Abstract
Ca-sensing receptor (CaSR), a member of the G protein-coupled receptor family, regulates the synthesis of parathyroid hormone in response to changes in serum Ca2+ concentrations. The functions of CaSR in human vascular smooth muscle cells are largely unknown. Here we sought to study CaSR activation and the underlying molecular mechanisms in human aortic smooth muscle cells (HASMC). Extracellular Ca2+ ([Ca2+]o) dose-dependently increased free cytosolic Ca2+ ([Ca2+]cyt) in HASMC, with a half-maximal response (EC50) of 0.52 mM and a Hill coefficient of 5.50. CaSR was expressed in HASMC, and the [Ca2+]o-induced [Ca2+]cyt rise was abolished by dominant negative mutants of CaSR. The CaSR-mediated increase in [Ca2+]cyt was also significantly inhibited by pertussis toxin, the phospholipase C inhibitor U-73122, or the general protein kinase C (PKC) inhibitor chelerythrine, but not by the conventional PKC inhibitor, Gö6976. Depletion of membrane cholesterol by pretreatment with methyl-β-cyclodextrin markedly decreased CaSR-induced increase in [Ca2+]cyt. Blockade of TRPC channels with 2-aminoethoxydiphenyl borate, SKF-96365, or La3 significantly inhibited [Ca2+]o entry, whereas activation of TRPC6 channels with flufenamic acid potentiated [Ca2+]o entry. Neither cyclopiazonic acid nor caffeine or ionomycin had any effect on [Ca2+]cyt in [Ca2+]o-free solutions. TRPC6 and PKCε mRNA and proteins were detected in HASMC, and [Ca2+]o induced PKCε phosphorylation, which could be prevented by chelerythrine. Our data suggest that CaSR activation mediates [Ca2+]o entry, likely through TRPC6-encoded receptor-operated channels that are regulated by a PLC/PKCε cascade. Our study therefore provides evidence not only for functional expression of CaSR, but also for a novel pathway whereby it regulates [Ca2+]o entry in HASMC.
Keywords: Ca2+ signaling, TRPC6 channels, PKCε phosphorylation, vascular smooth muscle cells
cytosolic free Ca2+ ([Ca2+]cyt) is known to play an essential role in various mammalian cells, regulating physiological functions such as neurotransmitter release, muscle contraction, fertilization, gene regulation, proliferation, and apoptosis (4, 10, 17, 28). Therefore, dysregulation of Ca2+ homeostasis in the body results in pathological changes in different systems (4, 22, 25). One of the regulators of Ca2+ homeostasis is the plasma membrane Ca-sensing receptor (CaSR), which is a plasma membrane protein with 1,078 amino acids that was initially cloned from bovine parathyroid cells (5–7). CaSR is a member of the G protein-coupled receptor (GPCR) family and regulates the synthesis of parathyroid hormone (6, 8, 15) in response to changes in serum Ca2+ concentrations. In addition, CaSR activation elicits complex intracellular signaling events through modulation of a wide range of intracellular mediators, including G proteins and phospholipase C (PLC). These, in turn, stimulate both protein kinase C (PKC) activation and inositol 1,4,5-trisphosphate (IP3) production, the latter inducing intracellular Ca2+ release (6, 15). Therefore, CaSR plays an important role in the regulation of serum Ca2+ concentrations in the body and [Ca2+]cyt homeostasis in mammalian cells. The plasma membrane CaSR can be directly activated by several CaSR agonists, such as extracellular Ca2+ ([Ca2+]o) and spermine (6–8, 14).
Although CaSR has been well documented to play important physiological roles in various types of mammalian cells, especially in parathyroid cells (6, 8, 15), the knowledge of its expression and function in the human cardiovascular system is limited. Recently, growing evidence suggests that CaSR is expressed in vascular smooth muscle cells (VSMC), likely modulates arterial blood pressure, and mediates VSMC proliferation through the MEK1/ERK1/2 signaling pathway (21, 32). However, the mechanism(s) whereby CaSR mediates physiological events in VSMC are not fully understood. Here, we sought to investigate the mechanisms underlying CaSR-mediated [Ca2+]cyt signaling pathway in human aortic smooth muscle cells (HASMC) as a model of VSMC because CaSR is known to be functionally expressed in this cell type (21).
MATERIALS AND METHODS
Cell culture.
HASMC (ScienceCell Research Laboratories, San Diego, CA) were cultured in smooth muscle growth medium (SmGM, ScienceCell) at 37°C in a humidified 5% CO2 plus 95% air atmosphere. SmGM was supplemented with 5% fetal bovine serum, 0.5 ng/ml human epidermal growth factor, 2 ng/ml human fibroblast growth factor, and 5 μg/ml insulin. The medium was changed every 48 h thereafter and cells of passages 1-10 were used for experiments (21).
Transfection of Flag-tagged dominant negative-CaSR constructs into HASMC.
We used two CaSR dominant negative mutants that are mutated at different locations of the wild-type CaSR, R185Q and R795W (gifts of Dr. Edward Brown, Harvard University). Flag protein was also cloned into these mutants. R185Q is characterized by mutation of the ligand binding region of the wild-type CaSR, while R795W is characterized by mutation of the transmembrane region. Both R185Q and R795W had substantially attenuated responses to [Ca2+]o although they exhibited normal patterns of receptor protein expression (1). R185Q showed a prominent “dominant negative” effect on the coexpressed wild-type receptor, while R795W mutation could potentially still bind to its respective G protein(s) but fail to activate them. HASMC were transfected with the plasmid DNA constructs with Fugene 6. Six-well plates containing 80–90% confluent cells in each well were transfected with a total of 2 μg/well plasmid DNA. [Ca2+]cyt measurement and membrane protein extraction for Western blots were performed 48 h after transfection.
[Ca2+]cyt measurement.
