Abstract
Response gene to complement 32 (RGC-32) is activated by transforming growth factor- β (TGF-β) and plays an important role in smooth muscle cell (SMC) differentiation from neural crest Monc-1 cells. The molecular mechanism governing TGF-β activation of RGC-32, however, remains to be determined. The present studies indicate that TGF-β regulates RGC-32 gene transcription. Sequence analysis revealed a Smad binding element (SBE) located in the region from −1344 to −1337 bp upstream of the transcription start site of RGC-32 gene. A polyomavirus enhancer activator (PEA3) binding site is adjacent to the SBE. Mutation at either SBE or PEA3 site significantly inhibited RGC-32 promoter activity. Mutations at both sites completely abolished TGF-β-induced promoter activity. Biochemically, TGF-β stimulated recruitment of Smad2, Smad4, and PEA3 to the RGC-32 promoter, as revealed by gel shift and chromatin immunoprecipitation analyses. Functionally, Smad2, but not Smad3, activated RGC-32 promoter. PEA3 appeared to enhance Smad2 activity. In agreement with their function, Smad2, but not Smad3, physically interacted with PEA3. In TGF-β-induced SMC differentiation of Monc-1 cells, knockdown of Smad2 by short hairpin RNA resulted in downregulation of RGC-32 and SMC marker genes. The downregulation of SMC markers, however, was rescued by exogenously introduced RGC-32. These results demonstrate that Smad2 regulation of RGC-32 transcription is essential for SMC differentiation from neural crest cells.
Keywords: response gene to complement 32, transforming growth factor-β, Smad2, polyomavirus enhancer activator 3, transcriptional regulation
vascular smooth muscle cell (SMC) differentiation is an important process during vasculogenesis and angiogenesis (22). The modulation of SMC phenotype plays a major role in a number of human diseases including atherosclerosis, hypertension, restenosis, and cancer (1, 20, 22, 28). Identification of the mechanisms controlling SMC differentiation will enhance our understanding of SMC phenotypic regulation and therefore will provide potential therapeutic or preventive tools for treating cardiovascular diseases.
Transforming growth factor-β (TGF-β) has been shown to play an important role in the regulation of embryonic vasculogenesis and SMC differentiation (15, 27, 30). In vivo studies by homologous recombination in mice have shown that TGF-β1, TGF-β receptors (including endoglin, TβRII, TβR-I, and Alk1), and signaling intermediates Smads are all critical for embryonic angiogenesis. Loss of any of these components leads to defects in SMC recruitment and/or differentiation during angiogenesis in the developing embryo or extraembryonic structures (17). TGF-β also plays an important role in SMC differentiation from neural crest cells. Although Choudhary and colleagues have reported that TGF-β type II receptor neural crest knockout does not cause SMC defect (10, 36), an earlier study demonstrates that neural crest-specific knockout of the type II receptor causes SMC defect in the aortopulmonary septum (10, 36). In addition, TGF-β induces primary cultured neural crest cells and neural crest cell line Joma1.3 to differentiate to SMC (26, 32). Our in vitro studies have demonstrated that TGF-β induces a contractile SMC phenotype from the neural crest cell line Monc-1 (8). Smad signaling and RhoA play critical roles in the differentiation of Monc-1 cells (6, 8).
TGF-β signaling is initiated by ligand binding to the transmembrane serine-threonine kinase receptors. The signaling is then transduced predominantly by phosphorylation of receptor-associated Smad proteins (R-Smads) (16, 24, 29). R-Smads (Smad2 and/or Smad3) combine with the common Smad Smad4 and translocate into the nucleus where they function as transcription factors alone or in association with other DNA binding factors (24). In addition to Smads, other pathways including those activated by mitogen-activated protein kinase, JNK kinases, phosphoinositide 3 kinase, as well as RhoA, are also involved in TGF-β action (6, 25, 34, 35).
To explore the molecular mechanisms controlling SMC differentiation, we identified a TGF-β downstream target, response gene to complement 32 (RGC-32), to be an important regulator of SMC differentiation of Monc-1 cells. Blockade of RGC-32 by small interfering RNA abolishes TGF-β-induced SMC marker expression. TGF-β markedly induces RGC-32 mRNA expression in Monc-1 cells, suggesting that TGF-β controls RGC-32 gene transcription (21), but the molecular mechanism governing Smad regulation of RGC-32 transcription remains to be determined.
In this study, we identified a Smad binding element in the 5′ upstream regulatory region to be critical for TGF-β regulation of RGC-32 gene. Additional studies revealed that Smad2, but not Smad3, is critical for TGF-β-induced RGC-32 transcription. In addition, we found that polyomavirus enhancer activator (PEA3) enhanced Smad function in RGC-32 transcription by interacting with Smad2. Furthermore, Smad2 regulation of RGC-32 transcription appears to be important for TGF-β-induced SMC differentiation from neural crest cells.
MATERIALS AND METHODS
Cell culture and reagents.
Monc-1 cells were cultured as previously described (7, 8). Smad1, Smad2, Smad3, Smad4, and Smad5 antibodies were generously provided by Dr. Peter ten Dijke (The Netherlands Cancer Institute, Amsterdam, The Netherlands). PEA3 antibodies were purchased from Santa Cruz (sc-113). Smad1, Smad3, Smad4, and Smad5 expression plasmids were previously described (11, 12). Smad2 expression plasmid was a generous gift from Dr. Ying Zhang (38).
Plasmid construction.
