B) while transfection with a vector containing myc-tagged RBM3 induced a 10-fold increase in total RBM3 protein abundance compared with the empty vector, pcDNA3 (Fig. 1A, right two lanes, and Fig. 1C). The morphology of the myoblasts changed in response to cell death inducers H2O2 (Fig. 2, B and G) and StSp (Fig. 2, D and I), by becoming more rounded with less extensions and by showing more cell clustering in the case of H2O2. These changes were attenuated in cells subjected to cold shock before H2O2 (Fig. 2C) or StSp (Fig. 2E) treatment or in cells transfected with myc-RBM3 before H2O2 (Fig. 2H) or StSp (Fig. 2J) treatment in which cases cells were less rounded and remained more flat. It should be noted that cells having undergone transient transfections in general look more stressed than nontransfected cells making it more difficult to see the difference with the interventions. Therefore further analyses investigating these changes were performed. The effect of RBM3 overexpression on cell number was investigated by a Trypan blue-based cell survival assay. Upon addition of H2O2 and StSp, cell survival decreased 65% and 74%, respectively, in the cells cultured under normal temperature conditions (Fig. 3A). When the cells were cultured under cold shock conditions there was an increase in cell survival of 30% and 40% with H2O2 and StSp, respectively, compared with no cold shock (Fig. 3A). Similarly, cell survival was decreased 49% and 72% after cells transfected with empty vector were subjected to H2O2 and StSp, respectively; RBM3 overexpression was associated with a significant increase in cell survival of 23% and 30% after treatment with H2O2 and StSp, respectively (Fig. 3B). Since an increase in cell survival as measured by Trypan blue can be due to an increase in cell proliferation, we performed a BrdU labeling assay in which BrdU-labeled cells were counted in response to cold shock (not shown) and RBM3 overexpression (Fig. 4B) compared with empty vector transfection (Fig. 4A). BrdU labeling was not different in cells subjected to cold shock or RBM3 overexpression (Fig. 4C), indicating that the increase in cell survival upon RBM3 overexpression or cold shock was due to a decrease in cell death.
Fig. 1.
RNA-binding protein (RBM3) abundance is increased in response to cold shock (CS) and upon transient transfection with c-myc-RBM3 construct. A: representative lanes of Western analysis. B: quantification of RBM3 abundance of CS (n = 9) compared with no CS (n = 8). C: quantification of RBM3 abundance of empty vector transfection (pcDNA3, n = 5) compared with transfection with c-myc-RBM3 containing vector (n = 6). *Significant difference from no CS (B) and pcDNA3 (C). Values are means ± SE; P < 0.05.
Fig. 2.
Cold shock and RBM3 overexpression protect from morphological changes. A–J: representative photographs from C2C12 myoblasts without a cell death inducer kept under control temperature (A) or transfected with pcDNA3 (F) compared with myoblasts treated with 1,000 μM H2O2 (B, C, G, and H) or 5 μM staurosporine (StSp; D, E, I, and J) either kept under control temperature (no CS: B and D) compared with cold shocked (C and E) or transfected with pcDNA3 (G and I) compared with transfected with RBM3 (H and J). Bar in J is for all pictures and indicates 100 μm.
Fig. 3.
Cold shock and RBM3 overexpression increase cell survival. Cell survival in response to H2O2 and StSp in myoblasts with (gray bars) and without (black bars) CS (A) and transfected with pcDNA3 (black bars) or RBM3 (gray bars; B) is depicted. [n = 4–6 in CS experiments (A) and n = 6 in all transfection experiments (B)]. #Significant difference from control conditions (0 μM); *significant difference from no CS in A and pcDNA3 transfected in B. Values are means ± SE; P < 0.05.
Fig. 4.
No change in proliferation rate in response to CS or transfection. A and B: representative pictures of 5-bromo-2-deoxyuridine (BrdU) incorporation in myoblasts transfected with pcDNA3 (A) and RBM3 (B). BrdU is in green and Hoechst is in blue. Bar in B is for both pictures and indicates 100 μm. C: quantification of cells exposed to CS or transfection. Values are means ± SE; n = 5–6 in experiments.