[Ca2+]cyt levels in HASMC were measured by fura-2 fluorescence ratio digital imaging as described previously (36). Briefly, HASMC were trypsinized and replated onto 10-mm round glass coverslips that had been precoated with 1 mg/ml poly-d-lysine (Sigma) at a density such that 70–90% confluence was achieved within 24 h. They were then loaded with 5 μM fura-2 acetoxymethyl ester (AM) [dissolved in 0.01% Pluronic F-127 plus 0.1% DMSO in normal physiological salt solution (PSS), described below] at room temperature for 50 min, then washed in PSS for at least 20 min. Thereafter, the coverslips with HASMC were mounted in a perfusion chamber on a Nikon microscope stage. Cells were initially superfused with PSS for 5 min and then switched to Ca2+-free or Ca2+ solutions containing drugs of interest. Fura-2 fluorescence ratio (510-nm light emission excited by 340- or 380-nm illuminations), as well as background fluorescence, was collected at room temperature (22°C) with the use of a ×40 Nikon UV-Fluor objective and an intensified CCD camera (ICCD200). The fluorescence signals emitted from the cells were monitored continuously using a MetaFluor Imaging System (Universal Imaging, Downingtown, PA) and recorded for later analysis. PSS used in digital Ca2+ measurement contained the following (in mM): 140 Na+, 5.0 K+, 2 Ca2+, 147 Cl−, 10 HEPES, and 10 glucose, pH 7.4. For the Ca2+-free PSS solution, Ca2+ was omitted, and 0.5 mM EGTA was added to prevent possible Ca2+ contamination. The osmolalities for all solutions were ∼300 mosM/l.
RT-PCR analysis of TRPC.
Briefly, total RNA (5 μg) from HASMC was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions and our previous publication (2). RNA were converted into cDNA with reverse transcriptase and PCR was performed using the following primers: TRPC1 (accession no. NM_003304) forward strand, 5′-TGCTTACCAAACTGCTGGTG-3′ and reverse strand, 5′-AACTGTTTTGCCGTTTGACC-3′; TRPC3 (accession no. NM_003305) forward strand, 5′-CTTTCCTTCAGGCAACGAAG-3′ and reverse strand, 5′-GTACGCAATCCGAGAGAAGC-3′; TRPC4 (accession no. NM_016179) forward strand, 5′-GCTGGAGGAGAAGACACTGG-3′ and reverse strand, 5′-GACCTGTCGATGTGCTGAGA-3′; and TRPC6 (accession no. NM_004621) forward strand, 5′-GCCAATGAGCATCTGGAAAT-3′ and reverse strand, 5′-AACCTCTTGCCTTCAGCAAA-3′. The conditions for PCR reactions for these genes were similar to those previously described (9). Primers for GAPDH were used as a control (forward strand, 5′-ACCACAGTCCATGCCATCAC-3′ and reverse strand, 5′-TCCACCACCCT-GTTGCTGTA-3′). The samples were amplified in an automated thermal cycler (GeneAmp 2400; Applied Biosystems). DNA amplification conditions included an initial 3-min denaturation step at 94°C, 35 cycles of 30 s at 94°C, 30 s at 57°C, 40 s at 72°C, and a final elongation step of 10 min at 72°C. The products were separated on a 1.5% agarose gel, stained with ethidium bromide, and then photographed under UV light.
Western blot analysis.
HASMC cells were washed three times with ice-cold PBS. Cells were then lysed with total lysis buffer [150 mM NaCl, 10 mM Tris·HCl (pH 7.8), 1 mM EDTA, 0.5% Triton X-100, and 1 mM sodium orthovanadate] containing protease inhibitors (1 μg/ml leupeptin and 100 μg/ml PMSF) and incubated at 4°C for 30 min with constant shaking. The cells were then scraped into microcentrifuge tubes and centrifuged at 12,000 g for 15 min to remove insoluble material. The protein content in each sample was determined and normalized. Cell lysates were then resuspended in 2× gel loading buffer, boiled for 5 min, and then separated by SDS-polyacrylamide gel electrophoresis (9% polyacrylamide). Resolved proteins were transferred overnight at 4°C onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA). Membranes were then blocked with a 5% solution of skim milk for 30 min at room temperature, followed by further incubation with monoclonal antibodies specific for CaSR (1:1,000, Abcam, Cambridge, MA), TRPC1, -4, and -6 (1:500, Alomone Lab, Israel), phospho-PKCε 1:1,000 (Upstate Biotechnology, Lake Placid, NY), PKCα 1:1,000 (BD Biosciences, San Jose, CA), or GAPDH 1:5,000 (Ambion, Austin, TX). After being washed with PBS with 1% Tween (PBST), the rabbit anti-mouse secondary antibody was applied to the membrane. After washing with PBST, the membrane was treated with a chemiluminescent solution (Fivephoton Biochemicals, San Diego, CA) according to manufacturer's instructions and exposed to X-ray film. Densitometric analysis of the blots was performed with the use of an AlphaImager digital imaging system (Alpha Innotech, San Leandro, CA).
Chemicals and solutions.
SKF-96365, U-73122, nifedipine, chelerythrine, and Gö6976 were purchased from Sigma. 2-Aminoethoxydiphenyl borate (2-APB) was purchased from Tocris Bioscience (Ellisville, MO). Chiral enantiomers of BEL (S- and R-BEL) were from Cayman Chemical (Ann Arbor, MI). Fura 2-AM was from Molecular Probes (Eugene, OR). The other chemicals were obtained from Fisher Scientific (Santa Clara, CA).
Statistical analysis.
Results are expressed as means ± SE. Differences between means were considered to be statistically significant at P < 0.05 using Student's t-test or one-way ANOVA followed by Newman-Keuls post hoc test, as appropriate.
RESULTS
CaSR activation induces Ca2+ signaling in HASMC.