RGC-32 promoter DNA were amplified from mouse genomic DNA (Promega) by PCR and cloned into luciferase reporter vector pGL3-basic (Promega) at the KpnI/BglII restriction site. Three RGC-32 promoter reporter plasmids were constructed as follows: p-2107/+844RGCluc contains the regulatory region from nucleotide (nt) −2107 to +844 bp of RGC-32 gene; p-2107RGCluc and p-1500RGCluc contain the regulatory regions from nt −2107 and −1500 to +22 bp of the RGC-32 gene, respectively. The transcription start site was determined based on RGC-32 gene sequence (accession number AF276981). For cloning purpose, the primers for each promoter DNA were flanked by a restriction site of either KpnI (forward primers) or BglII (reverse primers). The sequences of the primers for each construct are as follows (restriction enzyme sites are underlined): p-2107RGCluc: 5′-AAA AGG TAC CGT GAG GAG ATG AAG GAT ACA GCT CCC-3′; p-1500RGCluc: 5′-AAA AGG TAC CGC ATG CCA CAT TCT GAC ACC-3′; Reverse Primer 1 (+22): 5′-AAA AAG ATC TCG CTA GCT ACT CGC CTG AG-3′; Reverse Primer 2 (+844): 5′-AAA AAG ATC TTC ATA GTG GAA GTG GCG CTC GT-3′.
The mutant constructs were generated in the context of p-1500RGCluc using the Quikchange Multi Site-Directed Mutagenesis Kit (Stratagene) according to the manufacturer's recommendation. The PEA3 binding site AGGAAG (from nt −1379 to −1374 bp) was converted to ActtAG. The SBE GTCTGGAC (from nt −1344 to −1337 bp) was converted to GTtatGAC. The double mutations of PEA3 site and SBE were made according to the individually mutated sequences. All plasmid constructs and mutations were confirmed by sequencing.
The mouse PEA3 expression plasmid was generated by inserting the full-length PEA3 cDNA fragment into pcDNA3.0 expression vector at the HindIII and EcoRI sites. T7-tag was included at the 5′ end of PEA3 cDNA. The PEA3 full-length cDNA was amplified by PCR from the reverse transcription of mRNA extracted from Monc-1 cells using primer sequences of AAG CTT ACC ATG GCT AGC ATG ACT GGT GGA CAG CAA ATG GGT CGG ACT AAG TCT TCC AAC CAC (forward) and GAA TTC TCA CTA GTA AGA ATA TCC ACC TCT GTG (reverse). The PEA3 full-length cDNA was verified by sequencing.
Transient transfection and luciferase assay.
Monc-1 cells were plated at 2×105 cells/well in 12-well plates and incubated at 37°C in a 5% CO2 incubator until 80% confluency. Cells were then transiently transfected (in triplicate) with LipofectAMINE 2000 according to the manufacturer's recommendation (10, 11). Fifty percent of transfection efficiency was observed with enhanced green fluorescent protein plasmid transfection. Luciferase assays were performed with a dual luciferase reporter assay system (Promega) in a luminescence microplate reader (BioTek Instruments).
Quantitative polymerase chain reaction.
Total RNA was extracted using TRIzol reagent (Invitrogen) following the manufacturer's instruction. cDNA was synthesized using an iScript cDNA synthesis kit (Bio-Rad). Quantitative polymerase chain reaction (qPCR) was performed in MX3000P qPCR machine using SYBR Green qPCR Mastermix (Agilent). The primer sequences for RGC-32, Smad2, α-SMA, and SM22α were described previously (8, 18, 37).
Nuclear extract preparation.
Monc-1 cells were plated at a density of 3×106/150 mm dish and grown until 70–80% confluency. The cells were then treated with 5 ng/ml of TGF-β1 (R&D Biosystem) or vehicle for the times indicated. Nuclear extracts were prepared according to the published procedure (14). Protein concentrations were measured by the Bradford method (Bio-Rad).
Electrophoretic mobility shift assays.
Electrophoretic mobility shift assays (EMSA) reaction was performed as described (17). Supershift assays were performed by addition of 1 μg Smad antibody or 2 μg of PEA3 antibody. Protein-DNA complexes were separated in 5% nondenaturing polyacrylamide gel. Mutations for the mutant probes were the same as those generated in mutant luciferase plasmids as described above.
Coimmunoprecipitation assay (CoIP) and Western blot analysis were performed using standard procedure as described previously (4, 8).
Chromatin immunoprecipitation assay.
Chromatin immunoprecipitation assay (ChIP) assays were performed as previously described (37). Chromatin protein complexes were immunoprecipitated with 3 μg Smad2, Smad3 (37), or PEA3 antibody, whereas negative control was immunoprecipitated with normal rabbit IgG. Quantitative PCR was performed to amplify RGC-32 promoter region containing Smad and PEA3 binding sites using the following primer set: forward, 5′-CCAAAGATTCTGTGATGATGGC-3′ and reverse, 5′-AGTCTGAAGGCTCATCCTCAG -3′.
Statistical analysis.
All values are expressed as means ± SE. Data were analyzed using ANOVA with pairwise comparisons between groups. A level of P values <0.05 was considered statistically significant.
RESULTS
TGF-β regulates RGC-32 promoter activity.