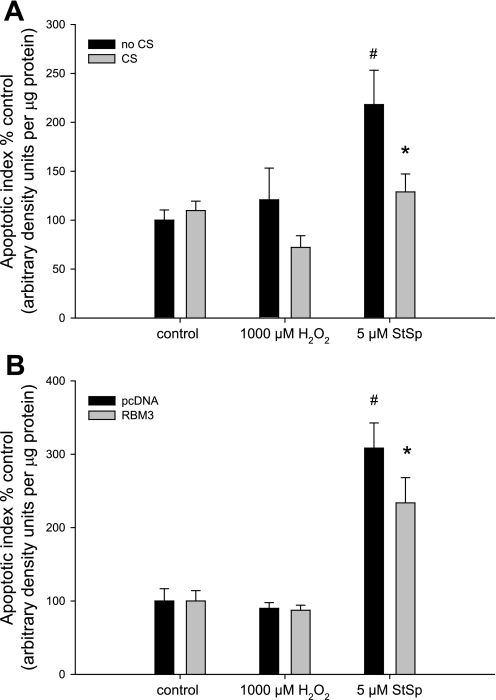
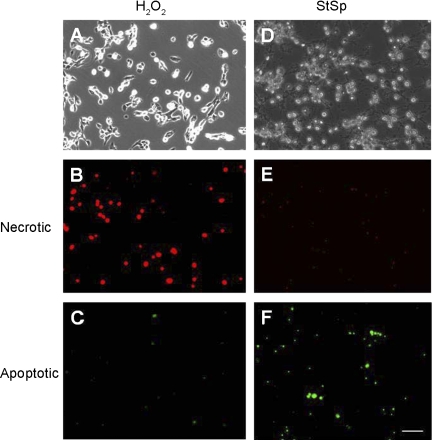
To investigate whether the increased abundance of RBM3 was associated with a decrease in apoptosis, a cell death ELISA assay was performed that measures DNA fragmentation in the cellular extracts. Under control temperature conditions, treatment with StSp was associated with a 2.1-fold increase in apoptosis, but treatment with H2O2 did not significantly increase the apoptotic index (Fig. 5A). Cold shock kept the apoptotic index to that of control vehicle-treated cells (Fig. 5A). Similarly, cells transfected with empty vector showed a threefold increase in the apoptotic index when treated with StSp, and this was attenuated in RBM3-transfected cells (P = 0.05) (Fig. 5B). To verify that indeed H2O2 treatment was not associated with an increase in apoptosis, we performed TUNEL assay. TUNEL-positive cells were increased dramatically upon StSp treatment, but H2O2 application was not associated with an increase in apoptosis (Table 1), confirming the cell death ELISA results and suggesting that cells treated with H2O2 are undergoing cell death through a different mechanism. To investigate whether cells subjected to H2O2 were undergoing necrosis instead of apoptosis, a cell death apoptosis assay was performed in which cells turn red when undergoing necrosis but turn green when undergoing apoptosis. Cells treated with H2O2 (Fig. 6A) and StSp (Fig. 6D) exhibited changes characteristic of cells undergoing cell death. In addition, cells subjected to H2O2 showed very high levels of red fluorescence (Fig. 6B) while cells incubated in StSp did not (Fig. 6E). In contrast, green fluorescence was higher in cells treated with StSp compared with those with H2O2 (Fig. 6, F and C, respectively). The ratio of red to green fluorescence (red/green) was calculated for the different treatments and compared with ethanol-treated cells which are pure necrotic. In ethanol-treated cells, the red/green was 23.5, while that for H2O2- and StSp-treated cells was 12.7 and 1.0, respectively. These data indicate that the process of cell death in response to H2O2 is more similar to necrosis, while that of StSp is apoptosis. We further investigated whether necrosis in H2O2-treated cells was associated with increased levels of markers of programmed necrosis (or necroptosis), RIP-1 and RIP-3. However, neither protein could be detected by Western analysis in myoblasts under any of the conditions (data not shown), suggesting that necroptosis is not activated but that classical necrosis is the cell death pathway by which H2O2 induces cell death in myoblasts. Therefore, our results indicate that upregulation of RBM3 expression protects cells from cell death through apoptotic and necrotic mechanisms. To ensure that we were not looking at a general stress response from exposing cells to different temperatures, we measured heat shock protein levels. HSP-25 and HSP-70 are not expressed under normal culture conditions in myoblasts, but are induced upon heat shock (Fig. 7). Protein abundance of HSP-25 or HSP-70 was not increased in myoblasts subjected to either cold shock or transfection with RBM3, indicating that the increase in cell survival is specific for cold shock and RBM3 (Fig. 7).
Fig. 5.
Cold shock and RBM3 overexpression decrease apoptosis in response to StSp. Apoptotic index as a percentage of control in response to H2O2 and StSp in myoblasts with (gray bars) and without (black bars) CS (n = 3–6) (A) and transfected with pcDNA3 (black bars) or RBM3 (gray bars) (n = 6) (B) is depicted. #Significant difference from control conditions (0 μM); *significant difference from no CS in A and pcDNA3 transfected in B. Values are means ± SE; P < 0.05.
Table 1.
Apoptosis as measured by TUNEL positive per total nuclei
| H2O2, % (1,000 μM) | StSp, % (5 μM) | |
|---|---|---|
| Control | 2.5 ± 0.5 | 2.2 ± 1.7 |
| Treated | 3.8 ± 0.7 | 42.3 ± 11.6* |
Values are means ± SE. TUNEL, terminal transferase dUTP nick end labeling; StSp, staurosporine.
P < 0.05, significantly different from all other groups.