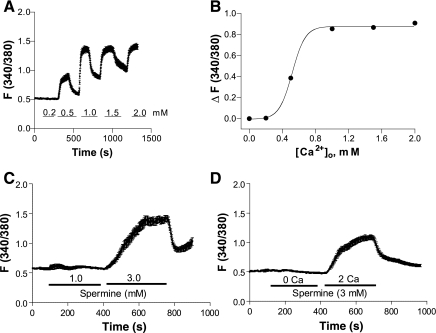
Although activation of CaSR resulted in ERK1/2 phosphorylation in HASMC (6, 21), little is known about Ca2+ signaling downstream of CaSR activation in this cell type. Therefore, [Ca2+]cyt levels in HASMC were measured, examining the dose-response of [Ca2+]cyt changes in HASMC stimulated with various levels of [Ca2+]o. Following a short exposure to Ca-free solutions (2–3 min), cells were superfused with different concentrations of [Ca2+]o (0.2–2.0 mM) (Fig. 1). While [Ca2+]o at 0.2 mM did not affect the basal [Ca2+]cyt, obvious increases were seen at higher levels of [Ca2+]o (Fig. 1A). Elevated [Ca2+]cyt was dependent on [Ca2+]o because it was promptly reversed when [Ca2+]o was removed (Fig. 1A). CaSR activation appeared to be cooperative, with a steep dose-response relationship (Fig. 1B). The half-maximal response (EC50) was seen when [Ca2+]o was 0.52 mM and the Hill coefficient for [Ca2+]o stimulation was 5.5 (Fig. 1B).
Fig. 1.
Extracellular Ca2+ ([Ca2+]o) and spermine stimulate an increase in free cytosolic Ca2+ ([Ca2+]cyt) in human aortic smooth muscle cells (HASMC). After HASMC were loaded with Fura-2 AM, [Ca2+]cyt in the cells was measured by a digital Ca2+ imaging system. A: time course of [Ca2+]cyt changes in HASMC induced by different concentrations of [Ca2+]o. B: summary data showing a dose-dependent relationship for the effect of [Ca2+]o stimulation or peak [Ca2+]cyt responses. C: time course of [Ca2+]cyt changes in HASMC induced by different concentrations of spermine in normal Ca2+ physiological salt solution (PSS). D: time course of [Ca2+]cyt changes in HASMC induced by same concentration of spermine (3 mM) in Ca2+-free or normal Ca2+ PSS. Data are shown as means ± SE of 40–50 cells.
Although [Ca2+]o stimulates CaSR, it may enter healthy cells through store-operated Ca2+ entry pathway (26), or may even directly leak into unhealthy cells through nonspecific pathways. We therefore tested these possibilities using spermine, another direct activator of CaSR (6, 21). While spermine at 1 mM did not affect the basal [Ca2+]cyt in normal PSS, obvious increases were seen at 3 mM (Fig. 1C). In addition, spermine (3 mM) did not affect the basal [Ca2+]cyt in Ca2+-free PSS, but significantly increased [Ca2+]cyt in normal Ca2+ PSS (Fig. 1D). These results provide further evidence for a functional role of CaSR in the regulation of [Ca2+]cyt in HASMC.
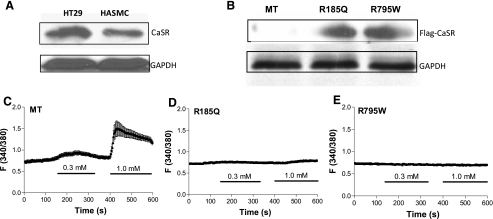
To demonstrate the presence of CaSR in HASMC, we performed Western blot analysis of lysates from HASMC and HT29, a human colonic cancer cell line used as a positive control. HASMC lysate produced a band of 160 kDa (Fig. 2A), consistent with the mature, full-size CaSR (21). To confirm the functional role of CaSR in the regulation of [Ca2+]cyt in HASMC, we used the CaSR mutants R185Q and R795W. The former showed a prominent dominant negative effect on the coexpressed wild-type receptor, while the latter could potentially still bind to its respective G protein(s) but fail to activate them (1). After transfection of dominant negative-CaSR constructs in HASMC for 48 h, expression of Flag-R185Q and Flag-R795W, but not empty (MT) vector, was seen (Fig. 2B). [Ca2+]o (0.3–1.0 mM) increased [Ca2+]cyt in MT-transfected HASMC (Fig. 2C) but not in R185Q- or R795W-transfected HASMC (Fig. 2, D and E). Together, these results confirm the functional role of CaSR in the regulation of [Ca2+]cyt in HASMC.
Fig. 2.
Protein expression and function of Ca-sensing receptor (CaSR) wild type and mutants in HASMC. A: HT29 (a human colonic cancer cell line used as a positive control) and HASMC were lysed and then Western blot analysis was performed to detect protein expression of CaSR wild type. B–E: after transfection of Flag-tagged empty (MT) vector, R185Q, or R795W construct in HASMC for 48 h, Western blot analysis was performed to detect protein expression of CaSR mutants (B), and digital Ca2+ imaging was performed to determine [Ca2+]o-induced [Ca2+]cyt signaling in HASMC transfected with MT (C), R185Q (D), or R795W construct (E). Data are shown as means ± SE of 30–40 cells.
CaSR functions as a GPCR.
CaSR activation elicits intracellular signaling events, including those mediated by G proteins, PLC and PKC, that in turn modulate [Ca2+]cyt (6, 15). Therefore, we sought to test the involvement of these pathways following CaSR activation in HASMC, using [Ca2+]o at both the threshold concentration (0.5 mM) and maximal concentration (1.0 mM). We first examined whether CaSR activation stimulates G proteins. HASMC were superfused with [Ca2+]o with or without pertussis toxin (PTX, 100 ng/ml) (Fig. 3A), a Gi/0 protein inhibitor (37). PTX significantly reduced the [Ca2+]o-induced Ca2+ signal (Fig. 3B). We next investigated the role of PLC using a selective PLC inhibitor (20), U-73122 (30 μM) (Fig. 3C). As shown in Fig. 3, C and D, the [Ca2+]o-induced Ca2+ signal was also significantly reduced by U-73122, at least at the lower level of [Ca2+]o. Taken together, both G proteins and PLC are involved in CaSR-mediated Ca2+ signaling in HASMC, which is consistent with other reports (5, 11).