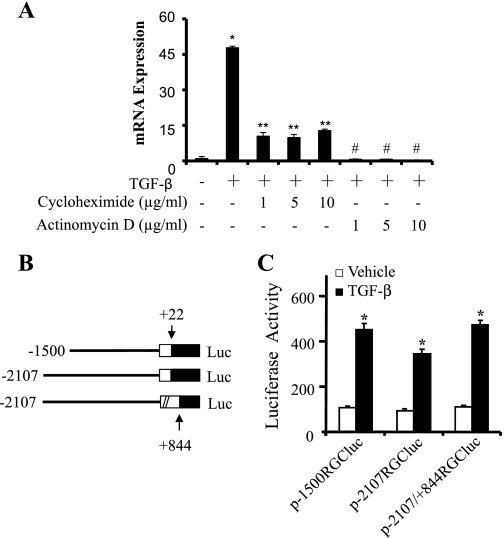
We previously reported that RGC-32 mRNA expression was significantly upregulated to 50-fold within 24 h after TGF-β induction of Monc-1 neural crest cells (21). To determine whether TGF-β regulates RGC-32 gene transcription, we treated TGF-β-induced cells with transcription inhibitor actinomycin D. Actinomycin D abolished RGC-32 mRNA expression, suggesting that TGF-β-induced response is indeed transcriptional (Fig. 1A). In addition, translational inhibitor cycloheximide, although lowered, did not block the TGF-β-induced RGC-32 mRNA expression, suggesting that TGF-β-induced response does not require de novo protein synthesis. The lowered expression of RGC-32 at 24 h of TGF-β treatment is probably due to the short half-life of TGF-β receptors. Since Smad signaling is the major pathway mediating TGF-β cellular functions (16), a transcription factor binding site screen was performed using the software pattern-defined regulatory island or PRI (9). Sequence analysis revealed a SBE (GTCTGGAC) (33) located in the region from −1344 to −1337 bp of the RGC-32 promoter. To confirm whether the RGC-32 response is transcriptional, we cloned three different regions of RGC-32 promoter containing this SBE into pGL3-basic luciferase reporter basic vector (Fig. 1B). These constructs were then transfected into Monc-1 cells followed by TGF-β treatment. As shown in Fig. 1C, TGF-β significantly upregulated the activities of RGC-32 promoter in these constructs (p-1500RGCluc, p-2107RGCluc, and p-2107RGCluc/+844). These data suggest that TGF-β regulates RGC-32 transcription through upstream regulatory elements, which are located in a region from −1500 to +22 bp of RGC-32 promoter. The first exon of RGC-32 gene appears not to be involved in TGF-β-induced RGC-32 transcription because the activity of the promoter region from −2107 to +884 bp is essentially the same as the region from −2107 to +22 bp (Fig. 1C).
Fig. 1.
Transforming growth factor-β (TGF-β) induction of response gene to complement 32 (RGC-32) promoter activity. A: TGF-β induction of RGC-32 mRNA expression is transcriptional and does not require de novo protein synthesis. Monc-1 cells were treated with cycloheximide or actinomycin as indicated for 30 min followed by TGF-β induction for 24 h. Quantitative polymerase chain reaction (qPCR) was performed to detect RGC-32 mRNA expression. *P < 0.01 compared with vehicle (−)-treated group; **, #P < 0.001 compared with TGF-β1 alone treated group (n = 3). B: schematic illustration of RGC-32 promoter reporter constructs. The promoter regions are shown. Numbers relative to the transcription start site of RGC-32 gene. C: TGF-β regulates RGC-32 promoter. RGC-32 promoter constructs were transfected into Monc-1 cells for 20 h as indicated. Cells were then treated with TGF-β1 (5 ng/ml) or vehicle for 18 h. Luciferase activities were determined. Data shown are the results of four independent experiments. *P < 0.001 compared with vehicle-treated group.
Both Smad and PEA3 are required for the formation of TGF-β-inducible transcription factor complex bound to RGC-32 promoter.
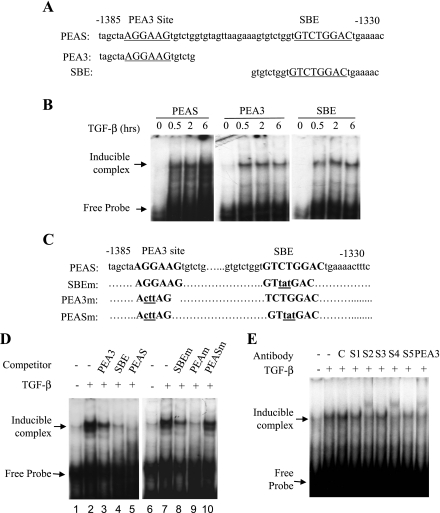
In natural promoters, interaction of Smad proteins with an adjacent transcription factor allows stable binding of Smad transcription complexes to the promoter (16). Sequence analysis identified a PEA3 binding site (AGGAAG) (23) located in the region from −1379 to −1374 bp, 35 bp upstream of the SBE. PEA3 is a transcription factor of E-twenty six (ETS) family. ETS proteins control the expression of genes that are critical for several biological processes, including cellular proliferation, differentiation, development, transformation, and apoptosis. ETS factors regulate hematopoiesis and the development of the central nervous system, bone and cartilage, mammary glands, and vasculature (3, 31). However, whether or not PEA3 interacts with Smads on RGC-32 promoter remains to be determined. To test the promoter-nuclear protein interaction in TGF-β regulation of RGC-32 transcription, we synthesized three DNA oligonucleotides containing SBE, PEA3 binding site, or both sites as shown in Fig. 2A and performed an EMSA with nuclear extracts from Monc-1 cells treated with vehicle or TGF-β. We found that TGF-β induced an interaction between nuclear factors and RGC-32 promoter as early as 30 min after the induction (Fig. 2B).
Fig. 2.
Smad2 and polyomavirus enhancer activator (PEA3) interact with RGC-32 promoter through Smad-binding element (SBE) and PEA3 binding sites. A: sequence scheme of DNA fragments used in electrophoretic mobility shift assay (EMSAs). Sequences for DNA fragments containing SBE, PEA3 binding site (PEA3), or both sites (PEAS) are shown. Numbers relative to the transcription start site. B: TGF-β induces RGC-32 promoter DNA-nuclear protein interaction. Monc-1 cells were treated with TGF-β (5 ng/ml) or vehicle (0) for the times indicated. Nuclear proteins were extracted followed by incubation with 32P-labeled PEAS, PEA3, or SBE probes. The protein-DNA complexes were resolved and detected as described in materials and methods. C: sequence scheme of DNA fragments used for 32P-labeled probe or competitors in EMSAs. Wild-type probe containing both SBE and PEAS, mutant DNA fragments with mutation at SBE (SBEm), or PEA3 binding site (PEA3m), or both sites (PEASm) are shown. The mutated sequences are shown in lower cases and underlined. Numbers relative to the transcription start site. D: competitive EMSAs. The EMSAs were performed using 32P-labeled PEAS and nuclear proteins extracted from Monc-1 cells treated with vehicle (−) or TGF-β1 (5 ng/ml) (+) for 2 h. Wild-type competitors are DNA fragments containing SBE, PEA3, or both sites as shown in A. 100 molar excess wild-type or mutant competitors were simultaneously added with the 32P-labeled probe to the EMSA reactions. E: supershift assay. The nuclear extracts were incubated with 1.0 μg of Smad1 (S1), Smad2 (S2), Smad3 (S3), Smad4 (S4), Smad5 (S5), or 2.0 μg of PEA3 antibody on ice for 30 min before the incubation with 32P-labeled PEAS probe. Normal goat IgG (1.0 μg) (Santa Cruz) served as control (C). The protein-DNA complexes were resolved and detected as described in materials and methods. Smad2, Smad4, and PEA3 antibodies recognize TGF-β-inducible complex.