Fig. 6.
Preferential induction of necrosis by H2O2. A–F: representative pictures from myoblasts subjected to H2O2 (A–C) and StSp (D–F) and photographed under bright field light conditions (A and D), under TRITC filter (red, 610 nm; B and E), or under FITC filter (green, 530 nm; C and F). Bar in F is for all pictures and indicates 100 μm.
Fig. 7.
No increase in heat shock proteins in response to CS or transfection. Shown are representative samples of myoblasts subjected to CS, transfection with pcDNA3 or RBM3, and heat shock at 42°C and immunoreacted with heat shock protein 70 (HSP-70) and HSP-25 antibodies.
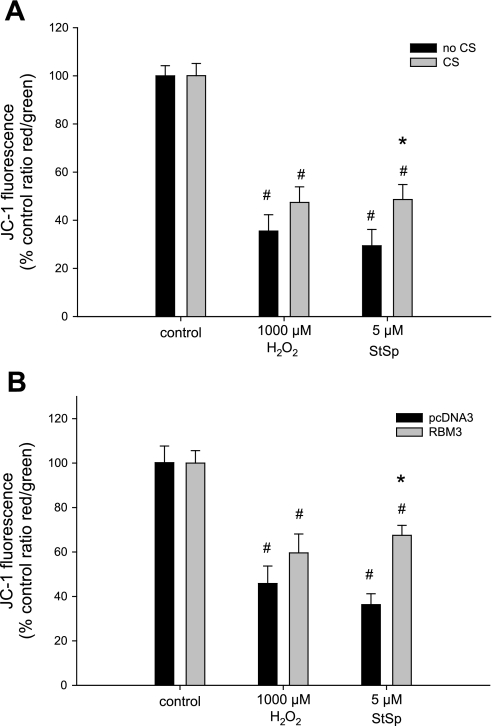
To investigate possible mechanisms by which RBM3 overexpression promotes survival of myoblasts, we assessed changes in mitochondrial membrane potential by use of JC-1 dye. Upon treatment with H2O2 and StSp, the mitochondrial membrane potential decreased by 65% and 70%, respectively, in cells that were cultured under normal temperature conditions (Fig. 8A). After cold shock, however, the decrease in mitochondrial membrane potential was significantly attenuated in the StSp-treated cells only (Fig. 8A). Similar results were found when cells were transfected with RBM3. H2O2 reduced membrane potential by 55%, and RBM3 overexpression did not have a significant effect on this reduction (Fig. 8B). However, when treated with StSp the mitochondrial membrane potential decreased 64% when cells were transfected with empty vector and only 33% when RBM3 was overexpressed (Fig. 8B). Thus, RBM3 overexpression is associated with protection from mitochondrial membrane collapse during apoptosis.
Fig. 8.
Protection of mitochondrial membrane potential dissipation by CS only in response to StSp. Decrease in mitochondrial membrane potential in myoblasts with (gray bars) and without (black bars) CS (n = 3–5) (A) or transfected with pcDNA3 (black bars) or RBM3 (gray bars) (n = 5–8) (B) in response to H2O2 and StSp is depicted. #Significant difference from control conditions; *significant difference from no CS. Values are means ± SE; P < 0.05.
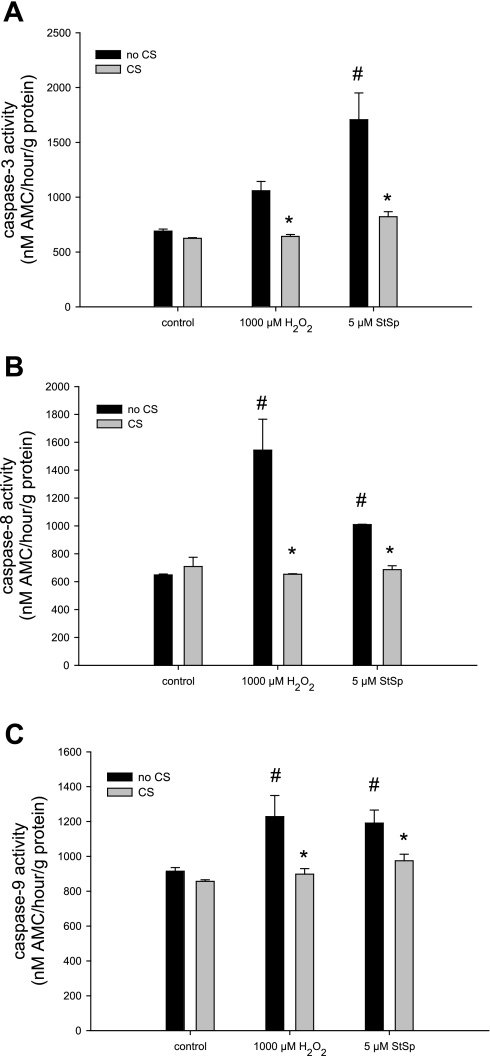
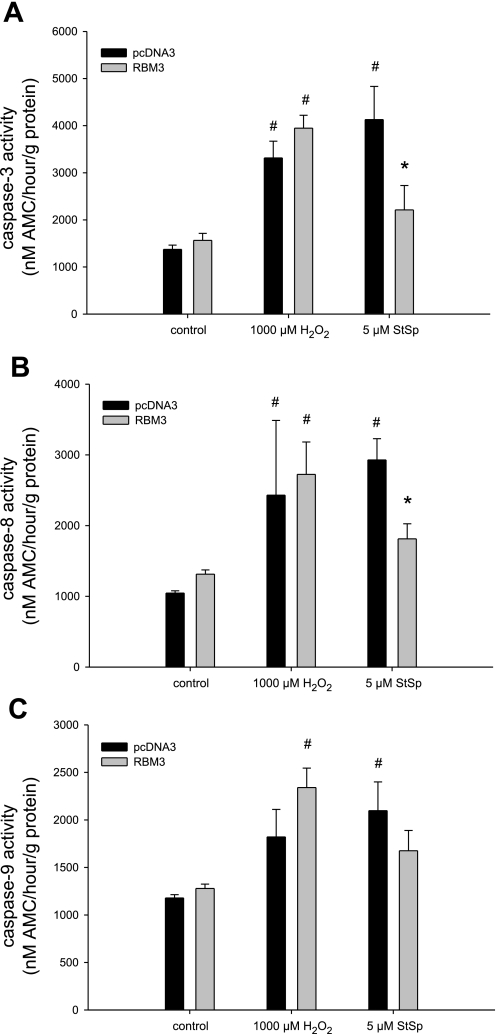
To further examine the role of RBM3 in conferring protection against apoptosis, we measured the activities of caspase-3, -8, and -9 to determine whether caspases, the classical proteolytic enzymes in apoptosis, were affected by cold shock and RBM3 overexpression. Caspase-3 was elevated in cells treated with StSp under no cold shock conditions, but the elevation failed to reach significance after H2O2 treatment (P = 0.05, Fig. 9A). Caspase-8 and -9 activities were significantly elevated after H2O2 and StSp treatment in cells under no cold shock conditions (Fig. 9, B and C, respectively). The activities of all three caspases studied were