Fig. 3.
Inhibition of G proteins or phospholipase C (PLC) attenuates [Ca2+]o-induced increase in [Ca2+]cyt in HASMC. A: time course of [Ca2+]cyt changes in HASMC induced by different concentrations of [Ca2+]o in control cells. B and C: time course of [Ca2+]cyt changes in HASMC induced by different concentrations of [Ca2+]o in the presence of pertussis toxin (PTX, 100 ng/ml) or U-73122 (30 μM). D: summary data showing inhibition of [Ca2+]o-induced increase in [Ca2+]cyt by PTX or U-73122. Data are shown as means ± SE of 40–50 cells for each group. **P < 0.01, ***P < 0.001 vs. control in the absence of inhibitors.
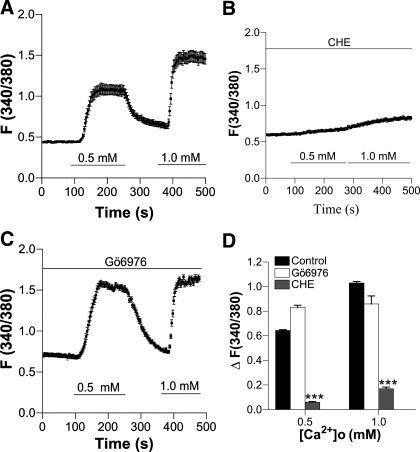
To test whether CaSR activation stimulates PKC, HASMC were superfused with [Ca2+]o in the absence (Fig. 4A) or the presence of chelerythrine (10 μM) (Fig. 4B), an inhibitor of the majority of PKC isoforms (39), or Gö6976 (10 μM) (Fig. 4C), a relatively selective inhibitor for conventional isoforms of PKC (39). As shown in Fig. 4D, the [Ca2+]o-induced Ca2+ signal in HASMC was prevented by chelerythrine, but not by Gö6976, suggesting that PKC isoforms other than conventional isoforms are involved in CaSR-mediated Ca2+ signaling in HASMC (35).
Fig. 4.
[Ca2+]o-induced increase in [Ca2+]cyt in HASMC is inhibited by chelerythrine (CHE) but not by Gö6976. A: time course of [Ca2+]cyt changes in HASMC induced by different concentrations of [Ca2+]o in control cells. B and C: time course of [Ca2+]cyt changes in HASMC induced by different concentrations of [Ca2+]o in the presence of chelerythrine (10 μM) or Gö6976 (10 μM). D: summary data showing effect of chelerythrine or Gö6976 on [Ca2+]o-induced increase in [Ca2+]cyt. Data are shown as means ± SE of 40–50 cells for each group. ***P < 0.001 vs. control in the absence of inhibitors.
PKCε activation plays a role in CaSR-mediated Ca2+ signaling.
More than 11 isoforms of PKC have been identified (23). To further our understanding of the expression of different isoforms of PKC in HASMC, we screened for expression of PKC isoforms using Western blot analysis of whole cell lysates. Like BxPc3 cells, pancreatic ductal cells that were used as positive controls (9), HASMC contained proteins immunoreactive with antibodies to PKCα, -β, and -γ in the conventional family, PKCε, -μ, and -δ in the novel family, and PKCι in the atypical family (Fig. 5).
Fig. 5.
Expression profiles of PKC isoforms in HASMC. HASMC and BxPc3 (a pancreatic ductal cell line used as a positive control) were lysed with lysis buffer and then Western blot analysis was performed. These data are representative of 3 experiments with similar results.
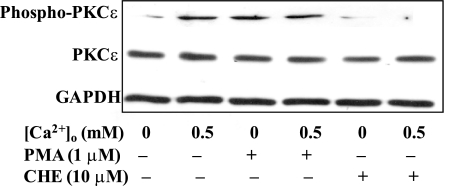
Since PKCε was previously implicated in the regulation of Ca2+ signaling in rabbit aortic smooth muscle cells (23), we focused on PKCε phosphorylation in HASMC in response to CaSR activation. As shown in Fig. 6, PMA (1 μM)-induced serine phosphorylation of PKCε in HASMC was reversed by chelerythrine (10 μM). Similarly, [Ca2+]o-induced (0.5 mM) PKCε serine phosphorylation was also prevented by chelerythrine (10 μM) (Fig. 6), suggesting that CaSR activation results in PKCε phosphorylation in HASMC (35).
Fig. 6.
[Ca2+]o-induced PKCε serine phosphorylation in HASMC. HASMC were pretreated with chelerythrine (10 μM) followed by treatment with or without [Ca2+]o (0.5 mM) for 30 min. PMA treatment was used as a positive control. Blots were then probed with an antibody to phosphorylated PKCε or total PKCε. GAPDH was used as a loading control. [Ca2+]o or PMA (1 μM) alone significantly induced PKCε serine phosphorylation, but the combination had no further effect. Chelerythrine alone did not affect PKCε serine phosphorylation but was able to suppress PKCε serine phosphorylation induced by [Ca2+]o or PMA. These data are representative of 3 experiments with similar results.
Source of Ca2+ mobilized by CaSR activation.