To determine whether SBE and/or PEA3 sites are important for TGF-β-induced DNA-protein interaction, we used the DNA oligonucleotides containing both SBE and PEA3 sites as probes to perform competitive EMSA. The wild-type or mutant oligonucleotides with mutation at SBE, PEA3 site, or both sites were used as competitors (Fig. 2C). As shown in Fig. 2D, wild-type DNA containing both Smad and PEA3 sites completely abolished the formation of TGF-β-induced complex (lane 5), whereas the competitors containing either site only partially inhibited the complex formation (lanes 3 and 4). Conversely, the mutation at either Smad or PEA3 site partially competed with the DNA-protein interaction (lanes 8 and 9), but the competitors with mutation at both sites completely lost the ability to compete for the interaction (lane 10). These data suggest that both SBE and PEA3 binding sites are essential for the interaction of TGF-β-inducible nuclear proteins with RGC-32 promoter.
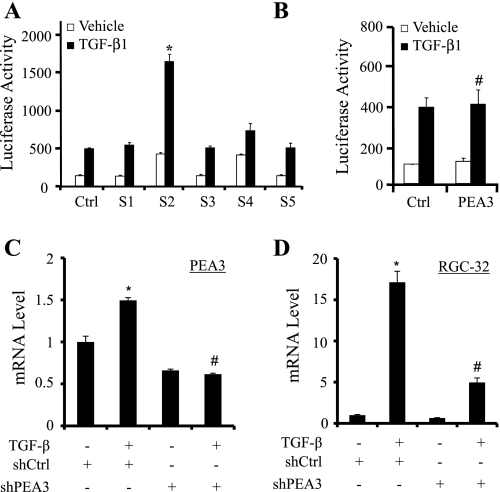
To determine whether Smad and/or PEA3 proteins participate in the formation of TGF-β-inducible nuclear complex formed with RGC-32 promoter and to determine which Smad is involved in the complex, we performed supershift EMSA using Smad and PEA3 antibodies. As shown in Fig. 2E, Smad1, Smad3, and Smad5 antibodies did not recognize the complex, therefore no supershift was observed. However, Smad2 and Smad4 antibodies interacted with the complex and supershifted the TGF-β-inducible complex (Fig. 2E). These data indicate that Smad2 and Smad4 are part of the TGF-β-inducible transcriptional complex. PEA3 antibody also recognized the TGF-β-inducible complex and supershifted the complex (Fig. 2E), suggesting that PEA3 is also part of TGF-β-induced transcriptional complex.
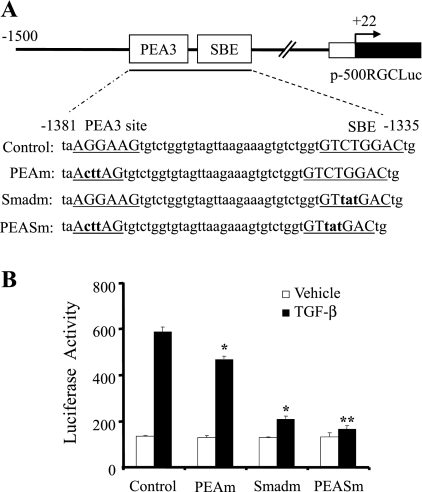
Both SBE and PEA3 binding sites are important for TGF-β regulation of RGC-32 promoter.
To determine whether the SBE and/or PEA3 binding sites play roles in TGF-β-induced RGC-32 promoter activation during SMC differentiation, we mutated the two sites individually and in combination by site-directed mutagenesis in the context of p-1500RGCluc as illustrated in Fig. 3A. The wild-type and mutant constructs were then transiently transfected into Monc-1 cells followed by TGF-β1 induction and luciferase assay. As shown in Fig. 3B, PEA3 binding site mutation significantly inhibited RGC-32 promoter activity. The SBE mutation appears to have a greater effect. Double mutations at both SBE and PEA3 sites resulted in a complete loss of TGF-β induction of the promoter (Fig. 3B). These data suggest that both SBE and PEA3 binding sites are critical for TGF-β-induced RGC-32 transcriptional activation.
Fig. 3.
Effects of SBE and/or PEA3 binding site mutations on RGC-32 promoter activity. A: sequence scheme of RGC-32 promoter region containing SBE and PEA3 binding sites in the promoter construct p-1500RGCluc. The SBE and PEA3 sites are underlined in the wild-type promoter construct (Control). Numbers relative to the transcription start site of RGC-32 gene. PEA3 site (PEAm), SBE (Smadm), or both sites (PEASm) mutations were generated in the context of p-1500RGCluc. The mutated nucleotides are shown as lower case and bold. B: functional analysis of SBE and PEA3 site mutations on RGC-32 promoter activity. The wild-type (Control) or mutant promoter constructs with PEAm, Smadm, or both sites (PEASm) were transiently transfected into Monc-1 cells for 20 h. Cells were then treated with TGF-β1 (5 ng/ml) or vehicle for 18 h followed by luciferase assay. Data shown are the results of three independent experiments. *P < 0.01 compared with TGF-β-treated control group; **P < 0.05 compared with the TGF-β-treated SBE mutation group (Smadm).