returned to control levels in cells subjected to cold shock (Fig. 9, A–C), suggesting that caspase-dependent apoptosis is inhibited by cold shock and potentially by RBM3. To test this further, we studied caspase activities in cells transfected with RBM3. Caspase-3 and -8 activities were elevated upon treatment with H2O2 and StSp in cells transfected with empty vector, and transfection with RBM3 returned caspase-3 and -8 activities to control levels only in cells treated with StSp, but not H2O2 (Fig. 10, A and B). The increase in caspase-9 activity in cells treated with H2O2 when transfected with empty vector did not reach significance. Surprisingly, however, in cells overexpressing RBM3, caspase-9 activity was significantly elevated by H2O2 treatment (Fig. 10C). In contrast, cells treated with StSp exhibited an increase in caspase-9 activity which was not different from control levels with RBM3 overexpression (Fig. 10C). These data suggest that cold shock and RBM3 overexpression inhibit the activation of caspases in the presence of classical apoptosis induced by StSp.
Fig. 9.
Cold shock reverses the increase in caspase activities with the induction of cell death. Caspase-3 (A), caspase-8 (B), and caspase-9 (C) activities in response to H2O2 and StSp with (gray bars) and without (black bars) CS are depicted. n = 3 for all experiments; #significant difference from control conditions; *significant difference from no CS. Values are means ± SE; P < 0.05.
Fig. 10.
Attenuation of caspase activities with RBM3 overexpression upon cell death induction. Caspase-3 (A), caspase-8 (B), and caspase-9 (C) activities in response to H2O2 and StSp with pcDNA3 (black bars) or RBM3 (gray bars) transfections are depicted. n = 3 for all experiments; #significant difference from control conditions; *significant difference from pcDNA3. Values are means ± SE; P < 0.05.
DISCUSSION
Results from this study show that RBM3 protects muscle cells from apoptotic as well as necrotic cell death. Elevation of RBM3 by overexpression of myc-RBM3 and by cold shock increased cell survival and inhibited necrosis along with apoptosis and apoptosis-associated caspase activation. Apoptosis is an important cell death pathway in skeletal muscle witnessed by the fact that it is increased in a number of pathological conditions, such as chronic heart failure, motor neuron disorders, denervation, spinal cord injury, muscular dystrophy, and atrophy due to hindlimb suspension, immobilization, and with aging (1, 2, 4, 10, 17, 22, 37, 45, 57, 59, 61, 65, 66). Similarly, necrosis is involved in the loss of muscle tissue due to injury associated with both trauma and disease. Since RBM3 protected cells from apoptotic as well as necrotic cell death it can be seen as a protein that is beneficial for the survival of muscle cells in general.