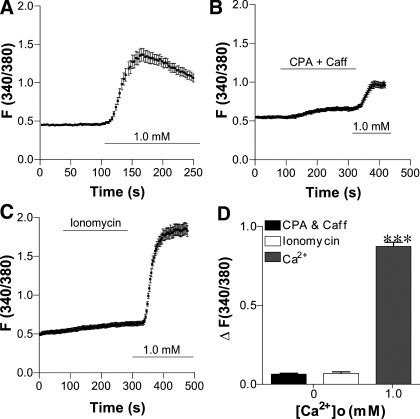
Since GPCR activation may mobilize different Ca2+ sources in different cell types, we investigated whether CaSR activation induces intracellular Ca2+ release, [Ca2+]o entry, or both in HASMC. To test whether intracellular Ca2+ release from the sarcoplasmic/endoplasmic reticulum (S/ER) plays a role, we used cyclopiazonic acid (CPA, 30 μM) to deplete intracellular Ca2+ stores (41), or ionomycin (10 μM) and caffeine (10 mM) to release total intracellular Ca2+ stores (18) in Ca2+-free solutions. None of these reagents induced any change in the basal [Ca2+]cyt (Fig. 7, B and C), but 1 mM [Ca2+]o induced significant [Ca2+]cyt signaling in HASMC (Fig. 7, A, C, and D). Interestingly, re-addition of [Ca2+]o after application of CPA plus caffeine did not induce a significant [Ca2+]cyt increase in HASMC (Fig. 7, B vs. A). Together, these results suggest that neither intracellular Ca2+ release mechanisms nor store-operated Ca2+ entry plays a significant role in regulating [Ca2+]cyt in HASMC.
Fig. 7.
[Ca2+]o mediates Ca2+ entry rather than intracellular Ca2+ release to raise [Ca2+]cyt in HASMC. A: time course of [Ca2+]cyt changes induced by 1 mM [Ca2+]o in control cells. B and C: time course of [Ca2+]cyt changes after addition of cyclopiazonic acid (CPA, 30 μM) and caffeine (Caff, 10 mM), ionomycin (10 μM), or [Ca2+]o in the Ca2+-free solutions. D: summary data showing that, although [Ca2+]o induces a marked increase in [Ca2+]cyt, CPA, caffeine, and ionomycin did not significantly affect basal [Ca2+]cyt in HASMC. Data are shown as means ± SE of 40–50 cells for each group. ***P < 0.001 vs. basal [Ca2+]cyt in HASMC.
Although voltage-gated Ca2+ channels (VGCC) in plasma membrane play an important role in regulating Ca2+ entry into VSMC, nifedipine (10 μM), which blocks VGCC, did not significantly affect [Ca2+]o-induced [Ca2+]cyt signaling in HASMC (data not shown), suggesting a minor role for VGCC in CaSR-mediated [Ca2+]cyt signaling.
TRPC-encoded nonselective cation channels are involved in CaSR-mediated Ca2+ entry.
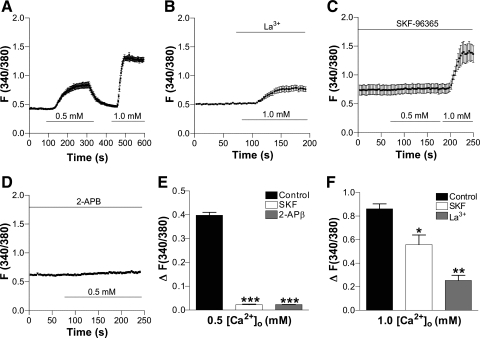
Since neither intracellular Ca2+ release from the S/ER nor VGCC appeared to be involved in CaSR-mediated [Ca2+]cyt signaling in HASMC, we further investigated whether other Ca2+ pathways, such as plasma membrane nonselective cation channels (NSCC), are involved. All of the TRPC-encoded NSCC blockers, including La3+ (10 μM), SKF-96365 (50 μM), or 2-APB (100 μM) (16), significantly inhibited CaSR-mediated [Ca2+]cyt signaling in HASMC (Fig. 8), While flufenamic acid (100 μM), an activator of TRPC6 channels (16), did not alter basal [Ca2+]cyt in Ca2+-free solutions, it significantly elevated [Ca2+]cyt in a [Ca2+]o-dependent manner, suggesting the involvement of TRPC6-encoded NSCC in CaSR-mediated Ca2+ entry into HASMC (Fig. 9).
Fig. 8.
[Ca2+]o-mediated Ca2+ entry is likely through receptor-operated Ca2+ channels in HASMC. A: time course of [Ca2+]cyt changes in HASMC induced by different concentrations of [Ca2+]o in control cells. B–D: time course of [Ca2+]cyt changes in HASMC induced by different concentrations of [Ca2+]o in the presence of La3+ (10 μM), SKF-96365 (50 μM), or 2-aminoethoxydiphenyl borate (2-APB; 100 μM). E and F: summary data showing effect of La3+, SKF-96365, or 2-APB [Ca2+]o-induced increase in [Ca2+]cyt. Data are shown as means ± SE of 40–50 cells for each group. *P < 0.05, **P < 0.01, ***P < 0.001 vs. the control in the absence of inhibitors.
Fig. 9.
[Ca2+]o-mediated Ca2+ entry through TRPC-encoded receptor-operated channels (ROC) and expression of TRPC isoforms in HASMC. A: time course of [Ca2+]cyt changes in HASMC induced by different concentrations of [Ca2+]o in control cells. B and C: time course of [Ca2+]cyt changes in HASMC induced by different concentrations of [Ca2+]o in the presence of flufenamic acid (FFA, 100 μM). D: summary data showing effect of flufenamic acid on different concentrations of [Ca2+]o-induced increase in [Ca2+]cyt. Please note that ΔF (340/380) in the 0.2 mM [Ca2+]o control group is close to zero. Data are shown as means ± SE of 40–50 cells for each group. ***P < 0.001 vs. the control in the absence of flufenamic acid. E and F: murine brains (used as a positive control) or HASMC were lysed with either TRIzol reagent or lysis buffer and then mRNA or protein expression of TRPC isoforms was detected by RT-PCR (E) or by Western blot analysis (F), respectively. GAPDH (GAP) was used as an internal standard. M, molecular weight marker. These data are representative of 3 experiments with similar results.