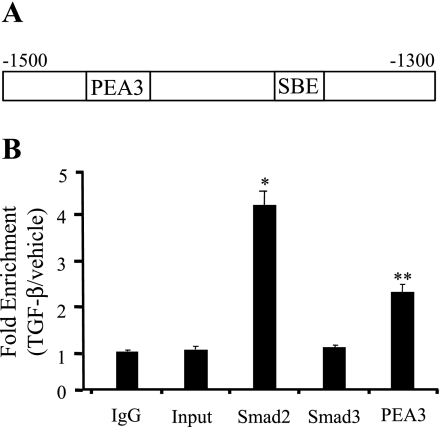
TGF-β induces an increased binding of Smad2 and PEA3 to the endogenous RGC-32 promoter.
To determine whether or not TGF-β induction enhances the binding of Smad2 and PEA3 to their binding sites in the endogenous RGC-32 promoter within intact chromatin, we performed ChIP analysis on chromatin isolated from Monc-1 cells treated with vehicle or TGF-β. As shown in Fig. 4, Smad2 binding was enhanced 4.2 ± 0.3-fold in Monc-1 cells treated with TGF-β compared with normal IgG or input controls. PEA3 binding was increased 2.3 ± 0.2-fold. However, Smad3 binding was not increased (Fig. 4). These results suggest that TGF-β may regulate RGC-32 gene transcription via a cooperative action of Smad2 and PEA3.
Fig. 4.
TGF-β induces increased interactions of Smad2 and PEA3 with the endogenous RGC-32 promoter within intact chromatin. A: schematic illustration of the location of SBE and PEA3 sites in RGC-32 promoter. The region for PCR amplification is shown. Numbers relative to the transcription start site of RGC-32 gene. B: chromatin immunoprecipitation (ChIP) assay. Monc-1 cells were treated with vehicle (−) or TGF-β (5 ng/ml) (+) for 30 min before isolating and harvesting chromatin for quantitative ChIP analysis as described in materials and methods. *P < 0.001, **P < 0.01 compared with control IgG group, input, or Smad3 (S3) group. Values shown are means ± SE of three independent experiments.
Both Smad2 and PEA3 are critical for TGF-β induction of RGC-32 promoter activity.
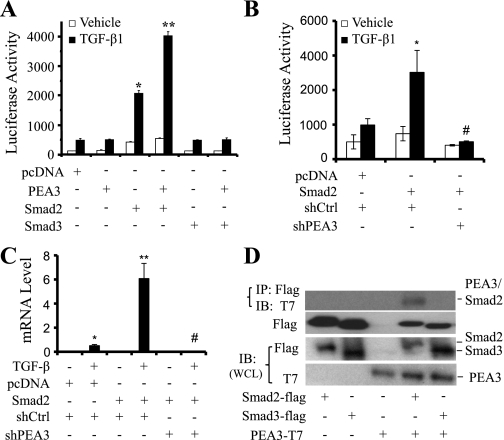
TGF-β signaling is predominantly mediated by Smad2 and Smad3 (16). As shown in the supershift assay (Fig. 3E), Smad2, but not Smad3, was present in the TGF-β-inducible complex formed with RGC-32 promoter. We sought to determine whether Smad2 is also functionally important for RGC-32 promoter activity. We cotransfected p-1500RGCluc plasmid with Smad1, Smad2, Smad3, Smad4, or Smad5 expression plasmids into Monc-1 cells followed by TGF-β induction. Luciferase assay showed that Smad1, Smad3, and Smad5 did not have any effect on RGC-32 promoter. However, Smad2 robustly increased the promoter activity (Fig. 5A). These data indicate that Smad2 is capable of activating RGC-32 promoter. As expected, Smad4 alone did not increase RGC-32 promoter activity.
Fig. 5.
Both Smad2 and PEA3 are important for RGC-32 promoter activity. A and B: Smad2 overexpression activates RGC-32 promoter. Smad1, Smad2, Smad3, Smad4, Smad5 (A), or PEA3 (B) expression plasmid was individually cotransfected with p-1500RGCluc promoter constructs into Monc-1 cells for 20 h, followed by TGF-β1 (5 ng/ml) or vehicle treatment for 18 h. Luciferase activity was then determined. Data are the results of three independent experiments. *P < 0.001, #P > 0.05 compared with the group with pcDNA (Ctrl) transfection treated with TGF-β. C and D: PEA3 knockdown blocks TGF-β-induced RGC-32 mRNA expression. Monc-1 cells were transfected with scramble (shCtrl) or PEA3 (shPEA3) short hairpin RNA (shRNA) followed by vehicle (−) or TGF-β induction for 24 h. qPCR was performed to detect PEA3 (C) or RGC-32 (D) mRNA expression. *P < 0.05 compared with vehicle-treated group; #P < 0.01 compared with TGF-β with control shRNA group (n = 3).
PEA3 overexpression did not increase TGF-β-induced RGC-32 promoter activity (Fig. 5B), suggesting that endogenous PEA3 may be sufficient for RGC-32 promoter activity. To determine whether PEA3 is essential for RGC-32 transcription, we blocked PEA3 expression in Monc-1 cells using short hairpin RNA (shRNA) and detected RGC-32 mRNA expression. qPCR analyses showed that PEA3 knockdown significantly inhibited TGF-β-induced RGC-32 mRNA expression (Fig. 5, C and D).
Smad2 interacts with PEA3 to regulate RGC-32 transcription.