It has been known for a long time that cold therapy is beneficial in the preservation of tissues such as brain and heart and therefore hypothermia was used frequently in ancient times. In modern medicine its use has been documented for over 200 years, and in more recent history, preclinical studies such as the one by Hinton et al. (31) in 1956 show that therapeutic hypothermia increased survival rates after hemorrhagic shock. Hypothermia has been used to preserve heart tissue after cardiac arrest, brain tissue after traumatic injury and oxygen deprivation during delivery, and spinal cord tissue after traumatic spinal cord injury (11, 15, 56). Indeed, the loss of neuronal function due to spinal cord injury, cerebral ischemia, and cerebral palsy has been shown to be reduced by hypothermia (7, 11, 15, 42, 46, 53, 54). The underlying mechanisms for the beneficial effect of cold therapy are largely unknown, but a decrease in cell death has been suggested in many situations (for review see Ref. 27). Mammalian cells exposed to a variety of cytotoxic and apoptotic stimuli were rescued by hypothermia (32°C), and this suppression was mediated partly through caspase-9-dependent and p53-dependent and -independent mechanisms (52). Similarly, apoptosis through the caspase-3-dependent pathway was attenuated in cardiomyocytes from hypothermia-treated rats in response to hemorrhagic shock compared with normothermic rats (56).
The cellular pathways through which this antiapoptotic effect of hypothermia is achieved are currently under investigation, but the increase of cold shock proteins in response to lower temperature has been suggested to be involved (27, 33). Cold shock proteins, such as RBM3 and CIRP in particular, are known modulators of apoptosis and are proposed proto-oncogenes (38, 72). RBM3 suppressed apoptosis in a polyglutamine-induced neuronal cell death model, but cellular apoptotic pathways were not investigated in this study (34). Recently, it was shown that the effect of hypothermia on neuronal survival is at least partly mediated through the actions of RBM3 (12). Also, RBM3 overexpression rescued human embryonic kidney cells from serum deprivation-induced cell death and knockdown of RBM3-induced cell death through a caspase-3-independent mechanism (68), indicating that RBM3 is a critical factor in cellular survival in this model. However, in colon cancer cells, RBM suppression induced cell death through a caspase-3-dependent mechanism (62) and therefore the underlying mechanisms of the protective effect of RBM3 may vary with both cell type examined and the specific cell death inducers. In our study, RBM3 overexpression rescued myoblasts from StSp-induced apoptosis and this was associated with an attenuation of Δψm and a reversal of caspase-3, -8, and -9 activation, indicating that the mitochondrial-dependent intrinsic apoptotic pathway was inhibited by RBM3. Interestingly, our work shows that H2O2-induced cell death was not associated with increased DNA fragmentation or expected activation of caspases and seemed to follow a classical necrotic death pathway, since programmed necrosis or necroptosis markers (RIP-1 and -3) could not be detected. Like apoptosis, the increase in cell death due to necrosis was also inhibited by increased levels of RBM3. In this case, the elevation of caspases, if present, was normalized with cold shock, but not with transfection of RBM3, indicating that other factors induced by cold shock may be responsible for the decrease in caspase activities associated with necrotic-type cell death in response to H2O2.
Since RBM3 is a RNA-binding protein, the question that remains unanswered is which RNA species are bound by RBM3 and how this binding is responsible for the cell-sparing effects observed in this study. It has been shown previously that RBM3 regulates the levels of miRNA-containing ribonucleoprotein complexes (18), raising the possibility that RBM3 exerts antiapoptotic effects via the miRNA pathway. In addition, it has been demonstrated that RBM3 binds to the 3′-untranslated region (UTR) of the cyclooxygenase-2 (COX-2) mRNA and that this binding increases RNA stability and translational efficiency of COX-2 (13, 62). RBM3 was bound in a complex with other RNA-binding proteins to the COX-2 mRNA through the AU-rich element (ARE) in the 3′-UTR and it is possible that other mRNAs containing AREs are also targets for RBM3. Interestingly, COX-2 was shown to be essential during early stages of skeletal muscle regeneration and this at least partly contributed to its effect on myoblast number (8). Furthermore, inhibition of COX-2 was associated with attenuated regrowth after atrophy and an inhibition of myoblast incorporation in soleus muscle (9), indicating a potential role for COX-2 in myoblast survival in vivo. By contrast, it was recently shown that inhibition of RBM3 led to cell death independent of COX-2 regulation (68), indicating that RBM3-binding to COX-2 may be cell type- or cell condition-specific.