We then examined the expression of TRPC channels in HASMC. Our RT-PCR data revealed transcripts for TRPC1, -3, -4, and -6 in HASMC (Fig. 9E), and Western blotting identified protein expression of TRPC1 and -6 (Fig. 9F). These data further support that TRPC-encoded NSCC may contribute to CaSR-mediated Ca2+ entry in HASMC.
Caveolae are involved in CaSR-mediated Ca2+ signaling.
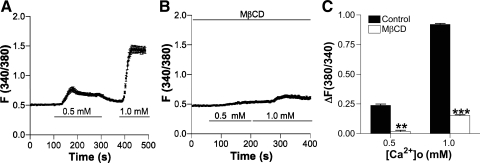
Spatially organized complexes of signaling molecules, such as caveolae, were found recently in microdomains of the plasma membrane in VSMC (13). These are important microstructures within the plasma membrane that contain signaling molecules such as caveolins, GPCR, and TRPC, all of which are involved in multiple cellular processes including Ca2+ homeostasis and signal transduction (27). Since caveolae are enriched in cholesterol, we tested whether caveolae were involved in CaSR-mediated Ca2+ entry by pretreating HASMC with MβCD (5 mM) for 1 h to deplete membrane cholesterol (27). Indeed, MβCD decreased CaSR-mediated Ca2+ entry in HASMC (Fig. 10), suggesting that CaSR in caveolae plays a role in [Ca2+]o-mediated [Ca2+]cyt signaling in HASMC.
Fig. 10.
Impairment of caveolae in the plasma membrane attenuates [Ca2+]o-induced increase in [Ca2+]cyt in HASMC. A: time course of [Ca2+]cyt changes in HASMC induced by different concentrations of [Ca2+]o in control cells. B: time course of [Ca2+]cyt changes in HASMC induced by different concentrations of [Ca2+]o in the presence of MβCD (5 mM) for 1 h to deplete membrane cholesterol. C: summary data showing effect of MβCD on [Ca2+]o-induced increases in [Ca2+]cyt. Data are shown as means ± SE of 40–50 cells for each group. **P < 0.01, ***P < 0.001 vs. the control in the absence of MβCD.
DISCUSSION
Here we found that 1) [Ca2+]o induces Ca2+ entry into HASMC likely via activation of CaSR; 2) CaSR activation mediates Ca2+ entry through receptor-operated channels (ROC); and 3) the PLC/PKCε pathway is involved in the CaSR-mediated [Ca2+]cyt rise in HASMC. Our study demonstrates a role for CaSR in regulating [Ca2+]o entry via ROC, and advances our understanding of the molecular mechanisms underlying CaSR-mediated [Ca2+]cyt rise in human VSMC.
Although CaSR was cloned from and characterized in bovine parathyroid cells in 1993 (5), it was recently shown also to be expressed in human VSMC and to function via the ERK signaling pathway (21). However, the proximal mechanism(s) by which CaSR transduces calcium-dependent signaling in VSMC are largely unknown. CaSR has physiological roles in the cardiovascular system by modulating myogenic tone in small arteries (24), arterial blood pressure (12, 34), and VSMC proliferation (32), through Ca2+ and/or ERK1/2 pathways (21, 32). In the present study, we found that both [Ca2+]o and spermine, two direct CaSR agonists with different molecular structures (33, 34), raised [Ca2+]cyt through CaSR activation in HASMC. We have also demonstrated that 1) CaSR proteins are expressed in HASMC, 2) [Ca2+]o-induced increase in [Ca2+]cyt was abolished in HASMC transfected with two CaSR mutant constructs that are mutated at different locations of the wild-type CaSR, and 3) [Ca2+]o-induced increase in [Ca2+]cyt was attenuated by inhibition of Gi/0 protein, PLC or PKC. Together, our results confirm the functional role of CaSR in the regulation of [Ca2+]cyt in HASMC. Similar to other GPCR, CaSR activation stimulates PLC, which cleaves phosphatidylinositol 4,5-bisphosphate into 1,2-diacylglycerol (DAG) and IP3 (6, 8, 33, 34). While DAG activates PKCε, and Ca2+ entry via receptor-operated calcium channels (ROC) (19, 29, 35, 38), IP3 causes intracellular Ca2+ release from S/ER stores and depletion of intracellular Ca2+ stores leads to Ca2+ entry via store-operated calcium channels (SOC) in different cell types (26, 30).
Both [Ca2+]o and [Ca2+]cyt play essential roles in numerous physiological process (10). [Ca2+]o is controlled by a complex homeostatic system, including the parathyroid glands and calcitonin-secreting C cells of various organs (6). In resting conditions, little or no Ca2+ crosses the plasma membrane passively, so as to maintain an almost 20,000-fold concentration gradient between [Ca2+]cyt (∼100 nM) and [Ca2+]o (∼1.2 mM) (6, 10). This is coordinated by activity of membrane ion channels, Ca2+ pumps, Na+/Ca2+ exchangers, and Ca2+ sequestration into the ER (10, 17). Our data suggest that [Ca2+]o specifically activates CaSR to induce Ca2+ entry via a specific PLC/PKCε pathway, rather than nonspecifically causing [Ca2+]o to leak into HASMC because 1) either [Ca2+]o or spermine dose-dependently raised [Ca2+]cyt in HASMC, which was inhibited by a G protein inhibitor or a PLC inhibitor; 2) a PKC inhibitor reduced CaSR-mediated [Ca2+]o entry; 3) [Ca2+]ob induced PKCε phosphorylation in HASMC, which was prevented by chelerythrine; 4) ROC blockers reduced, but a TRPC activator potentiated, CaSR-mediated [Ca2+]o entry; and 5) mRNA and protein for TRPC were identified in HASMC.