As indicated above, TGF-β-induced nuclear factor-promoter interaction requires both SBE and PEA3 binding sites (Fig. 2D). PEA3 is present in the TGF-β-induced transcriptional complex (Fig. 2E). PEA3 is indeed required for RGC-32 gene transcription (Fig. 5, C and D). To determine whether Smad2 functionally interacts with PEA3, p-1500RGCluc was cotransfected with PEA3, Smad2, or Smad3 expression plasmids individually or in combination into Monc-1 cells followed by TGF-β treatment. As shown in Fig. 6A, overexpression of PEA3 or Smad3 did not alter RGC-32 promoter activity. Coexpression of PEA3 and Smad3 also did not affect the promoter. However, Smad2 dramatically increased RGC-32 promoter activity. Cotransfection of PEA3 and Smad2 significantly increased the promoter activity compared with Smad2 alone (Fig. 6A). These data suggest that PEA3 enhances Smad2 function. Additional studies with PEA3 shRNA showed that PEA3 knockdown significantly blocked Smad2-mediated RGC-32 promoter activity (Fig. 6B) and RGC-32 mRNA expression (Fig. 6C).
Fig. 6.
Smad2 functionally and physically interacts with PEA3. A: Smad2 interaction with PEA3 in regulating RGC-32 promoter. Smad2, Smad3, and PEA3 expression plasmids were cotransfected individually or in combination with p-1500RGCluc promoter into Monc-1 cells for 20 h, followed by TGF-β1 (5 ng/ml) or vehicle treatment for 18 h. Luciferase activities were determined. Data are the results of three independent experiments. *P < 0.001 compared with pcDNA-transfected group treated with TGF-β. **P < 0.001 compared with Smad2-transfected group treated with TGF-β. B: PEA3 knockdown blocks Smad2-induced RGC-32 promoter activity. p-1500RGCluc promoter was cotransfected with Smad2 and control (shCtrl) or PEA3 shRNA (shPEA3) into Monc-1 cells for 48 h followed by TGF-β induction for 18 h. Luciferase assay performed. *P < 0.01 compared with pcDNA transfected and TGF-β-treated group. #P < 0.01 compared with Smad2/shCtrl and TGF-β-treated group. C: PEA3 knockdown blocks Smad2-induced RGC-32 mRNA expression. Cells were treated similarly as in B but without p-1500RGCluc promoter transfection. RGC-32 mRNA expression was detected by qPCR. *P < 0.01 compared with pcDNA transfected and TGF-β-treated group. #P < 0.01 compared with Smad2/shCtrl and TGF-β-treated group. D: PEA3 physically interacts with Smad2. Flag-tagged Smad2, flag-tagged Smad3, and T7-tagged PEA3 plasmids were transfected into Cos-7 cells individually or in combination as indicated and coimmunoprecipitation (CoIP) was performed. Smad-interacting proteins were pulled down with Flag antibody (IP: Flag), and the immunoprecipitates were blotted (IB) with T7 and Flag antibody. Smad2 (58 kDa), Smad3 (50 kDa) or PEA3 expression was monitored in whole cell lysates (WCL IB) with flag or T7 antibodies as indicated.
Since PEA3 enhanced the activity of Smad2 in regulating RGC-32 promoter, PEA3 and Smad2 were also present in the same transcription complex binding to RGC-32 promoter (Fig. 2E); we sought to confirm if PEA3 physically interacts with Smad2. We transfected flag-tagged Smad2, Smad3, and T7-tagged PEA3 expression plasmids individually or in combination into Cos-7 cells and performed a CoIP assay by using anti-flag antibody to pull down Smad2/3-interacting proteins and using T7 antibody to blot PEA3. Our results show that PEA3 did not interact with Smad3. However, PEA3 interacted with Smad2 (Fig. 6D), which is consistent with the function of PEA3 in enhancing Smad2 activity (Fig. 6A).
Smad2 regulation of RGC-32 is important for TGF-β-induced SMC differentiation.
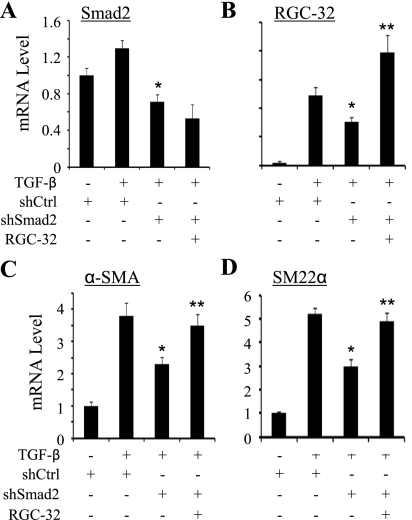
To determine whether Smad2 regulation of RGC-32 transcription is relevant to SMC differentiation, we tested if RGC-32 is able to rescue the expression of SMC marker genes downregulated by Smad2 knockdown. As shown in Fig. 7, Smad2 shRNA (18) significantly inhibited the expression of RGC-32 and endogenous SMC marker genes α-SMA and SM22α. Overexpression of RGC-32 (Fig. 7B), however, reversed the effect of Smad2 shRNA and restored the expression of SMC markers (Fig. 7, C and D). These data indicate that Smad2 regulation of RGC-32 expression is an important process in TGF-β-induced SMC differentiation of neural crest cells.
Fig. 7.
Smad2 regulation of RGC-32 is important for SMC marker gene expression. Control (shCtrl) or Smad2 shRNA (shSmad2) was cotransfected with pcDNA 3.0 (−) or RGC-32 cDNA into Monc-1 cells followed by vehicle (−) or TGF-β induction (5 ng/ml) as indicated. qPCR was performed to quantify the mRNA expression of Smad2 (A), RGC-32 (B), α-SMA (C), and SM22α (D). *P < 0.01 compared with shCtrl and TGF-β-treated groups. **P < 0.01 compared with shSmad2 and TGF-β-treated groups.