Currently, no known RNA targets for RBM3 have been identified in skeletal muscle cells and therefore the precise mechanisms for the cell death-reducing actions of RBM3 remain to be determined. In the current study we began investigating these mechanisms in myoblasts instead of myotubes, because of the relative ease of inducing cell death in myoblasts. However, mechanisms of cell death in myoblasts and myotubes have similarities as well as differences (70), and therefore future studies will need to be directed toward investigating the effects of RBM3 on myotubes and muscles in vivo. However, since RBM3 protected the elevation in caspase activities which are involved in muscle atrophy (19), we suggest that it may play a role in this process and is a good candidate for the muscle sparing effect during hibernation.
In summary, overexpression of RBM3 in skeletal muscle myoblasts is associated with an increase in cell survival through inhibitory actions on both apoptosis and necrosis. These two processes are involved in dysfunction of skeletal muscle due to atrophy and/or muscle diseases and therefore RBM3 can be seen as a potential interventional target for the treatment of these conditions.
GRANTS
This work was supported by National Institutes of Health Grants AG028925 (to E. E. Dupont-Versteegden) and NS066053 (to P. W. Vanderklish).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Adams V, Jiang H, Yu J, Mobius-Winkler S, Fiehn E, Linke A, Weigl C, Schuler G, Hambrecht R. Apoptosis in skeletal myocytes of patients with chronic heart failure is associated with exercise intolerance. J Am Coll Cardiol 33: 959–965, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Adhihetty PJ, O'Leary MF, Chabi B, Wicks KL, Hood DA. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol 102: 1143–1151, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Al-Fageeh MB, Smales CM. Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems. Biochem J 397: 247–259, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol Cell Physiol 273: C579–C587, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Alway SE, Siu PM. Nuclear apoptosis contributes to sarcopenia. Exerc Sport Sci Rev 36: 51–57, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anker SD, Steinborn W, Strassburg S. Cardiac cachexia. Ann Med 36: 518–529, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 346: 557–563, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Bondesen BA, Mills ST, Kegley KM, Pavlath GK. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am J Physiol Cell Physiol 287: C475–C483, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Bondesen BA, Mills ST, Pavlath GK. The COX-2 pathway regulates growth of atrophied muscle via multiple mechanisms. Am J Physiol Cell Physiol 290: C1651–C1659, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Borisov AB, Carlson BM. Cell death in denervated skeletal muscle is distinct from classical apoptosis. Anat Rec 258: 305–318, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Cappuccino A, Bisson LJ, Carpenter B, Marzo J, Dietrich IIID, Cappuccino H. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine 35: E57–E62, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Chip S, Zelmer A, Ogunshola OO, Felderhoff-Mueser U, Nitsch C, Buhrer C, Wellmann S. The RNA-binding protein RBM3 is involved in hypothermia induced neuroprotection. Neurobiol Dis 43: 388–396, 2011. [DOI] [PubMed] [Google Scholar]
- 13. Cok SJ, Acton SJ, Sexton AE, Morrison AR. Identification of RNA-binding proteins in RAW 264.7 cells that recognize a lipopolysaccharide-responsive element in the 3-untranslated region of the murine cyclooxygenase-2 mRNA. J Biol Chem 279: 8196–8205, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Danno S, Nishiyama H, Higashitsuji H, Yokoi H, Xue JH, Itoh K, Matsuda T, Fujita J. Increased transcript level of RBM3, a member of the glycine-rich RNA-binding protein family, in human cells in response to cold stress. Biochem Biophys Res Commun 236: 804–807, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Degos V, Loron G, Mantz J, Gressens P. Neuroprotective strategies for the neonatal brain. Anesth Analg 106: 1670–1680, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Derry JM, Kerns JA, Francke U. RBM3, a novel human gene in Xp11.23 with a putative RNA-binding domain. Hum Mol Genet 4: 2307–2311, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol 282: R519–R527, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Dresios J, Aschrafi A, Owens GC, Vanderklish PW, Edelman GM, Mauro VP. Cold stress-induced protein Rbm3 binds 60S ribosomal subunits, alters microRNA levels, and enhances global protein synthesis. Proc Natl Acad Sci USA 102: 1865–1870, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, Price SR, Mitch WE. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 113: 115–123, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dupont-Versteegden EE. Apoptosis in muscle atrophy: relevance to sarcopenia. Exp Gerontol 40: 473–481, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Dupont-Versteegden EE. Apoptosis in skeletal muscle and its relevance to atrophy. World J Gastroenterol 12: 7463–7466, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dupont-Versteegden EE, Murphy RJ, Houle JD, Gurley CM, Peterson CA. Activated satellite cells fail to restore myonuclear number in spinal cord transected and exercised rats. Am J Physiol Cell Physiol 277: C589–C597, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Dupont-Versteegden EE, Nagarajan R, Beggs ML, Bearden ED, Simpson PM, Peterson CA. Identification of cold-shock protein RBM3 as a possible regulator of skeletal muscle size through expression profiling. Am J Physiol Regul Integr Comp Physiol 295: R1263–R1273, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dupont-Versteegden EE, Strotman BA, Gurley CM, Gaddy D, Knox M, Fluckey JD, Peterson CA. Nuclear translocation of EndoG at the initiation of disuse muscle atrophy and apoptosis is specific to myonuclei. Am J Physiol Regul Integr Comp Physiol 291: R1730–R1740, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Favier FB, Benoit H, Freyssenet D. Cellular and molecular events controlling skeletal muscle mass in response to altered use. Pflügers Arch 456: 587–600, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Fedorov VB, Goropashnaya AV, Toien O, Stewart NC, Gracey AY, Chang C, Qin S, Pertea G, Quackenbush J, Showe LC, Showe MK, Boyer BB, Barnes BM. Elevated expression of protein biosynthesis genes in liver and muscle of hibernating black bears (Ursus americanus). Physiol Genomics 37: 108–118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gonzalez-Ibarra FP, Varon J, Lopez-Meza EG. Therapeutic hypothermia: critical review of the molecular mechanisms of action. Front Neurol 2: 4, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graumann PL, Marahiel MA. A superfamily of proteins that contain the cold-shock domain. Trends Biochem Sci 23: 286–290, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Gualerzi CO, Giuliodori AM, Pon CL. Transcriptional and post-transcriptional control of cold-shock genes. J Mol Biol 331: 527–539, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Harlow HJ, Lohuis T, Beck TD, Iaizzo PA. Muscle strength in overwintering bears. Nature 409: 997, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Hinton JW, Postel AH, Reid LC. Effect of hypothermia in irreversible hemorrhagic shock. Circ Res 4: 594–598, 1956 [DOI] [PubMed] [Google Scholar]
- 32. Hudson NJ, Lehnert SA, Ingham AB, Symonds B, Franklin CE, Harper GS. Lessons from an estivating frog: sparing muscle protein despite starvation and disuse. Am J Physiol Regul Integr Comp Physiol 290: R836–R843, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Jones PG, Inouye M. The cold-shock response–a hot topic. Mol Microbiol 11: 811–818, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Kita H, Carmichael J, Swartz J, Muro S, Wyttenbach A, Matsubara K, Rubinsztein DC, Kato K. Modulation of polyglutamine-induced cell death by genes identified by expression profiling. Hum Mol Genet 11: 2279–2287, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Lee K, Park JY, Yoo W, Gwag T, Lee JW, Byun MW, Choi I. Overcoming muscle atrophy in a hibernating mammal despite prolonged disuse in dormancy: proteomic and molecular assessment. J Cell Biochem 104: 642–656, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Leeuwenburgh C. Role of apoptosis in sarcopenia. J Gerontol A Biol Sci Med Sci 58: 999–1001, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol 288: R1288–R1296, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Lleonart ME. A new generation of proto-oncogenes: cold-inducible RNA binding proteins. Biochim Biophys Acta 1805: 43–52, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Lohuis TD, Harlow HJ, Beck TD, Iaizzo PA. Hibernating bears conserve muscle strength and maintain fatigue resistance. Physiol Biochem Zool 80: 257–269, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci 57: B359–B365, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Mihailovich M, Militti C, Gabaldon T, Gebauer F. Eukaryotic cold shock domain proteins: highly versatile regulators of gene expression. Bioessays 32: 109–118, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Nelson KB, Chang T. Is cerebral palsy preventable? Curr Opin Neurol 21: 129–135, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Phadtare S, Alsina J, Inouye M. Cold-shock response and cold-shock proteins. Curr Opin Microbiol 2: 175–180, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273: E99–E107, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Podhorska-Okolow M, Sandri M, Zampieri S, Brun B, Rossini K, Carraro U. Apoptosis of myofibres and satellite cells: exercise-induced damage in skeletal muscle of the mouse. Neuropathol Appl Neurobiol 24: 518–531, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 37: S186–S202, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Pollack M, Leeuwenburgh C. Apoptosis and aging: role of the mitochondria. J Gerontol A Biol Sci Med Sci 56: B475–B482, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Rantanen T, Guralnik JM, Sakari-Rantala R, Leveille S, Simonsick EM, Ling S, Fried LP. Disability, physical activity, and muscle strength in older women: the women's health and aging study. Arch Phys Med Rehabil 80: 130–135, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, Guralnik JM. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci 55: M168–M173, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci 55: M716–M724, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Rourke BC, Yokoyama Y, Milsom WK, Caiozzo VJ. Myosin isoform expression and MAFbx mRNA levels in hibernating golden-mantled ground squirrels (Spermophilus lateralis). Physiol Biochem Zool 77: 582–593, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Sakurai T, Itoh K, Liu Y, Higashitsuji H, Sumitomo Y, Sakamaki K, Fujita J. Low temperature protects mammalian cells from apoptosis initiated by various stimuli in vitro. Exp Cell Res 309: 264–272, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Sato K, Kimura T, Nishikawa T, Tobe Y, Masaki Y. Neuroprotective effects of a combination of dexmedetomidine and hypothermia after incomplete cerebral ischemia in rats. Acta Anaesthesiol Scand 54: 377–382, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Schulzke SM, Rao S, Patole SK. A systematic review of cooling for neuroprotection in neonates with hypoxic ischemic encephalopathy-are we there yet? BMC Pediatr 7: 30, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shavlakadze T, Grounds M. Of bears, frogs, meat, mice and men: complexity of factors affecting skeletal muscle mass and fat. Bioessays 28: 994–1009, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Shuja F, Tabbara M, Li Y, Liu B, Butt MU, Velmahos GC, DeMoya M, Alam HB. Profound hypothermia decreases cardiac apoptosis through Akt survival pathway. J Am Coll Surg 209: 89–99, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Siu PM, Pistilli EE, Butler DC, Alway SE. Aging influences the cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol Cell Physiol 288: C338–C349, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Smart F, Aschrafi A, Atkins A, Owens GC, Pilotte J, Cunningham BA, Vanderklish PW. Two isoforms of the cold-inducible mRNA-binding protein RBM3 localize to dendrites and promote translation. J Neurochem 101: 1367–1379, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Smith HK, Maxwell L, Martyn JA, Bass JJ. Nuclear DNA fragmentation and morphological alterations in adult rabbit skeletal muscle after short-term immobilization. Cell Tissue Res 302: 235–241, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Sonna LA, Fujita J, Gaffin SL, Lilly CM. Invited review: effects of heat and cold stress on mammalian gene expression. J Appl Physiol 92: 1725–1742, 2002 [DOI] [PubMed] [Google Scholar]
- 61. Strasser H, Tiefenthaler M, Steinlechner M, Eder I, Bartsch G, Konwalinka G. Age dependent apoptosis and loss of rhabdosphincter cells. J Urol 164: 1781–1785, 2000 [PubMed] [Google Scholar]
- 62. Sureban SM, Ramalingam S, Natarajan G, May R, Subramaniam D, Bishnupuri KS, Morrison AR, Dieckgraefe BK, Brackett DJ, Postier RG, Houchen CW, Anant S. Translation regulatory factor RBM3 is a proto-oncogene that prevents mitotic catastrophe. Oncogene 27: 4544–4556, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Szulc P. Rapid loss of appendicular skeletal muscle mass is associated with higher all-cause mortality in older men: the prospective MINOS study. Am J Clin Nutr 91: 1227–1236, 2010 [DOI] [PubMed] [Google Scholar]
- 64. Taylor JM, Dupont-Versteegden EE, Davies JD, Hassell JA, Houle JD, Gurley CM, Peterson CA. A role for the ETS domain transcription factor PEA3 in myogenic differentiation. Mol Cell Biol 17: 5550–5558, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tews DS, Goebel HH, Meinck HM. DNA-fragmentation and apoptosis-related proteins of muscle cells in motor neuron disorders. Acta Neurol Scand 96: 380–386, 1997 [DOI] [PubMed] [Google Scholar]
- 66. Tidball JG, Albrecht DE, Lokensgard BE, Spencer MJ. Apoptosis precedes necrosis of dystrophin-deficient muscle. J Cell Sci 108: 2197–2204, 1995 [DOI] [PubMed] [Google Scholar]
- 67. Wellmann S, Buhrer C, Moderegger E, Zelmer A, Kirschner R, Koehne P, Fujita J, Seeger K. Oxygen-regulated expression of the RNA-binding proteins RBM3 and CIRP by a HIF-1-independent mechanism. J Cell Sci 117: 1785–1794, 2004 [DOI] [PubMed] [Google Scholar]
- 68. Wellmann S, Truss M, Bruder E, Tornillo L, Zelmer A, Seeger K, Buhrer C. The RNA-binding protein RBM3 is required for cell proliferation and protects against serum deprivation-induced cell death. Pediatr Res 67: 35–41, 2010 [DOI] [PubMed] [Google Scholar]
- 69. Williams DR, Epperson LE, Li W, Hughes MA, Taylor R, Rogers J, Martin SL, Cossins AR, Gracey AY. Seasonally hibernating phenotype assessed through transcript screening. Physiol Genomics 24: 13–22, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Xiao R, Ferry AL, Dupont-Versteegden EE. Cell death-resistance of differentiated myotubes is associated with enhanced anti-apoptotic mechanisms compared to myoblasts. Apoptosis 16: 221–234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yan J, Barnes BM, Kohl F, Marr TG. Modulation of gene expression in hibernating arctic ground squirrels. Physiol Genomics 32: 170–181, 2008 [DOI] [PubMed] [Google Scholar]
- 72. Zeng Y, Kulkarni P, Inoue T, Getzenberg RH. Down-regulating cold shock protein genes impairs cancer cell survival and enhances chemosensitivity. J Cell Biochem 107: 179–188, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]