Our data show a Hill coefficient for CaSR activation by [Ca2+]o of 5.50 in HASMC, which is close to that of 4.73 in HEK-293 cells transfected with the human CaSR as reported by Quinn et al. (31), suggesting that multiple CaSR binding sites for [Ca2+]o in HASMC (1, 31). However, the EC50 for [Ca2+]o to raise [Ca2+]cyt in HASMC (0.52 mM) is about fourfold lower than seen in CaSR-transfected human embryonic kidney (HEK)-293 cells (∼2.0 mM) (31), suggesting that CaSR endogenously expressed in native HASMC might be more sensitive to [Ca2+]o than that heterologously overexpressed in HEK-293 cells. Our findings in HASMC also support an important role for the PLC/PKCε cascade during CaSR activation in different types of VSMC (34, 35, 38).
CaSR-mediated [Ca2+]o entry has previously been studied mostly in vascular endothelial cells (3, 42), but not in vascular smooth muscle cells. Moreover, little was known about [Ca2+]o entry mechanisms upon activation of CaSR. Although CaSR activation was found to enhance the activity of permeable NSCC in HEK-293 cells stably transfected with CaSR (40), the precise mechanisms underlying CaSR-mediated [Ca2+]cyt homeostasis in VSMC were largely unknown. After excluding the involvement of VGCC, we focused on SOC and ROC, both of which exist in VSMC. The major differences between SOC and ROC are 1) SOC is known to be functionally activated by depletion of intracellular Ca2+ stores, but ROC is activated via a PLC/PKCε cascade (19, 29, 35, 38); and 2) TRPC1 is a molecular candidate for SOC in VSMC, compared with TRPC6 for ROC (16, 19, 29, 38). SOC is unlikely involved in the [Ca2+]o-induced [Ca2+]cyt increase we observed in the present study because 1) the [Ca2+]cyt signal was not only induced by [Ca2+]o in Ca2+-free solutions, but also induced by spermine in Ca2+-containing solutions; 2) [Ca2+]o-induced [Ca2+]cyt increase was abolished in HASMC transfected with two different CaSR dominant negative mutants; and 3) [Ca2+]o re-addition did not induce a significant [Ca2+]cyt signal in HASMC after application of CPA plus caffeine. Together, our data suggest that TRPC1- or Orai1-mediated SOC does not play a major role in [Ca2+]o-induced [Ca2+]cyt increase in HASMC although Orai1 is another molecular candidate of SOC (2).
We therefore tested whether ROC participate in [Ca2+]o entry because they are NSCC that have been characterized in VSMC. Here, we showed that ROC blockers with different chemical structures significantly inhibited CaSR-mediated Ca2+ entry in HASMC, suggesting an involvement of ROC in this process. Moreover, we also found that TRPC6 is expressed in HASMC and that CaSR-mediated [Ca2+]o entry into HASMC was significantly enhanced by flufenamic acid, an activator of TRPC6 channels (16). Since TRPC6 satisfies many of the functional criteria of ROC and thus has been considered a ROC candidate in VSMC (16, 19, 29, 38), our findings support that TRPC6-encoded ROC may play a key role in CaSR-mediated [Ca2+]o entry into HASMC.
In summary, we demonstrate that [Ca2+]o activates CaSR in HASMC, and mediates [Ca2+]o entry into the cell interior likely through TRPC6-encoded ROC. We have also provided evidence that CaSR-mediated Ca2+ entry is regulated by the PLC/PKCε signaling cascade. Further studies are needed to assess the physiological role of CaSR-mediated [Ca2+]cyt in modulating cardiovascular function, and eventually to discover a novel therapeutic target for the treatment of cardiovascular diseases.
GRANTS
This work was supported by American Heart Association Beginning Grant-in-Aid Award (0565025Y) and American Lung Association research grant to H. Dong and by the University of California San Diego Digestive Diseases Research Development Center Grant DK-080506, in which H. Dong serves as the director of cell imaging core. It was also partially supported by National Institutes of Health Grant DK-073090 and the University of California San Diego Academic Senate Research Grant RH154H to J. Y. C. Chow.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Bai M, Quinn S, Trivedi S, Kifor O, Pearce SH, Pollak MR, Krapcho K, Hebert SC, Brown EM. Expression and characterization of inactivating and activating mutations in the human Ca2+o-sensing receptor. J Biol Chem 271: 19537–19545, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Baryshnikov SG, Pulina MV, Zulian A, Linde CI, Golovina VA. Orai1, a critical component of store-operated Ca2+ entry, is functionally associated with Na+/Ca2+ exchanger and plasma membrane Ca2+ pump in proliferating human arterial myocytes. Am J Physiol Cell Physiol 297: C1103–C1112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berra Romani R, Raqeeb A, Laforenza U, Scaffino MF, Moccia F, Avelino-Cruz JE, Oldani A, Coltrini D, Milesi V, Taglietti V, Tanzi F. Cardiac microvascular endothelial cells express a functional Ca+-sensing receptor. J Vasc Res 46: 73–82, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81: 239–297, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Brown EM, Pollak M, Chou YH, Seidman CE, Seidman JG, Hebert SC. The cloning of extracellular Ca(2+)-sensing receptors from parathyroid and kidney: molecular mechanisms of extracellular Ca(2+)-sensing. J Nutr 125: 1965S–1970S, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Brown EM, Vassilev PM, Hebert SC. Calcium ions as extracellular messengers. Cell 83: 679–682, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Chow JY, Dong H, Quach KT, Van Nguyen PN, Chen K, Carethers JM. TGF-β mediates PTEN suppression and cell motility through calcium-dependent PKC-α activation in pancreatic cancer cells. Am J Physiol Gastrointest Liver Physiol 294: G899–G905, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clapham DE. Calcium signaling. Cell 131: 1047–1058, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Emanuel RL, Adler GK, Kifor O, Quinn SJ, Fuller F, Krapcho K, Brown EM. Calcium-sensing receptor expression and regulation by extracellular calcium in the AtT-20 pituitary cell line. Mol Endocrinol 10: 555–565, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Fryer RM, Segreti JA, Widomski DL, Franklin PH, Banfor PN, Koch KA, Nakane M, Wu-Wong JR, Cox BF, Reinhart GA. Systemic activation of the calcium sensing receptor produces acute effects on vascular tone and circulatory function in uremic and normal rats: focus on central versus peripheral control of vascular tone and blood pressure by cinacalcet. J Pharmacol Exp Ther 323: 217–226, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res 94: 1408–1417, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Hebert SC, Brown EM. The extracellular calcium receptor. Curr Opin Cell Biol 7: 484–492, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol 4: 530–538, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca(2+)-permeable cation channel. Circ Res 88: 325–332, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ, Sato K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev 49: 157–230, 1997 [PubMed] [Google Scholar]