DISCUSSION
TGF-β plays an important role in SMC differentiation from neural crest Monc-1 cells (6–8). The signaling molecules mediating TGF-β function in this process have been identified to be Smads and RhoA (6, 8). Our recent studies demonstrated that RGC-32 is a TGF-β downstream target important for the regulation of SMC differentiation of neural crest cells (21). Interestingly, RGC-32 was not induced by TGF-β in human umbilical vein endothelial cells. Instead, it is induced by vascular endothelial growth factor (VEGF). VEGF-induced RGC-32 inhibits endothelial cell proliferation, thus impairs hypoxia-regulated angiogenesis (2). It is unknown, however, if TGF-β plays a role in RGC-32 induction in endothelial cells during vascular development.
Both Smad and RhoA signaling appear to be important for RGC-32 activation (21), but how Smad and RhoA regulate RGC-32 gene is unclear. In the present study, we demonstrate for the first time that Smad2 and PEA3 cooperatively regulate RGC-32 gene transcription in the TGF-β-induced SMC differentiation of neural crest cells. Several lines of evidence support our observation. First, TGF-β triggers an interaction of nuclear factors with RGC-32 promoter. Both Smad and PEA3 binding sites are essential for this protein-promoter interaction. The presence of either site only partially competes, but the presence of both sites completely competes for the interaction (Fig. 2D, left). Likewise, mutation of either site only partially inhibits the interaction and promoter activity, but mutation of both sites completely abolishes the interaction and promoter activity (Fig. 2D, right, and Fig. 3). Second, Smad2 (along with Smad4) and PEA3 are present in the TGF-β-inducible complex as recognized by their specific antibodies. The interactions of Smad2 and PEA3 with RGC-32 promoter are confirmed in chromatin setting by ChIP assay. TGF-β induces a dramatic increase of Smad2 and PEA3 binding to endogenous RGC-32 promoter. Third, both Smad2 and PEA3 are important for RGC-32 promoter activity, and Smad2 functionally interacts with PEA3 to enhance RGC-32 transcription. Fourth, Smad2 physically interacts with PEA3. Finally, RGC-32 restores the SMC marker gene expression downregulated by Smad2 shRNA. These data clearly demonstrate that Smad2 regulation of RGC-32 gene transcription is essential for TGF-β-induced SMC differentiation from neural crest cells.
Smad2 and Smad3 are direct mediators of TGF-β signaling. Many studies have shown that Smad2 and Smad3 play distinct roles in mediating TGF-β signaling and regulating gene transcription (5). We found that TGF-β regulation of RGC-32 gene in neural crest cells is Smad2 dependent. Overexpression of Smad2 and Smad4, but not Smad3, significantly increases RGC-32 promoter activity. Smad2, but not Smad3, is present in the TGF-β-inducible complex formed by nuclear proteins with RGC-32 promoter. TGF-β induces an enrichment of Smad2, but not Smad3, binding to endogenous RGC-32 promoter. Moreover, Smad2, but not Smad3, physically interacts with PEA3. Collectively, our results show that Smad2 is critical for RGC-32 gene transcription, whereas Smad3 appears to have no activity although both Smad2 and Smad3 are simultaneously activated after 15 min of TGF-β induction (8).
Smad transcriptional activity can be modulated by the recruitment of coactivators or corepressors (5). Interestingly, SBEs often appear adjacent to binding sites for other transcription factors (13). The SBE in RGC-32 promoter is adjacent to the PEA3 binding site. TGF-β induces an enhanced binding of PEA3 to RGC-32 promoter (Figs. 2 and 4). PEA3 appears to play a role in modulating Smad2 activity. Since Smad2 does not directly bind promoter sequence, the possible underlying mechanism for Smad2 function is that Smad4 binds the SBE and PEA3 binds its nearby site. Smad2, by interacting with both PEA3 and Smad4, provide a bridge between the two DNA-bound proteins. These proteins cooperatively activate RGC-32 promoter, similar to the mechanism governing the regulation of Mixl1 promoter by FOXH1, Smad2, and Smad4 (19). Smad2-mediated RGC-32 transcription appears to be very important for TGF-β-induced SMC differentiation because the downregulation of SMC marker genes due to Smad2 knockdown can be rescued by RGC-32 expression in Monc-1 cells.
Taken together, our results provide novel information about the mechanism underlying TGF-β regulation of RGC-32 promoter in SMC differentiation; i.e., Smad2 interacts with PEA3 to regulate RGC-32 transcription, which further regulates SMC differentiation from neural crest cells.
GRANTS
This work was supported, in whole or in part, by the National Institutes of Health (Grants HL-093429 and HL-107526 to S. Y. Chen and DK-039308, HL-092196, and HL-23081 to P. A. Jose) and Chinese National Nature Science Foundation (30871177 to W. Y. Huang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Xuedong Liu for helping analyze transcription factor binding bites of the RGC-32 promoter.
REFERENCES
- 1. Aikawa M, Sivam PN, Kuro-o M, Kimura K, Nakahara K, Takewaki S, Ueda M, Yamaguchi H, Yazaki Y, Periasamy M. Human smooth muscle myosin heavy chain isoforms as molecular markers for vascular development and atherosclerosis. Circ Res 73: 1000–1012, 1993 [DOI] [PubMed] [Google Scholar]
- 2. An X, Jin Y, Guo H, Foo SY, Cully BL, Wu J, Zeng H, Rosenzweig A, Li J. Response gene to complement 32, a novel hypoxia-regulated angiogenic inhibitor. Circulation 120: 617–627, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bartel FO, Higuchi T, Spyropoulos DD. Mouse models in the study of the Ets family of transcription factors. Oncogene 19: 6443–6454, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Briones VR, Chen S, Riegel AT, Lechleider RJ. Mechanism of fibroblast growth factor-binding protein 1 repression by TGF-beta. Biochem Biophys Res Commun 345: 595–601, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Brown KA, Pietenpol JA, Moses HL. A tale of two proteins: Differential roles and regulation of Smad2 and Smad3 in TGF-beta signaling. J Cell Biochem 101: 9–33, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Chen S, Crawford M, Day RM, Briones VR, Leader JE, Jose PA, Lechleider RJ. RhoA modulates Smad signaling during transforming growth factor-beta-induced smooth muscle differentiation. J Biol Chem 281: 1765–1770, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen S, Kulik M, Lechleider RJ. Smad proteins regulate transcriptional induction of the SM22alpha gene by TGF-beta. Nucleic Acids Res 31: 1302–1310, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen S, Lechleider RJ. Transforming growth factor-beta-induced differentiation of smooth muscle from a neural crest stem cell line. Circ Res 94: 1195–1202, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Cheung TH, Barthel KK, Kwan YL, Liu X. Identifying pattern-defined regulatory islands in mammalian genomes. Proc Natl Acad Sci USA 104: 10116–10121, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choudhary B, Ito Y, Makita T, Sasaki T, Chai Y, Sucov HM. Cardiovascular malformations with normal smooth muscle differentiation in neural crest-specific type II TGFbeta receptor (Tgfbr2) mutant mice. Dev Biol 289: 420–429, 2006 [DOI] [PubMed] [Google Scholar]
- 11. de Caestecker MP, Parks WT, Frank CJ, Castagnino P, Bottaro DP, Roberts AB, Lechleider RJ. Smad2 transduces common signals from receptor serine-threonine and tyrosine kinases. Genes Dev 12: 1587–1592, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Caestecker MP, Yahata T, Wang D, Parks WT, Huang S, Hill CS, Shioda T, Roberts AB, Lechleider RJ. The Smad4 activation domain (SAD) is a proline-rich, p300-dependent transcriptional activation domain. J Biol Chem 275: 2115–2122, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J 17: 3091–3100, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11: 1475–1489, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feinberg MW, Watanabe M, Lebedeva MA, Depina AS, Hanai J, Mammoto T, Frederick JP, Wang XF, Sukhatme VP, Jain MK. Transforming growth factor-beta1 inhibition of vascular smooth muscle cell activation is mediated via Smad3. J Biol Chem 279: 16388–16393, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol 21: 659–693, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Goumans MJ, Mummery C. Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol 44: 253–265, 2000 [PubMed] [Google Scholar]
- 18. Guo X, Jose PA, Chen SY. Response gene to complement 32 interacts with Smad3 to promote epithelial-mesenchymal transition of human renal tubular cells. Am J Physiol Cell Physiol 300: C1415–C1421, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hart AH, Willson TA, Wong M, Parker K, Robb L. Transcriptional regulation of the homeobox gene Mixl1 by TGF-beta and FoxH1. Biochem Biophys Res Commun 333: 1361–1369, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Kocher O, Gabbiani G. Cytoskeletal features of normal and atheromatous human arterial smooth muscle cells. Hum Pathol 17: 875–880, 1986 [DOI] [PubMed] [Google Scholar]
- 21. Li F, Luo Z, Huang W, Lu Q, Wilcox CS, Jose PA, Chen S. Response gene to complement 32, a novel regulator for transforming growth factor-beta-induced smooth muscle differentiation of neural crest cells. J Biol Chem 282: 10133–10137, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol 27: 1248–1258, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Martin ME, Piette J, Yaniv M, Tang WJ, Folk WR. Activation of the polyomavirus enhancer by a murine activator protein 1 (AP1) homolog and two contiguous proteins. Proc Natl Acad Sci USA 85: 5839–5843, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J 19: 1745–1754, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsuura I, Wang G, He D, Liu F. Identification and characterization of ERK MAP kinase phosphorylation sites in Smad3. Biochemistry 44: 12546–12553, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Maurer J, Fuchs S, Jager R, Kurz B, Sommer L, Schorle H. Establishment and controlled differentiation of neural crest stem cell lines using conditional transgenesis. Differentiation 75: 580–591, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Michon IN, Penning LC, Molenaar TJ, van Berkel TJ, Biessen EA, Kuiper J. The effect of TGF-beta receptor binding peptides on smooth muscle cells. Biochem Biophys Res Commun 293: 1279–1286, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Piek E, Heldin CH, Ten Dijke P. Specificity, diversity, and regulation in TGF-beta superfamily signaling. FASEB J 13: 2105–2124, 1999 [PubMed] [Google Scholar]
- 30. Raida M, Clement JH, Leek RD, Ameri K, Bicknell R, Niederwieser D, Harris AL. Bone morphogenetic protein 2 (BMP-2) and induction of tumor angiogenesis. J Cancer Res Clin Oncol 131: 741–750, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Sementchenko VI, Watson DK. Ets target genes: past, present and future. Oncogene 19: 6533–6548, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Shah NM, Groves AK, Anderson DJ. Alternative neural crest cell fates are instructively promoted by TGFbeta superfamily members. Cell 85: 331–343, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Stroschein SL, Wang W, Luo K. Cooperative binding of Smad proteins to two adjacent DNA elements in the plasminogen activator inhibitor-1 promoter mediates transforming growth factor beta-induced smad-dependent transcriptional activation. J Biol Chem 274: 9431–9441, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Wamhoff BR, Bowles DK, McDonald OG, Sinha S, Somlyo AP, Somlyo AV, Owens GK. L-type voltage-gated Ca2+ channels modulate expression of smooth muscle differentiation marker genes via a rho kinase/myocardin/SRF-dependent mechanism. Circ Res 95: 406–414, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Wang G, Matsuura I, He D, Liu F. Transforming growth factorβ-inducible phosphorylation of Smad3. J Biol Chem 284: 9663–9673, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wurdak H, Ittner LM, Lang KS, Leveen P, Suter U, Fischer JA, Karlsson S, Born W, Sommer L. Inactivation of TGFbeta signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome. Genes Dev 19: 530–535, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xie WB, Li Z, Miano JM, Long X, Chen SY. Smad3-mediated myocardin silencing, a novel mechanism governing the initiation of smooth muscle differentiation. J Biol Chem 286: 15050–15057, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature 394: 909–913, 1998 [DOI] [PubMed] [Google Scholar]