- 18. Karaki H, Weiss GB. Calcium release in smooth muscle. Life Sci 42: 111–122, 1988 [DOI] [PubMed] [Google Scholar]
- 19. Lemos VS, Poburko D, Liao CH, Cole WC, van Breemen C. Na+ entry via TRPC6 causes Ca2+ entry via NCX reversal in ATP stimulated smooth muscle cells. Biochem Biophys Res Commun 352: 130–134, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Molostvov G, Fletcher S, Bland R, Zehnder D. Extracellular calcium-sensing receptor mediated signalling is involved in human vascular smooth muscle cell proliferation and apoptosis. Cell Physiol Biochem 22: 413–422, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Molostvov G, James S, Fletcher S, Bennett J, Lehnert H, Bland R, Zehnder D. Extracellular calcium-sensing receptor is functionally expressed in human artery. Am J Physiol Renal Physiol 293: F946–F955, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer 7: 519–530, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258: 607–614, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Ohanian J, Gatfield KM, Ward DT, Ohanian V. Evidence for a functional calcium-sensing receptor that modulates myogenic tone in rat subcutaneous small arteries. Am J Physiol Heart Circ Physiol 288: H1756–H1762, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4: 552–565, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev 85: 757–810, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Patel HH, Zhang S, Murray F, Suda RY, Head BP, Yokoyama U, Swaney JS, Niesman IR, Schermuly RT, Pullamsetti SS, Thistlethwaite PA, Miyanohara A, Farquhar MG, Yuan JX, Insel PA. Increased smooth muscle cell expression of caveolin-1 and caveolae contribute to the pathophysiology of idiopathic pulmonary arterial hypertension. FASEB J 21: 2970–2979, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Petersen OH, Michalak M, Verkhratsky A. Calcium signalling: past, present and future. Cell Calcium 38: 161–169, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Poburko D, Liao CH, Lemos VS, Lin E, Maruyama Y, Cole WC, van Breemen C. Transient receptor potential channel 6-mediated, localized cytosolic [Na+] transients drive Na+/Ca2+ exchanger-mediated Ca2+ entry in purinergically stimulated aorta smooth muscle cells. Circ Res 101: 1030–1038, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Putney JW., Jr Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here). Cell Calcium 42: 103–110, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quinn SJ, Ye CP, Diaz R, Kifor O, Bai M, Vassilev P, Brown E. The Ca2+-sensing receptor: a target for polyamines. Am J Physiol Cell Physiol 273: C1315–C1323, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Smajilovic S, Hansen JL, Christoffersen TE, Lewin E, Sheikh SP, Terwilliger EF, Brown EM, Haunso S, Tfelt-Hansen J. Extracellular calcium sensing in rat aortic vascular smooth muscle cells. Biochem Biophys Res Commun 348: 1215–1223, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Smajilovic S, Tfelt-Hansen J. Calcium acts as a first messenger through the calcium-sensing receptor in the cardiovascular system. Cardiovasc Res 75: 457–467, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Smajilovic S, Tfelt-Hansen J. Novel role of the calcium-sensing receptor in blood pressure modulation. Hypertension 52: 994–1000, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Smani T, Patel T, Bolotina VM. Complex regulation of store-operated Ca2+ entry pathway by PKC-ε in vascular SMCs. Am J Physiol Cell Physiol 294: C1499–C1508, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Smith AJ, Chappell AE, Buret AG, Barrett KE, Dong H. 5-Hydroxytryptamine contributes significantly to a reflex pathway by which the duodenal mucosa protects itself from gastric acid injury. FASEB J 20: 2486–2495, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Su JY, Vo AC. 2-Arachidonylglyceryl ether and abnormal cannabidiol-induced vascular smooth muscle relaxation in rabbit pulmonary arteries via receptor-pertussis toxin sensitive G proteins-ERK1/2 signaling. Eur J Pharmacol 559: 189–195, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Syyong HT, Poburko D, Fameli N, van Breemen C. ATP promotes NCX-reversal in aortic smooth muscle cells by DAG-activated Na+ entry. Biochem Biophys Res Commun 357: 1177–1182, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Wang YX, Ding YJ, Zhu YZ, Shi Y, Yao T, Zhu YC. Role of PKC in the novel synergistic action of urotensin II and angiotensin II and in urotensin II-induced vasoconstriction. Am J Physiol Heart Circ Physiol 292: H348–H359, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Ye C, Rogers K, Bai M, Quinn SJ, Brown EM, Vassilev PM. Agonists of the Ca(2+)-sensing receptor (CaR) activate nonselective cation channels in HEK293 cells stably transfected with the human CaR. Biochem Biophys Res Commun 226: 572–579, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Zhang S, Yuan JX, Barrett KE, Dong H. Role of Na+/Ca2+ exchange in regulating cytosolic Ca2+ in cultured human pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 288: C245–C252, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Ziegelstein RC, Xiong Y, He C, Hu Q. Expression of a functional extracellular calcium-sensing receptor in human aortic endothelial cells. Biochem Biophys Res Commun 342: 153–163, 2006 [DOI] [PubMed] [Google Scholar]